Affiliation:
1Nucleic Acids Research Lab, Department of Chemistry, University of Delhi (North Campus), Delhi 110007, India
ORCID: https://orcid.org/0000-0003-4980-0669
Affiliation:
2Genomics and Molecular Medicine Unit, CSIR-Institute of Genomics and Integrative Biology (IGIB), Delhi 110007, India
3Academy of Scientific and Innovative Research (AcSIR), CSIR-Institute of Genomics and Integrative Biology (CSIR-IGIB) Campus, Delhi 110007, India
ORCID: https://orcid.org/0000-0002-6968-1129
Affiliation:
4Department of Physiology and Pharmacology “Vittorio Erspamer”, Sapienza University of Rome, 00185 Rome, Italy
ORCID: https://orcid.org/0000-0003-4530-8706
Affiliation:
1Nucleic Acids Research Lab, Department of Chemistry, University of Delhi (North Campus), Delhi 110007, India
Email: skukreti@chemistry.du.ac.in
ORCID: https://orcid.org/0000-0002-7305-8364
Explor Med. 2025;6:1001372 DOI: https://doi.org/10.37349/emed.2025.1001372
Received: July 22, 2025 Accepted: October 13, 2025 Published: November 25, 2025
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
The article belongs to the special issue Lipid Peroxidation and Cancer
The brain lipid profile is a complex and dynamic system playing a critical role in regulating various functions, including mood swings, perception, and emotional behavior. Explicitly, the enrichment of polyunsaturated fatty acids (PUFAs) in the brain and membranes exposes them to reactive free radical species [reactive oxygen species (ROS)/reactive nitrogen species (RNS)], leading to lipid peroxidation (LP), which may result in disruption of cell fluidity and membrane permeability, hindering cellular functions. An increase in LP end products specifically triggers apoptosis and necrosis, potentially resulting in the onset of serious ailments such as neurodegenerative diseases, cancer, atherosclerosis, and diabetes. Cells are equipped with antioxidant defense systems to combat and scavenge harmful reactive free radical species, thereby maintaining redox homeostasis. Indisputably, the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) is a key player in regulating the cellular antioxidant response by controlling gene expression related to oxidative and electrophilic stress. Nrf2 also influences various cellular processes such as metabolism, inflammation, drug detoxification, and DNA repair. In recent years, several compounds have emerged as Nrf2 modulators, including curcumin, quercetin, anthocyanins, tea polyphenols, kaempferol, hesperetin, and icariin. These compounds play a vital role in regulating various essential Nrf2 upstream activators, thereby modulating Nrf2 pathways, predominantly upregulated by several phytochemical compounds, such as terpenoids like monoterpenes (aucubin, catapol), diterpenes (ginkgolides), triterpenes (ginsenosides), and carotenoids (astaxanthin, lycopene). This review is a modest attempt to provide a comprehensive literature appraisal, facilitating a deeper understanding of the significant role of Nrf2 modulators in obstructing LP and treating serious diseases such as cancer.
Free radical species [reactive oxygen species (ROS)/reactive nitrogen species (RNS)] are entities specifically involved in oxygen and nitrogen radicals, continuously generated during the mitochondrial respiration process. A moderate level of ROS is substantial for the signaling and other cellular processes but is detrimental in excess [1, 2]. A plethora of studies are indicative of the interlinking of free radicals such as superoxide radicals, singlet oxygen (1O2), hydrogen peroxide (H2O2), hydroxyl, and RNS like peroxynitrite, etc., in the etiology of cancer and are categorically named as ‘prooxidants’ [3]. The redox status of the cell is maintained by controlling the generation of reactive free radical species and their scavenging process via the antioxidant system of cellular machinery, such as glutathione, superoxide dismutase (SOD), catalase (CAT), vitamin C, vitamin E, etc. Reduction of the antioxidant defense system leads to excessive accumulation of ROS/RNS in the cell, thus resulting in deleterious effects on macromolecules, i.e., nucleic acids, proteins, and lipids [4].
Lipid enrichment in cells is undeniably reflected in its crucial role in various biological processes, i.e., as a signaling molecule, energy reservoir, and protective covering for nerve cells [5, 6]. Enrichment in polyunsaturated fatty acids (PUFAs) cellular and organelle membranes poses a threat to lipid peroxidation (LP), which is a malicious process that is triggered by ROS and RNS that react with lipid molecules present in cellular organelles, thus leading to the production of lipid peroxides. Various end products of LP, including 4-hydroxy-2-nonenal (4-HNE) and malondialdehyde (MDA), are predominantly linked to the progression of serious ailments such as cancer, cardiovascular diseases, diabetes, and neurodegeneration [7, 8]. Recent reports have suggested that ROS-triggered LP also promotes apoptosis and autophagy [9]. It is now established that LP plays a key role in carcinogenesis and disease progression, along with its existence in fat-enriched cancerous tissue [10–12]. There are contrasting reports that LP might aid in an anticarcinogenic mechanism via activating apoptotic pathways, resulting in cell death [13, 14]. This is supported by the hypothesis that radiotherapy and chemotherapy are mediated through oxidative stress (OS) generation by inducing LP, eliminating tumor cells [15, 16]. Also, it is well documented that the higher level of LP and its secondary metabolic products in breast cancer patients is observed than in non-tumor samples [17–20]. Additionally, other studies are indicative of the relation of elevated levels of LP with disease recurrence, progressive disease stage, and aggressive molecular subtypes [21–23]. LP is substantially linked to carcinogenesis and needs to be targeted with the required combinatorial therapy. Figure 1 depicts the sources of ROS and aberrations caused in biomolecules (DNA, protein, and lipid) and morbidity caused by ROS.
