Affiliation:
School of Pharmacy, Desh Bhagat University, Mandi Gobindgarh 147301, Punjab, India
ORCID: https://orcid.org/0009-0003-5830-8323
Affiliation:
School of Pharmacy, Desh Bhagat University, Mandi Gobindgarh 147301, Punjab, India
ORCID: https://orcid.org/0009-0005-9534-2218
Affiliation:
School of Pharmacy, Desh Bhagat University, Mandi Gobindgarh 147301, Punjab, India
ORCID: https://orcid.org/0009-0005-6849-7556
Affiliation:
School of Pharmacy, Desh Bhagat University, Mandi Gobindgarh 147301, Punjab, India
Email: anishsingh7421@gmail.com
ORCID: https://orcid.org/0000-0002-8547-2263
Explor Neurosci. 2025;4:100682 DOI: https://doi.org/10.37349/en.2025.100682
Received: January 04, 2025 Accepted: March 11, 2025 Published: April 02, 2025
Academic Editor: Marcello Iriti, Milan State University, Italy
The article belongs to the special issue Medicinal Plants and Bioactive Phytochemicals in Neuroprotection
Alzheimer’s disease (AD), the term “dementia”, describes a specific neuropathology together with the development and progression of age-related cognitive and functional loss. Formononetin is naturally occurring isoflavone recognized for its potential health benefits, anti-oxidant, anti-inflammatory, anti-cancer, and anti-apoptotic properties. Neurodegenerative disorders arise from the gradual loss of function and eventual death of nerve cells in the brain or peripheral nervous system. Astragalus membranaceus is a traditional plant with a variety of pharmacological and biochemical properties, including antiviral, anti-hyperglycaemic, and immunomodulatory effects. Moreover, the expression of membrane-bound and soluble receptor for advanced glycation end products (RAGE) is enhanced in the AD brain due to increased levels of soluble and insoluble amyloid-beta (Aβ) peptides. Additionally, in inflammatory circumstances, leukocytes’ firm attachment and transmigration to endothelial cells are regulated by intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1). Formononetin also possesses anti-bacterial, anti-inflammatory, anti-cancer, anti-oxidant, and estrogenic activity. Formononetin has emerged as a promising agent in the modulation of mediators involved in neurodegenerative disease. Formononetin might modulate nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling pathway to potentiate the anti-Alzheimer’s activity. Additionally, formononetin might inhibit the Aβ/RAGE interaction which further inactivates the activity of extracellular signal regulated kinase (ERK), Janus kinase (JNK) signaling pathway that results in the reduction of nuclear translocation of nuclear factor kappa B (NF-κB) and also reduces the cytokines level to ameliorate AD. It might inhibit the ICAM, VCAM, and THP-1 proteins. Therefore, this compound offers potential therapeutic benefits by reducing cytokine levels to ameliorate AD. This review article is designed to explore the mechanistic interplay underlying the anti-Alzheimer’s effect of A. membranaceus, especially formononetin.
Neurodegenerative diseases are disorders that cause nerve cells, mostly in the brain, to gradually deteriorate. With little progress achieved in the previous ten years in creating viable treatments, neurodegenerative illnesses of the brain represent a significant and growing worldwide health concern [1]. The sixth-most prevalent cause of mortality worldwide is Alzheimer’s disease (AD), an incurable neurological illness that causes a slow decline in brain function [2]. Up to 80% of dementia diagnoses are due to AD, which is by far the most prevalent cause of dementia [3]. The number of sick people is predicted to reach about 152 million by 2050, with emerging nations experiencing the largest rise [4]. With an emphasis on the role of non-physician health care providers and caregivers, the special report examines the broader health care system for older persons with cognitive impairments. Currently, an estimated 6.9 million Americans 65 and older suffer from AD. By 2060, this figure may rise to 13.8 million if no new treatments are developed to prevent or treat AD [5]. The brain produces a protein that takes the shape of “plaques” and “tangles”. The peptide amyloid-beta (Aβ) is created when fragments of the natural amyloid precursor protein (APP) split off, and α-secretase, β-secretase, and γ-secretase all play a part [6]. AD is indicated by neurotic plaques that form Aβ peptide (Aβ42) and neurofibrillary tangles (NFTs) made of hyperphosphorylated tau