Affiliation:
Gastroenterology, Hepatology & Digestive Endoscopy Section, Department of Medicine & Surgery, University of Perugia, 06156 Perugia, Italy
Email: gabassot@gmail.com
ORCID: https://orcid.org/0000-0002-0237-1812
Explor Neurosci. 2025;4:100683 DOI: https://doi.org/10.37349/en.2025.100683
Received: February 01, 2025 Accepted: March 21, 2025 Published: April 11, 2025
Academic Editor: Janine Gronewold, University Hospital Essen, Germany
The article belongs to the special issue Enteric Neuro-Gliopathies: Ready for Prime Time?
“Functional” gut disorders are clinical conditions frequently encountered in clinical practice, often characterized by abnormalities of the intestinal sensory and motor functions. Although traditionally believed not harboring organic abnormalities, some of these disorders have been demonstrated to have more or less subtle involvement of the enteric nervous system. This involvement has been especially documented for enteric glial cells, even though other elements may be involved. Given the pivotal role of enteric glial cells in gut pathophysiology and their evident abnormalities in some disorders of gut-brain interaction, it may be time to reconsider their role and recognize them as an important pathophysiological factor in these conditions. Thus, due to the prominent neuronal and glial involvement in some clinically severe forms, it is proposed that at least some of the “functional” gut disorders should be reclassified as enteric neuro-gliopathies.
“Functional” gastrointestinal disorders are clinicopathological conditions frequently encountered in clinical practice, often characterized by sensory/motor intestinal disturbances [1]. These conditions are associated with poor quality of life and high healthcare costs [2, 3]. Of interest, there has been recent literature re-assertion that the diagnosis of these disorders should be based on “excluding organic etiologies of the patient's symptoms” [1]. This statement maintains the old distinction between “functional” and “organic” disorders of the gastrointestinal tract, proposed many years ago [4]. This distinction, however, disregards the accumulating evidence of an organic basis for at least some of these clinicopathological conditions. Indeed, in the last years, it has progressively emerged that some “functional” gut disorders, presently re-named “disorders of gut-brain interaction (DGBI)” [5], may in fact have an organic background when appropriately investigated. Thus, at least for some of them a more appropriate label, such as “entero-gliopathies” has been proposed [6]. In this article, the perspective supporting this claim will be analyzed under the light of these evidences.
By widely accepted definition the term “functional”, when applied to a disease, implies the absence of underlying organic pathophysiological mechanisms or conditions, whether anatomic, inflammatory, neoplastic or otherwise. Thus, the DGBIs (formerly “functional gut disorders”) are usually classified according to the Rome IV criteria [7] and require the exclusion of an underlying or associated organic condition to be diagnosed [1, 7]. However, since the original appearance of Rome criteria in the 80’s, the knowledge about these disorders has considerably increased and the firm belief that no organic abnormality is present has been shaken by solid evidences. In fact, at least some (or some subtypes of) DGBIs may actually display some underlying subtle organic pathophysiological mechanisms as responsible for their clinical manifestations [8, 9]. These abnormalities chiefly involve the cell populations constituting the enteric nervous system (ENS) and/or the mucosal immune system [10, 11], and are usually detected with specific pathologic techniques [12], thereby explaining why in past the routinary assessment by hematoxylin-eosin stains has failed to show them.
The ENS, also known as “the brain of the gut”, regulates the main intestinal functions (motility, secretion, perception) by means of a complex neural network arranged in several interconnected plexuses. Two of these plexuses are ganglionated and are represented by the submucosal (Meissner’s) and the myenteric (Auerbach’s) plexus, whose cellular components are constituted by a dense array of enteric neurons (EN) and enteric glial cells (EGC) [13]. These latter cells have received in recent years enormous attention by researchers and, due to their multiple functions, are presently considered as a crossway of paramount importance for the homeostasis of the gut [14] (Figure 1). Together with the interstitial cells of Cajal (ICC) [15], responsible for pacemaker activity, EN and EGC govern (by means of their bidirectional communications involving their multiple functions) the complex motor activity of the gut [13]. Thus, as we will see below, it is not surprising that abnormalities involving one or both cell populations may have important pathophysiological consequences, especially concerning intestinal motility [12].
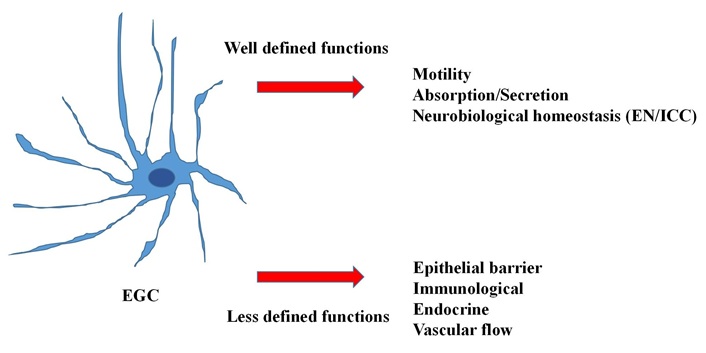
The multiple functions of the enteric glial cells. EGC: enteric glial cells; EN: enteric neurons; ICC: interstitial cells of Cajal
In recent years, the attention of researchers has focused on abnormalities involving the cell subpopulations that constitute the ENS. The main findings will be summarized below.