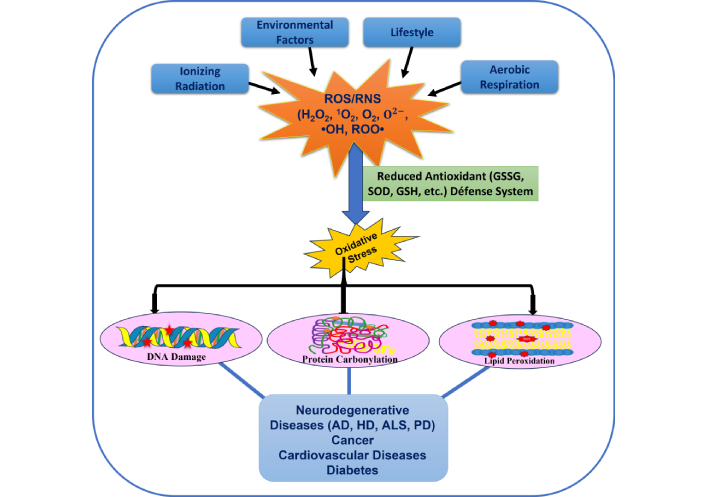
Schematic representation of sources of ROS, effects on biomolecules (nucleic acids, proteins, lipids), and diseases. ROS: reactive oxygen species; RNS: reactive nitrogen species; H2O2: hydrogen peroxide; GSSG: oxidized glutathione; GSH: glutathione; SOD: superoxide dismutase; AD: Alzheimer’s disease; HD: Huntington’s disease; ALS: amyotrophic lateral sclerosis; PD: Parkinson’s disease.
The cell has an endogenous antioxidant defense system that systematically regulates the level of ROS/RNS, including the transcription factor nuclear factor erythroid 2-related factor 2 (Nrf2) [24]. The Nrf2/Kelch-like ECH-associated protein 1 (Keap1) pathways protect cells from ROS/RNS-mediated OS damage. Nrf2 has been found to regulate the expression of more than 300 target genes, crucially involved in antioxidant and anti-inflammatory roles, cell metabolism, proliferation, and differentiation, thus leading to cytoprotection [25, 26]. The role of Nrf2 is also associated with preventing and promoting cancer, but it is unclear whether it suppresses cancer promotion or exerts pro-oncogenic functions. Literature is rich in reports that several modulators exist that effectively regulate the expression of Nrf2 efficiently. This review presents a modest attempt to provide an overview of the exact role of Nrf2 and its modulators in combating LP and carcinogenesis.
ROS is the most important chemical entity playing a dual role in biological systems, acting as both a cell’s signaling molecule and an agent of cellular damage. These are oxygen derivatives such as superoxide anion (O2−), H2O2, hydroxyl radical (•OH), peroxyl radicals (ROO•), and 1O2, etc. There is a misconception regarding ROS that all free radical species perform a similar function in the cell; however, this is not so. Apart from cell signaling, an elevated level of ROS is indicative of the transition from normal, healthy tissue to the invasion of carcinoma [27]. It is produced during cellular metabolic reactions in mitochondria and NADPH oxidases (NOX) [28, 29]. Under normal physiological conditions, an optimum level of ROS is beneficial for cell signaling and to maintain cell homeostasis. An excess level of ROS extends adverse effects on cell components, biomolecules (DNA, protein, and lipids), and aggravates disease conditions. LP is defined as the spontaneous oxidative deterioration of unsaturated fatty acids by adding oxygen molecules to the non-polar lipids’ unsaturated fatty acyl chain. It is a chain reaction and can be manifested via two pathways, i.e., enzymatic and nonenzymatic.