protein [7]. Due to its 20% higher oxygen consumption than other organs, the brain is more vulnerable to reactive oxygen species (ROS) and reactive nitrogen species (RNS) which interact with neurons which results in mitochondrial malfunction, lipid oxidation, or changes in the redox potential of Aβ metal ions, which causes nerve cell death [8]. When receptor for advanced glycation end products (RAGE) and Aβ interact, inflammatory signaling pathways are triggered, ROS are released to create oxidative stress, and neuroinflammation results, which leads to mitochondrial and neuronal malfunction [9]. Prior studies showed a clear correlation between cognitive functions and the novel function of the hippocampus vascular cell adhesion molecule 1 (VCAM-1) in brain aging control [10]. Whereas, platelet, white blood cell, and vascular endothelial adhesion can be mediated by the inflammatory factor intercellular adhesion molecule 1 (ICAM-1), which is released by vascular endothelium [11]. According to the existing clinical evidence, the beneficial benefits of statins are mediated by changes in the metabolism of tau and Aβ, as well as other clinical characteristics of AD and genetic and lifestyle risk factors [12]. Since beta site APP cleaving enzyme 1 (BACE1) plays a part in the amyloidogenic cleavage of APP, which produces Aβ, it is a logical target in the development of drugs for AD [13]. Moreover, the two primary categories of phytoestrogens are flavonoids and non-flavonoids, which are naturally occurring nonsteroidal phenolic plant chemicals. Additionally, isoflavones can be regarded as endocrine disruptors that could have detrimental effects on the environment or the health of a certain population [14]. Formononetin is an isoflavone that is mostly derived from certain leguminous plants, including Caulis Spatholobi, Pueraria lobata, and Astragalus membranaceus [15]. Evidence suggests that a neuroprotective ingredient in herbal remedies like A. membranaceus and Glycyrrhiza uralensis, formononetin has the ability to target many pathways [16]. Several theories revealed that the neuroprotective potential of the O-methylated isoflavone formononetin has drawn a lot of attention and has been the subject of several studies. Whereas, to deliver neuroprotection, formononetin alters a number of endogenous mediators [17]. Additionally, it has been demonstrated to reduce oxidative stress and apoptosis in brain tissue after ischemia injury, aiding in the recovery from neurodegeneration brought on by stroke [18]. It has a variety of pharmacological actions, including anti-oxidant, anti-inflammatory, anti-cancer, and anti-apoptotic qualities [18]. Various lines of evidence indicate that formononetin modulates the Aβ/RAGE mediated pro-inflammatory cytokines to ameliorate AD [19]. In addition to this, formononetin significantly decreased the VCAM-1 and ICAM-1 levels in cells, the percentage of nuclear p65 protein level, and decreased the localization of nuclear factor kappa B (NF-κB) [20]. Additionally, formononetin modulates the oxidative stress mediated nuclear factor erythroid 2-related factor 2 (Nrf-2) signaling pathway to potentiate the anti-Alzheimer’s activity [21]. Therefore, by synthesizing the published reports on the neuroprotective capabilities of formononetin, we aim to give an insight into the current status of its mechanistic potential in neuroprotection.
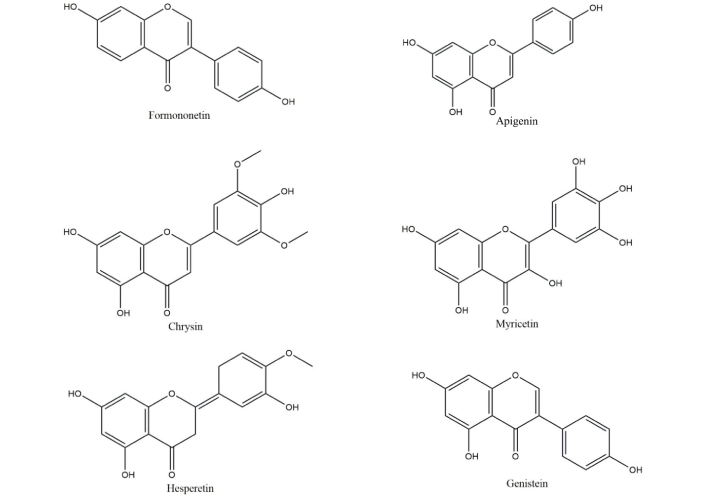
A. membranaceus has been found to contain over 52 different flavonoid components, including flavones, isoflavones, flavanones, flavonols, chalcones, and anthocyanidins [22]. With a total of over 20,000, triterpenes and their saponins are the second largest secondary metabolites found in nature. They are found in fungi, pteridophytes, higher plants, dicotyledons, monocotyledons, and marine organisms [23], while A. membranaceus frequently contains tetracyclic and pentacyclic triterpenoids, which are triterpene saponins. Important tetracyclic triterpenoids, astragalosides, have significant pharmacological effects in addition to a variety of biological activity [24]. Astragaloside and calycosin-7-O-β-D-glucoside (CG) are frequently utilized as “marker components” in the Chinese Pharmacopoeia [25] and are thought to be the most significant bioactive components that belong to the triterpene saponins and flavonoids in A. membranaceus, respectively. Generally, flavonoids are classified as