This has traditionally been considered as the prototype “functional” gut disorder, and it represents without doubts one of the most frequently encountered DGBI in the daily clinical practice. Irritable bowel syndrome (IBS) is clinically defined by abdominal pain/discomfort associated with variations of bowel habits (constipation/diarrhea) [7]. Although the diagnosis of IBS is still one of exclusion, some authors, a few years ago, have convincingly demonstrated that in patients with severe IBS may be actually present a low-grade inflammation involving the intestinal ENS [16]. Since then, evidence has mounted confirming that this entity may actually recognize more or less subtle and different abnormalities of the ENS as responsible for at least some of the symptoms [17], especially those related to abnormal gut motility/perception and to the regulation of epithelial barrier functions [18]. Even though most of the evidence had been obtained from animal studies, human investigations have also shown altered interactions between EGC and mast cells and abnormal glioplasticity in these patients [19].
One of the most frequent DGBIs in the clinical practice, chronic constipation (CC) is an umbrella term including several clinical subtypes, ranging from mild symptoms to very severe forms presenting an important decrease (up to the total absence) of colonic propulsive activity [20]. These patients are frequently unresponsive to most therapeutic options [21], and may require surgery to alleviate symptoms. There is presently good evidence, obtained in surgically treated patients with severe and medically refractory CC, that assessment of the ENS may reveal several abnormalities. For instance, most patients with severe slow-transit constipation undergoing colectomy for medically refractory constipation have a significant decrease of EN, EGC, and ICC in the colon, compared to controls [22]. Of interest, these abnormalities are also present in the small bowel [23], providing a possible explanation to the gut dysmotilities frequently reported in these subjects [24]. It is worth noting that a significant decrease of EGC has been also documented in patients undergoing surgery for intractable constipation due to obstructed defecation [25]. Of note, in these patients the EGC and glial filaments were close to degranulated mast cells, suggesting a possible common pathophysiologic event [26].
A significant decrease of colonic EGC has been also reported in surgical samples obtained from constipated patients with Chagasic and idiopathic megacolon undergoing colectomy for severe symptoms [27].
All in all, the above observations suggest that several conditions clinically characterized by the presence of constipation are linked to abnormalities of colonic EGC.
An infrequent but potentially lethal condition, chronic intestinal pseudo-obstruction (CIPO) is a clinical syndrome characterized by signs and symptoms of intestinal obstruction in the absence of mechanical obstruction [28]. Although several cases of CIPO may be secondary to other pathological conditions (e.g., scleroderma), many patients are classified as “functional gut disorders”. This is due to the fact that usually there are no apparently detectable macro- or microscopic abnormalities in the tissues examined after surgical procedures are carried out in these patients. However, in recent years the diagnostic approach to CIPO has benefitted from more in-depth pathological analyses. For instance, some studies showed an abnormal activity of the EGC as possibly responsible for the intestinal motor abnormalities documented in these patients [17]. Interestingly, a study reported that the localization of some viruses in the EGC of patients with CIPO might suggest a possible pathophysiological role of pathogens in the origin of symptoms in these patients [29]. Other studies showed abnormal glial signaling in these patients, and suggested a possible role for EGC in the genesis of intestinal dysmotilities in subjects with CIPO [30]. The above observations are of paramount importance to address potential new issues for treating these complex conditions [31], often unresponsive even to multiple therapeutic approaches.
The field of neurogastroenterology is ebullient, with new discoveries and new concepts being continuously proposed to the research and the clinical communities. Indeed, the technological advancements to investigate the multi-faceted aspects of the ENS are quite common and rapid. In fact, new methodologies are continuously being proposed and used, such as those developed to profile the enteric trascriptome and cell lineages in animal and human models [32, 33], which could aid future clinical management. Thus, a more precise classification of some DGBIs may contribute to paradigm shifts and breakthroughs in future research.
At present, there is accumulating evidence that at least some of the previously defined “functional” gut disorders (recently renamed DGBI) may in fact not be “functional” at all. In fact, when properly investigated tissues from some subcategories of these patients may reveal the presence of more or less subtle organic abnormalities. These abnormalities have been mainly described in the ENS of the small bowel and (especially) the colon even though, from time to time, may be also present in the esophagus [34]. However, since these findings have been usually reported in patients with severe clinical involvement requiring a surgical approach to alleviate the symptoms, it is obvious that the results should not be automatically translated to all subjects belonging to these categories (in particular, to IBS and constipated patients).
Although the described anomalies encompass with more or less intensity all the main cell populations of the ENS (i.e., neurons, ICC, and EGC) there are instances in which the constant abnormality only relates to EGC. In particular, it is worth noting the fact that EGC are abnormally reduced in different measure in all patients so far investigated for different subtypes of CC, suggesting a likely pathophysiological role for this cell population. For this reason, it is suggested that the term “neuro-gliopathies” should be added to the classification of at least some subtypes of DGBIs. Of course, further solid evidence is needed to firmly establish this definition, but the time is ripe to move forward and think outside the old schemes.
CC: chronic constipation
CIPO: chronic intestinal pseudo-obstruction
DGBI: disorders of gut-brain interaction
EGC: enteric glial cells
EN: enteric neurons
ENS: enteric nervous system
IBS: irritable bowel syndrome
ICC: interstitial cells of Cajal
GB: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
Gabrio Bassotti who is the Guest Editor of Exploration of Neuroscience had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1789
Download: 20
Times Cited: 0
Luxita Sharma, Dhananjay Sharma
Ravi Philip Rajkumar
Momin Ahmed ... Brandon Lucke-Wold