In this pathway, a specific category of proteins, such as lipoxygenase (LOX), cyclooxygenase (COX), and cytochrome P450, which contains peroxidase activity, catalyze LP, where it is also involved in the formation of R-OOH. The proteins that have pseudo-peroxidase activity (cytochrome c) under some conditions can also catalyze LP via binding to cardiolipin [30–32]. LOX specifically catalyzes the oxidation of abundantly found arachidonic (C20:4) and linoleic (C18:2) polyenoic fatty acids by molecular oxygen to form hydroperoxyl groups at distinct carbon positions of acyl chains [33]. Several reports have shown that among COX-1 and COX-2 isoforms, the latter is crucially expressed in tumoral tissues [34–36]. Some contrasting reports revealed the existence of the enzyme overexpression product eicosanoids in breast, lung, and pancreatic cancer. The proinflammatory product prostaglandin E2 (PGE2) is found to be produced via COX-2 in mutagenesis, angiogenesis, and cell migration, all linked with cancer. The COX-2 mechanism pathway has been proven using biological cell lines, i.e., human colorectal HT-29 and human prostate carcinoma DU145 cell lines [37, 38]. A crucial interlink is found between the production of PGE2 along with tumor cell resistance to programmed cell death via the activation of a cascade of P2Y2 P2Y2/Src/p38 [P2Y2 nucleotide receptor, Src is the Src protein-tyrosine kinase, and p38 refers to the p38 mitogen-activated protein kinase (MAPK) signaling pathway]. Through this membrane released arachidonic acid (AA) by PLA2 overexpression, simultaneously with the overexpression of COX-2, with PGE2 production [38]. Figure 2 illustrates the conversion of membrane phospholipids into AA, as well as the involvement of COX-1, COX-2, and LOX in tumor growth, and the role of inhibitors in the apoptosis of cancerous cells.
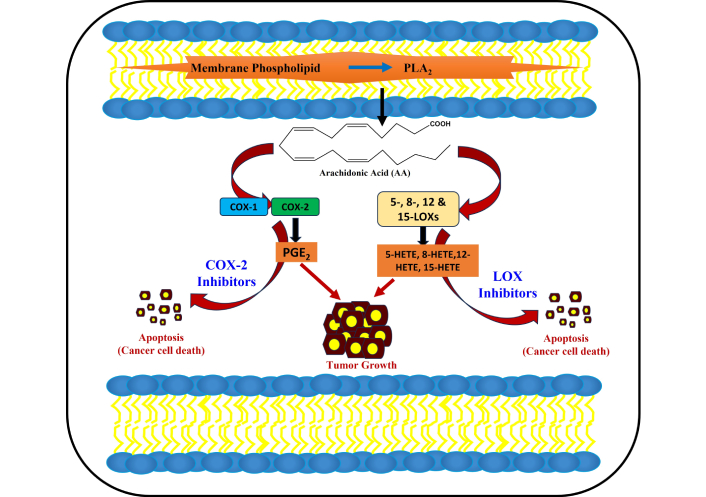
Membrane phospholipids are converted into phospholipase A, which results in arachidonic acid. Tumor progression results from the activation of LOX and COX-2, and is suppressed by LOX and COX-2 inhibitors, resulting in apoptosis (cancer cell death). COX: cyclooxygenase; LOX: lipoxygenase; PGE2: prostaglandin E2; HETE: hydroxyeicosatetraenoic acid.
In non-enzymatic reactions, the initiation of radical chain reactions is essential for LP mediated via the Fenton reaction by involvement of transition metals, i.e., iron. Iron is highly reactive within cells and generates •OH by reacting with endogenously produced H2O2. The production of hydroxyl and ROO• initiates the lipid production. Similarly, like H2O2, PLOOH is also involved in the Fenton reaction, ultimately leading by a series of reactions to the generation of lipid hydroxy radical (PLO•) and lipid peroxyl radical (PLOO•) respectively [39, 40]. If PLOOH is neutralized, LP cannot take place; if not scavenged by antioxidants, propagation of LP takes place to the neighboring PUFA-phospholipids. This propagation leads to the deterioration of the plasma membrane, and LP causes fluidity and leakage in the cell membrane and obstructs membrane-bound enzyme activity [41]. In the absence of an efficient antioxidant defense system, lipid hydroperoxides become unstable and can lead to the production of secondary products, i.e., MDA, 4-HNE, and other reactive aldehydes [42, 43]. These deleterious effects lead to a reduction in cellular processes and aggravate cytotoxicity. Ultimately, uncontrolled cellular growth and lump formation take place, leading to apoptosis [8, 44]. Though, optimum level of LP is substantial for physiological processes and cell signaling, elevated LP causes morbidity of cells via involvement in several pathological conditions like neurodegenerative diseases, cardiovascular diseases, inflammation, and cancers [43, 45–47].
Mitochondria are known as the powerhouse of eukaryotic cells owing to their pivotal role in ATP production through oxidative phosphorylation. A great number of superoxides are produced at the complexes I and III of the electron transport chain (ETC). This is why it is significantly called the hub of ROS generation; consequently, ROS is substantially involved in LP. Although the role of OS in cancer has been well reported in the literature, the amount of LP products in cancer cells has also been a matter of investigation by many groups. This section encompasses the role of LP products in cancer.
LP results in secondary metabolic products, MDA, also known as MDA, which can be produced via either peroxidation of PUFAs present in the membrane or prostaglandin production [48, 49]. MDA is a highly reactive and more toxic aldehyde, and when accumulated, it leads to membrane permeability and hinders the membrane fluidity of the bilayer lipid membrane. It is very mutagenic and reacts with biomolecules, specifically forming adducts with deoxyadenosine(dA) and deoxyguanosine(dG) of DNA, resulting in the formation of DNA adducts [50–52]. MDA-DNA adduct formation results in strand breaks, cell cycle arrest, and ultimately cell death. This is also linked to the development and progression of carcinogenesis. MDA is significantly used as a biomarker for the identification of LP [53, 54].