chrysin, kaempferol, luteolin, myricetin, taxifolin, apigenin, eriodictyol, hesperetin, naringenin, genistein; isoflavones are classified as genistein, daidzein, equol, coumestrol, formononetin, calycosin as shown in Figure 1. To date, various studies revealed that A. membranaceus possesses different types of therapeutic effects, especially neuroprotection [26–30]. The plant A. membranaceus and the Chinese medicinal herb red clover (Trifolium pratense) are the sources of the phytoestrogen known as formononetin. It may have hepatoprotective, neuroprotective, and anti-neoplastic effects, according to evidence [31]. The red clover plant extract contains biochanin A, formononetin (biochanin B), genistein, and daidzein. Formononetin [7-hydroxy-3-(4-methoxyphenyl)-4H-1-benzopyran-4-one], one of the primary bioactive substances isolated from red clover, has been identified as the primary compound responsible for the extract’s medicinal actions. It is only found in the Fabaceae family and is taken from the roots of Glycyrrhiza glabra, A. membranaceus, and T. pratense [32]. Recuperation from stroke-induced neurodegeneration has also been demonstrated to be aided by the mitigation of oxidative stress and apoptosis in brain tissue after ischemia injury [33]. Studies on neurological disorders have revealed that formononetin may have neuroprotective benefits, such as reducing inflammation and inhibiting the production of ROS, which may help treat AD and cerebral ischemic stroke [34]. Hence, formononetin molecule which is an isoflavone extracted from A. membranaceus also shows neuroprotective effect.
Diagramatically representation of various extracts from radius Astragalus membranaceus
In certain publications, toxicity investigations of formononetin and its derivatives have been conducted. An acute toxicity investigation of sodium formononetin-3-sulphonate in rats was conducted by Li et al. [35]. Results show that a sodium formononetin-3-sulphonate dosage of 2,000 mg/kg did not cause significant changes in body weight, obvious toxic effects, or death in rats [35]. Research on the acute and subacute toxicity of formononetin was recently conducted by another team. There was no discernible effect on behavior after 14 days of intraperitoneal formononetin treatment at various doses—5 mg/kg, 50 mg/kg, 100 mg/kg, 150 mg/kg, 200 mg/kg, and 300 mg/kg. Formononetin’s LD50 value was determined to be 103.6 mg/kg of body weight, while at 50 mg/kg of body weight, no observed adverse effect level (NOAEL) was detected. There were no signs of side effects or death in the subacute trial. Additionally, hematological and biochemical measurements, as well as body weight, food, and water intake, did not change [36]. Singh et al. [33] investigated the permeability of formononetin using the parallel artificial membrane permeability assay (PAMPA) methodology. According to the PAMPA study, formononetin’s permeability [Pe 106 (cm/sec)] was greater at pH 4 (11.30) and pH 7 (16.50).
Singh et al. [33] also used the plasma ultra-filtration technology to investigate formononetin’s binding to plasma proteins. The results revealed that the plasma levels were 93.61 ± 0.44% and 96.14 ± 0.15% protein binding at two different concentrations, that is 50 ng/mL and 150 ng/mL, respectively [37]. Additionally, the work by Luo et al. [38] shed light on the pharmacokinetic properties of formononetin when it was given to rats orally and intravenously. When formononetin was dissolved in 0.5% CMC-Na, its bioavailability was found to be 21.8%, and it was absorbed in several gastrointestinal tract regions with differing permeability levels. Furthermore, formononetin was shown to be modestly absorbed by passive diffusion using the Caco-2 cell monolayer model [38]. The pharmacokinetic analyses of three formononetin formulations—that is, formononetin dissolved in 1% hydroxypropyl methylcellulose, co-crystal form, and solid dispersion form—were recently carried out by Kim et al. [39] in male Sprague Dawley rats at a dosage of 20 mg/kg. The results showed that there was no significant difference between the co-crystal formulation’s Cmax (58.34 ± 10.98 nmol/L) and AUCinf (278.47 ± 34.40 h × nmol/L) and the 1% hydroxypropyl methylcellulose (Cmax: 110.03 ± 158.20 nmol/L). In contrast to the 1% hydroxypropyl methylcellulose group, the solid dispersion formulation demonstrated a considerable increase in the Cmax (1,343.06 ± 876.85 nmol/L) and AUCinf (2,267.55 ± 904.88 h × nmol/L), or 12 and 3 times, respectively [39].
Several pieces of evidence suggest that increased Aβ level in the brain increases the RAGE. RAGE serve as the receptor for Aβ. Interaction with Aβ/RAGE confers to increased pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-18 by increasing ROS and oxidative stress [40]. To date, numerous in vitro and in vivo activities revealed that formononetin ameliorates AD as shown in Table 1.
Various in vitro and in vivo studies of formononetin might ameliorate Alzheimer’s disease
| Sr. no | Model | Dose | Effect | Reference |
|---|---|---|---|---|
| 1. | HBEMC cell model | 1–20 µM | Decreased the VCAM and ICAMDecreased the percentage of NF-κBIncreased the Nrf-2 levelAmeliorated the adhesion of THP-1 protein | [20] |
| 2. | APP/PS1 mouse model | 10 mg/kg | Increased swimming lengthDecreased latency and APP synthesisIncreased the production of AβIncreased LRP1 and ApoJ levelsDecreased RAGE and NF-κB p65Increased IL-6 and TNF-α levelDecreased mRNA and protein levels | [21] |
| 3. | Cell culture model | 0.1–100 µM | Increased living cellDecreased the cell death rate, LDH release, and caspase-3 activityIncreased cell viability in the cell culture and the level of sAβPPαIncreased α-secretaseIncreased levels of ADAM10 in cellsN2a-AβPP cells were treated and increased both ADAM10 and mRNA level | [22] |
VCAM: vascular cell adhesion molecule; ICAM: intercellular adhesion molecule; NF-κB: nuclear factor kappa B; Nrf-2: nuclear factor erythroid 2-related factor 2; APP: amyloid precursor protein; Aβ: amyloid-beta; RAGE: receptor for advanced glycation end products; IL: interleukin; TNF: tumor necrosis factor
By Using APP/PS1 mouse model, Fei et al. [21] documented that formononetin significantly increased swimming length and decreased latency. It also reduced Aβ burden by decreasing APP synthesis in the brain of mice. Moreover, significantly there is an increase in the production of Aβ in familial AD. Western blot analysis revealed that it also increased LRP1 and ApoJ levels. Additionally, it also significantly decreased the protein expressions of RAGE, NF-κB p65, oxidative stress injury, inflammatory response, and neuronal cell death. Furthermore, it also increased IL-6 and TNF-α levels in the brains of mice. Moreover, it also reduced protein levels and also decreased mRNA expressions [21]. Using HBMEC cell model, Fan et al. [20] documented that formononetin (1–20 µM) significantly decreased the VCAM-1 and ICAM-1 levels in cells [20]. Moreover, it decreased the percentage of nuclear p65 protein level, localization of NF-κB (p65) in the nucleus of HBMECs and nuclear p65 levels in NC siRNA-transfected HBMECs. Furthermore, it also ameliorated the adhesion of THP-1 protein to cells [20]. Furthermore, it also reduced the caspase-3 activity and increased the cell viability in the cell culture. It also increased the level of sAβPPα. The increase in sAβPPα was conducted by shifting AβPP processing to the non-amyloid cleavage pathway. It also showed a threefold increase of α-secretase as compared to the hypoxia group. In addition to this, it also increased the levels of both premature and mature ADAM10 in cells. In the normoxia condition, the N2a-AβPP cells were treated with it and the total protein level of ADAM10 and mRNA level were both increased as shown in Figure 2 [22].