LP of PUFAs produces an array of secondary metabolic products and lipid electrophiles. Among all the lipid byproducts, 4-HNE is one of the most extensively studied lipid oxidative products. Though the biological consequences of 4-HNE are well established, the exact pathway via which its formation takes place is still not clear [55]. Many groups have suggested that 4-HNE is produced from the decomposition of the hydroperoxide of ω-6 PUFAs at the sn-2 position of glycerophospholipids present in cellular lipid membranes. It was found that most of the 4-HNE production is contributed by linoleic acid (LA, 18:2, ω-6) and AA (20:4, ω-6), which are abundantly present in cellular membranes [56]. A significant amount of HNE was also produced from the oxidation of mitochondrial phospholipids and cardiolipin, along with other oxidation products. HNE, linked with biomolecules and forms adducts, thus leading to the deterioration of structural proteins and DNA. The HNE adducts substantially impact key signaling pathways such as the mitogen-activated kinase signaling pathway, Nrf2, activating protein AP-2, and NF-κB, etc. [57]. Microtubule dysfunction and deteriorated synaptic system are also significantly affected by the abnormality in glucose uptake at synapses, crucially intervened by HNE [58].
Established reports also indicate the key role of HNE in controlling cell proliferation and differentiation. It has been demonstrated that cell line K562 derived from human erythroleukemia, cell proliferation is found to be decreased, as well as c-myc oncogene expression is blocked significantly by HNE [59, 60]. Pizzimenti et al. [61] and Rinaldi et al. [62] have reported that HNE is found to be involved in hindering the c-myc expression in U937, ML1 human leukemic cells, and murine erythroleukemic (MEL) cells. In tumor cell line SK-N-BE (neuroblastoma cells), HNE efficiently blocks cell proliferation and elicits apoptosis. In tumor cells that significantly express p53 and its family members (p63, p73), HNE is crucially found to be involved in inducing expression of these cells, thus resulting in inhibiting proliferation [63, 64]. HNE-mediated inhibition of human colon tumor cells through regulation of MAP kinase and PPAR gamma pathways has also been reported [65, 66]. Similarly, inhibition of cell proliferation is also reported in breast cancer cells, i.e., MCF7, by treatment with conjugated LA, leading to the elevation of endogenous levels of HNE [67] and in osteosarcoma cells treated with HNE [68]. Numerous reports reflect the pivotal role of HNE in controlling cell proliferation in tumor cells, whereas it does not affect the normal differentiation and growth of normal cells [69].
Among all the LP products, acrolein is the most reactive peroxidation product of PUFAs. It is also generated from the partial combustion of organic materials or fuel such as coal, petrol, and wood [70]. Apart from this, acrolein can also be present in cigarette smoke and sources like cyclophosphamide bioactivation. Threonine metabolism by myeloperoxidase of activated phagocytes is also involved in acrolein production [71]. It consists of three carbon atoms bonded via a double bond and is present in 40 times more concentration than any other transient reactive oxidant species [72]. Acrolein is also an electrophile and binds to the nucleophilic sites of basic cellular enzymes, DNA, and proteins, specifically to histidyl, lysyl, and cysteinyl residues, along with the N-terminal amino group of proteins. Acrolein also forms adducts with cysteinyl residues of enzymes (which are crucially involved in the catalytic activity of enzymes) and hinders the enzyme activity via interfering with substrate binding, thus leading to enzyme deactivation [73].
Neurofilament aggregation is also found to be induced by acrolein, which plays a vital role in cross-linking, resulting in OS induced production of a large amount of protein carbonylation, leading to neurodegenerative diseases [74]. Feng et al. [75] have established that acrolein is a major contributing agent for cigarette smoke-related lung cancer, playing a malicious role in DNA damage and obstructing DNA repair. Others have demonstrated that acrolein suppresses anticancer drug-induced cytotoxicity via the overexpression of CLDN1. Enhanced CLDN1 expression also upregulates the Nrf2 signaling pathway. Acrolein-mediated silencing of CLDN1 is directly linked to less sensitivity towards anticancer drugs, thus leading to cancer progression [76]. A report by Tsai et al. [77] showed that acrolein-induced DNA damage is found to be significantly higher in colorectal cancer tissues than in normal epithelial cells in colorectal cancer patients. Acrolein plays a crucial role in oncogenic transformation through activation of the RAS/MAPK signaling pathway, thus resulting in colon tumorigenesis [77, 78]. The underlying mechanisms are established by using cDNA microarray analysis with Ingenuity Pathway Analysis in NIH/3T3 Acr-clone#4 cells to establish that acrolein is involved in oncogenic transformation. The Tsai group [77] successfully demonstrated that four genes (Rnd1, Rras2, myc, and PI3Kcb) involved in the RAS/MAPK signaling pathway were upregulated in acrolein-transformed clone #4 (NIH/3T3 Acr-clone #4). These results were further supported by Western blot analysis, along with the finding that acrolein activated the RAS/MAPK signaling pathway and increased c-myc in NIH/3T3 cells and the human normal colon epithelium CCD-841CoN. Surprisingly, acrolein was found to induce cell proliferation, colony formation activity, and cell migration capacity in CCD-841CoN cells. RAS/MAP signaling pathways are crucially activated in the CCD-841CoN Acr clone as well as in human colon cancer cell lines, SW480 and HCT116. The study demonstrated that acrolein prominently mediated and induced oncogenic transformation through activation of the RAS/MAPK pathways [77].