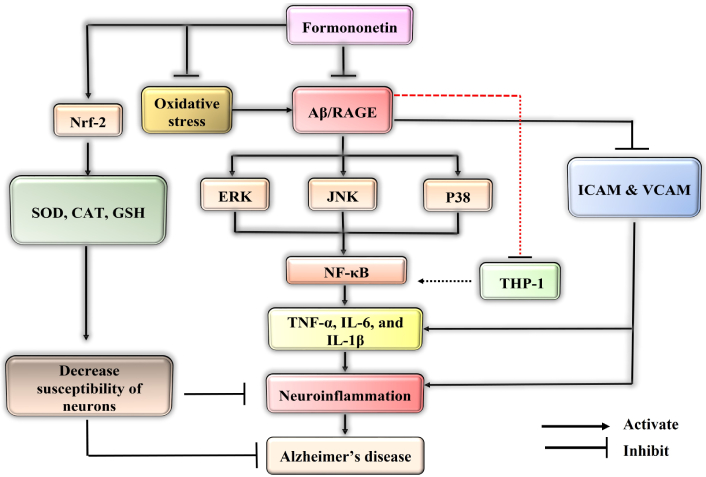
Mechanistic interplay of formononetin by various pathways to confer protection against Alzheimer’s disease (AD). Formononetin might inhibit the amyloid-beta (Aβ)/receptor for advanced glycation end products (RAGE) interaction which further inactivates the activity of extracellular signal regulated kinase (ERK), Janus kinase (JNK), P38 that results in the reduction of nuclear translocation of nuclear factor kappa B (NF-κB) and also reduces the cytokines level to ameliorate AD. It might inhibit the intercellular adhesion molecule (ICAM), vascular cell adhesion molecule (VCAM), and THP-1 proteins. It inhibited the oxidative stress that activates Aβ/RAGE which generally increase neuroinflammation. It also increased the nuclear factor erythroid 2-related factor 2 (Nrf-2) factor that leads to neuroprotection. TNF: tumor necrosis factor; IL: interleukin
Several pieces of evidence suggest that increased oxidative stress confer to inactivate the Keap1 leading to the phosphorylation of Nrf-2. Generally, Nrf-2 plays a crucial role in the regulation of anti-oxidant defense within the basic leucine zipper transcription factor family [40]. Using HBMEC cell model, author demonstrated that formononetin (1–20 µM) significantly increased the Nrf-2 protein levels. Moreover, Nrf-2 undergoes ubiquitination with Keap1 protein. Western blots revealed that it significantly reduced the interaction of Nrf-2 with Keap1. Generally, Nrf-2 transcriptional pathway plays a pivotal role in the detoxification and removal of ROS [40, 41]. Using a cell culture model, Sun et al. [22] documented that formononetin (0.1 µM, 1 µM, 10 µM, 20 µM, 50 µM, and 100 µM) significantly reduced cell viability under hypoxia conditions, however, formononetin also increased living cell number and decreased the cell death rate. It also reduced the hypoxia-mediated LDH released by nearly 40% [42].
AD dementia describes specific neuropathology along with an age-related start and progression of cognitive and functional impairment. Formononetin provides neuroprotection by modifying a number of endogenous mediators. By reducing the amount of ROS and pro-inflammatory cytokines, it inhibits RAGE activation, which in turn inhibits neuronal damage. Formononetin might inhibit the Aβ/RAGE interaction which further inactivates the activity of extracellular signal regulated kinase (ERK), Janus kinase (JNK), P38 that results in the reduction of nuclear translocation of NF-κB and also reduces the cytokines level to ameliorate AD. It might inhibit the ICAM, VCAM, and THP-1 proteins. Formononetin might activate Nrf-2 signaling pathway that activates the anti-oxidant mechanism that leads to the increased levels of GSH, SOD, CAT to potentiate the anti-Alzheimer’s activity. Formononetin also possesses anti-bacterial, anti-inflammatory, anti-cancer, anti-oxidant, and estrogenic activity. Additionally, exploring the potential synergistic effects of formononetin in neuroprotection possesses novel therapeutic interventions for neurodegenerative diseases. The lack of clinical studies and pharmacokinetic profiling of metabolites of formononetin are the major hindrances to its development as a therapeutic agent. In the future, by conducting preclinical and clinical studies to validate their efficacy, potential as therapeutic agents for neurodegenerative disorders could be increased.
The primary obstacle to developing formononetin as a therapeutic agent is the absence of clinical studies and a pharmacokinetic profile of its metabolites. Therefore, more research is necessary to understand the detailed pharmacokinetic profile of formononetin, along with conducting clinical trials. However, it is crucial to recognize that translating laboratory findings to clinical applications can be intricate and challenging. Another challenge is that these metabolites can show different levels of bioactivity and unique pharmacokinetic characteristics, which can significantly impact the overall pharmacological outcomes.
A. membranaceus: Astragalus membranaceus
AD: Alzheimer’s disease
APP: amyloid precursor protein
Aβ: amyloid-beta
ICAM-1: intercellular adhesion molecule 1
IL: interleukin
NF-κB: nuclear factor kappa B
Nrf-2: nuclear factor erythroid 2-related factor 2
RAGE: receptor for advanced glycation end products
ROS: reactive oxygen species
VCAM-1: vascular cell adhesion molecule 1
MK: Writing—original draft, Investigation. SS and Avikramjeet S: Writing—review & editing, Investigation. Anish S: Conceptualization, Writing—original draft, Writing—review & editing, Investigation.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Deepa A V, Dennis Thomas T
Neha Kamboj ... Rahul Kumar
Suvendu Ghosh ... Debosree Ghosh
Apoorva A. Bankar ... Nazma N. Inamdar