In 1990, one more peroxidation product was identified, which resembles prostaglandin compounds generated from the peroxidation of AA in situ in phospholipids known as F2-isoprostanes (F2-IsoPs) [79]. Unlike other LP products, Isoprostanes are prone to detection easily owing to chemical and metabolic stability in a number of biological samples such as urine, plasma, and tissues, respectively [56]. Isoprostanes can be accumulated and aggregated in cell membranes, thus impairing the fluidity of membranes and aiding in the etiology of serious ailments such as neurodegenerative diseases, cancers, etc. F2-IsoPs elevated level is analyzed by employing gas chromatography/negative ion chemical ionization mass spectrometry for OS level [80–82]. Figure 3 depicts a schematic representation of the LP and the formation of the secondary metabolic product of LP.
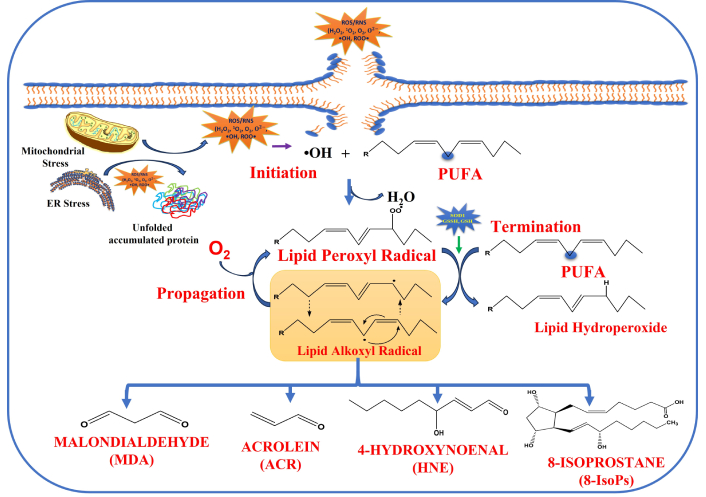
Lipid peroxidation via free radicals is generated extracellularly and intracellularly, the mechanism of lipid peroxidation leads to the formation of secondary metabolic products such as MDA, HNE, ACR, and 8-IsoPs, respectively. ROS: reactive oxygen species; RNS: reactive nitrogen species; H2O2: hydrogen peroxide; PUFA: polyunsaturated fatty acids; HNE: hydroxy-2-nonenal; MDA: malondialdehyde.
Many groups are engaged extensively to prove the involvement of isoprostanes with cancers [83–85]. Ma et al. [86] have reported that the UPLC-MS/MS-based method is an efficient tool to measure the 8-isoprostane plasma concentration and identified as a biomarker for early lung cancer screening [86]. Rasool et al. [87] have established the key role of isoprostanes and matrix metalloproteinase-7 (MMP7) in the development of colorectal cancer in males. More studies are underway to unravel the exact pathway for the link between isoprostanes and carcinogenesis.
An optimum level of ROS/RNS is inevitable for the normal functioning of the cells. An elevated level of ROS/RNS exerts a high level of OS, which in turn is found to be one of the characteristics of cancer cells [88]. Human cells have an adequate defensive system to combat and scavenge the ROS/RNS, which are continuously produced in biological systems both intracellularly and extracellularly. The most significant cellular defense system against OS is the Nrf2/Keap1 signaling cascade [89].
Nrf2 is a basic leucine zipper transcription factor, also most commonly known as Cap “n” Collar (CNC) transcription factor, crucially responsible for the critical regulation of cytoprotective response against OS [90]. Nrf2 orchestrates metabolic stability, proteostasis, and redox balance, and has emerged significantly as a key regulator of cellular homeostasis, required for the proper functioning and vitality of an organism. Nrf2 binds to the antioxidant response elements (AREs) in the promoters of target genes, thus mediating the upregulation of antioxidants and phase II detoxifying enzymes such as glutamate-cysteine ligase catalytic subunit (GCLC), haem oxygenase 1 (HO-1), and NAD(P)H-quinone oxidoreductase 1 (NQO1), etc. Figure 4 depicts the role of Nrf2 in the regulation of various genes involved in myriad metabolic functions.
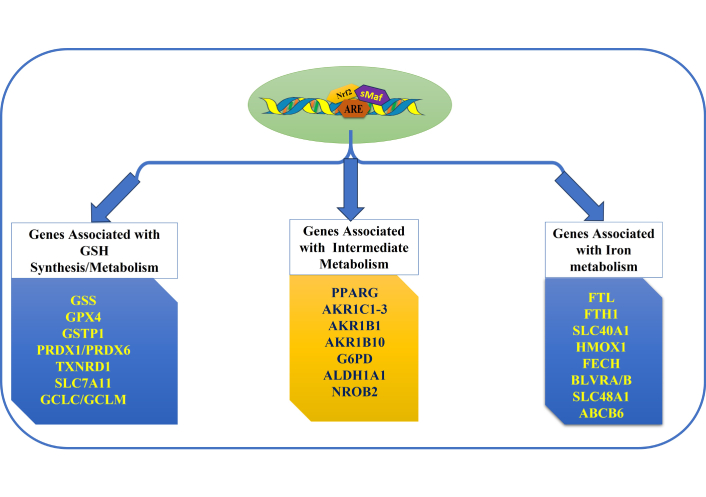
Role of Nrf2 in the regulation of target genes involved in myriad metabolic functions. Nrf2: nuclear factor erythroid 2-related factor 2; ARE: antioxidant response element.
Under normal physiological conditions, Nrf2 is kept inactive by Keap1, which significantly keeps a surveillance on Nrf2 and retains it in the cytoplasm, and gradually Nrf2 undergoes proteasomal degradation (Figure 5).
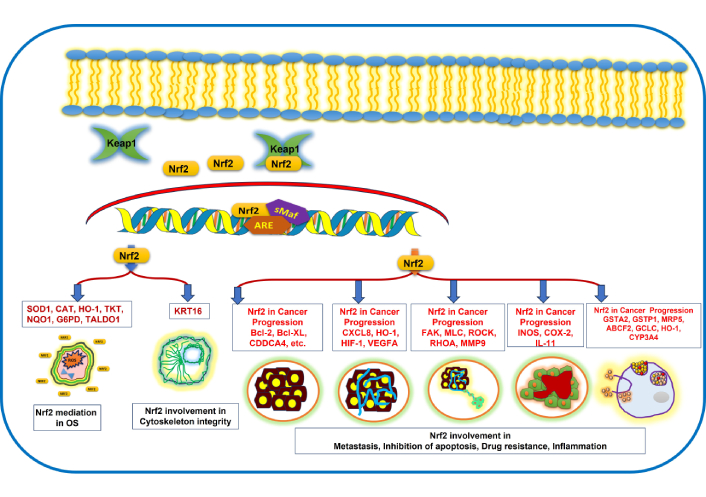
Role of Nrf2 in various cellular processes and signaling, as well as anticarcinogenic activity. Keap1: Kelch-like ECH-associated protein 1; Nrf2: nuclear factor erythroid 2-related factor 2; SOD1: superoxide dismutase 1; CAT: catalase; HO-1: haem oxygenase 1; TKT: transketolase; NQO1: NAD(P)H-quinone oxidoreductase 1; G6PD: glucose-6-phosphate dehydrogenase; TALDO1: transaldolase 1; KRT16: keratin 16; Bcl-2: B-cell lymphoma 2; Bcl-XL: B-cell lymphoma-extra-large; CDDCA4: cluster of differentiation 4; CXCL8: C-X-C motif chemokine ligand 8; HIF-1: hypoxia-inducible factor 1; VEGFA: vascular endothelial growth factor A; FAK: focal adhesion kinase; MLC: myosin light chain; ROCK: Rho-associated coiled-coil containing kinase; RHOA: Ras homolog gene family member A; MMP9: matrix metalloproteinase-9; INOS: inducible nitric oxide synthase; COX-2: cyclooxygenase-2; IL-11: interleukin-11; GSTA2: glutathione s-transferase alpha 2; GSTP1: glutathione S-transferase pi 1; MRP5: multidrug resistance protein 5; ABCF2: ATP-binding cassette subfamily F member 2; GCLC: glutamate-cysteine ligase catalytic subunit; CYP3A4: cytochrome P450 family 3 subfamily A member 4.
Due to its degradation, Nrf2 is unable to bind to the nuclear ARE; thus, it is not available for the facilitation of transcription of various antioxidants and detoxifying enzymes [91, 92]. An elevation of ROS/RNS leads to OS, and on the onset of this, Nrf2 is lodged in the nucleus and, on attaching itself to sMaf protein to form a heterodimer, this complex in turn binds to the ARE sequences, leading to the regulation of transcription of several antioxidant genes [91]. Nrf2 was launched to safeguard cells against malicious hazardous materials, and many chemoprotective agents have been identified that modulate activation of Nrf2 [93]. It is noteworthy that though enrichment of documents reflects that Nrf2 target genes are substantially linked to the obstruction of LP products, thus leading to the inhibition of carcinogenesis, many reactive lipid species, such as HNE, are shown to form adducts with cysteines of Keap1, which is a negative regulator of Nrf2 leading to over expression of Nrf2 target genes [94]. Reports are suggestive that modification in Keap1 facilitates the activation of Nrf2 to intercept LP, in contrast, many reactive lipid species are found to suppress/inhibit the function of Nrf2 target genes, which in turn aggravate tumorigenesis and other serious ailments [95–102]. It is worth mentioning here that in diseases like cancer, where Nrf2 is high in tumor cells, the defensive antioxidant system can be utilized as a protective strategy against LP and secondary LP products accumulation, whereas in neurodegenerative diseases, Nrf2 is low, and LP is significantly enhanced, leading to disease progression. Owing to its potential mediation in promoting cell survival, Nrf2 and its associated target gene inhibition are linked to suppressed responsiveness to cellular stress and apoptosis. Recently, the term ferroptosis has emerged and been extensively studied as a novel cell death cascade, which is iron-dependent, lipid-peroxide-mediated, non-apoptotic regulated cell death. It has been crucially found to be a promising therapeutic target for cancer therapies, due to its association with elevated iron and lipid peroxide levels as well as Nrf2 dysfunction [103–105]. The role of Nrf2 in normal cells as well as in cancer cells is schematically represented in Figure 6.
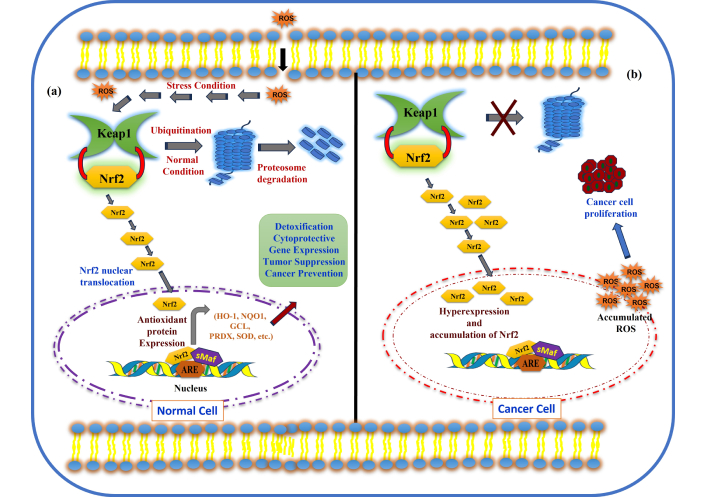
Nrf2 activation in normal cells and cancer cells. (a) Under normal conditions, and (b) oxidative stress conditions (hyperactivation of Nrf2 and cancer cell proliferation in cancer cells). Nrf2: nuclear factor erythroid 2-related factor 2.
Mounting evidence revealed that Nrf2 is identified as suppressing, as well as mitigating the tumors, and thus acts as a double-edged sword, as briefly described in the following section.
Undeniably, Nrf2 plays a substantial role in anti-tumor activity by sustaining redox homeostasis, modulating cell growth, cell differentiation, and anti-inflammatory activities [106]. Reports revealed that the Nrf2 signaling pathway is crucially involved in detoxification of ROS/RNS via modulating drug-metabolizing enzymes, thus leading to the suppression of the OS-mediated cancer development. It is well documented that Nrf2 activation is perceived in several cancers, such as bladder cancer, cervical cancer, breast cancer, colon cancer, glioblastoma, glioma, hepatocellular carcinoma, lung cancer, pancreatic cancer, multiple myeloma, ovarian cancer, and gastric cancer [107, 108].
One piece of evidence has suggested that in the Nrf2 knockout model, Nrf2 facilitated protection in the skin, bladder, and stomach against chemically induced carcinogenesis. In another report, on exposure to benzopyrene, Nrf2-null mice were shown to develop gastric neoplasia [109]. Multiple reports have established that upon exposure to azoxymethane followed by dextran sodium sulfate, intestinal tumors develop significantly more in Nrf2-deficient mice than in the wild-type mice [110, 111]. Reports also indicate that many cancers, such as mammary, skin, and colorectal cancers, have increased substantially with administration of carcinogenic molecules [112]. The exact pathway of Nrf2-mediated preventive mechanism of cells against carcinogenesis is via reducing ROS/RNS levels, leading to the inhibition of lipid peroxide products and DNA damage. Additional experimental evidences bolster the claim that Nrf2 reflects protective behavior towards carcinogenesis. An experiment demonstrated in mice, possessing a single nucleotide polymorphism (SNP) in the promoter location of the murine Nrf2 gene, revealed the reduced expression of Nrf2, posing a threat for hyperoxia-induced lung morbidity [113]. Suzuki et al. [114] and Zhao et al. [115] have reported that owing to this SNP, the mRNA expression level of Nrf2 declined, linking to the elevated vulnerability towards non-small-cell lung cancer (NSCLC). A plethora of evidence has illustrated the preventive impact of the Nrf2 signaling pathway against several cancers [116–118].
Though the preventive effect of Nrf2 is well established, the dark side and detrimental effects of Nrf2 in cancer activation and progression are also well demonstrated by many reports. Modulation of Nrf2 activation plays a crucial role in disease progression. The emergence of the negative effect of Nrf2 in carcinogenesis and the progression of several cancers fueled controversy about the beneficial role of Nrf2 in cancer prevention or tumor suppression. Nrf2 hyperactivation within tumor cells raised questions on its preventive measures as antioxidants, as well as anticancer effect or chemoprevention [119]. Reports are suggestive of an antagonistic effect of overexpression of Nrf2 in the survival of cancer cells, thus leading to vital involvement in cancer progression [120], and it was evident that cancer cells employed Nrf2 as a self-protective mechanism for survival [121].
Nrf2 actively participates in cancer cell proliferation and invasion, along with suppression of the antiapoptotic activity of cells via the overstimulation of anti-apoptotic protein Bcl-2. Nrf2 hyperactivation and increased levels of Nrf2 were envisaged in several tumors and malignancies, such as bladder, ovarian, pancreatic, prostate, lung, breast, and endometrium, respectively [122]. Nrf2 activation induced by radiotherapy and chemotherapy resistance is also significantly observed, reflecting its malicious role in cancer progression. Numerous reports documented the deleterious role of Nrf2 in cell proliferation, cancer progression, and suppressing cell death [123–126].
The main challenge for the eradication of cancer is chemoresistance and drug efflux. Nowadays, ROS and LP products like HNE are targeted for cancer therapy along with conventional therapies such as chemotherapy and radiotherapy. The irony of the treatment is the toxicity of chemotherapy drugs and their toxicity towards non-malignant cells because they not only kill cancer cells, but drugs may also cause morbidity to normal cells. Earlier studies suggest that the use of antioxidant supplementation should be practiced to mitigate the side effects of anticancer therapies, like chemotherapies and radiation. It is advised to choose wisely and cautiously the antioxidant supplements because they have a detrimental effect during cancer therapy [127].
The Nrf2-Keap1 pathway is also targeted for treating cancer owing to its cytoprotective responses. It is worth mentioning that Nrf2 is identified as a double-headed arrow owing to its cytoprotective role and response at early stages of carcinogenesis via activation of antioxidant genes for scavenging ROS and balancing redox homeostasis, which leads to stopping DNA mutation and cell proliferation, whereas it poses a detrimental effect at later stages of carcinogenesis by imposing resistance to chemotherapy/radiotherapy. Several natural as well as synthetic compounds have been identified as Nrf2 modulators and inhibitors in chemoprevention and therapeutic strategies. Brief accounts of Nrf2 activators and inhibitors, along with their sources, actions, and cancer types, are summarized in Table 1.
Nrf2 activators and inhibitors.
| S. NO. | Compound | Source | Type | Mechanism of action | Cancer type | References |
|---|---|---|---|---|---|---|
| Nrf2 activators | Sulforaphane (SFN) [1-isothiocyanato-4-(methylsulfonyl)-butane] | Broccoli, Brussels sprouts | Isothiocyanate | Electrophilic modification of Keap1-Cys-151 | Bladder cancer | [128] |
| Oltipraz (4-methyl-5-[2-pyrazinyl]-1,2-dithiole-3-thione) (OPZ) | Cruciferous vegetables | Organosulfur compound | Electrophilic modification of Keap1-Cys-151 | Various cancers in animal models | [129] | |
| Epigallocatechin gallate (EGCG) | Green tea | Catechin | Oxidizing the cysteine thiols of Keap1 | Prostate cancer, head and neck, and lung cancer cell lines | [130] | |
| Dimethyl fumarate (tecfidera or DMF) | Synthetic drug | Fumaric acid ester | Electrophilic modification of Keap1-Cys-151 | Breast, colon, melanoma, and pancreatic cancer | [131] | |
| Diallyl trisulfide (DATS) | Garlic | Isothiocyanate | Modification of Keap1-Cys-288 | Colorectal, breast, and bladder cancer | [132] | |
| Curcumin (CUR) | Dried rhizome of Curcuma longa | Stilbene | Electrophilic modification of Keap1-Cys-151 | Gastric cancer | [133] | |
| 2-Cyano-3,12-dioxooleana-1,9(11)-dien-28-oic acid (CDDO) | Synthetic derivative of oleanolic acid, a naturally occurring plant compound | Synthetic triterpenoids | Electrophilic modification of Keap1-Cys-151 | Pancreatic cancer (e.g., Panc1, Panc28 cells), colon cancer, ovarian cancer, and diffuse large B-cell lymphoma | [134] | |
| Apigenin (4,5,7-trihydroxyflavone (Api)) | Celery, parsley | Plant flavone | Epigenetic modifications of Nrf2 | Breast, colorectal, prostate, liver, lung, skin, and oral cancers | [135] | |
| Resveratrol (3,5,4’-trihydroxystilbene (RES)) | Grapes, berries | (E)-Stilbene derivate | Electrophilic modification of Keap1-Cys-151 | Breast, prostate, colon, ovarian, thyroid, and melanoma | [136] | |
| Carotenoids | Fruits and vegetables | Natural pigments | Potential for cancer prevention by activating the Nrf2 pathway | Initial stage of cancer | [137] | |
| Nrf2 inhibitors | Brusatol (BRU) | Brucea javanica (an evergreen shrub) | Triterpene lactone compound | Stimulation of Nrf2 poly-ubiquitination | Colorectal, pancreatic, lung, liver, leukemia, and glioblastoma | [138] |
| Luteolin (3’,4’,5,7-tetrahydroxyflavone (LUT)) | Celery, parsley, green pepper | Plant flavone | Nrf2 mRNA degradation, reduction of Nrf2 binding to AREs | Lung, breast, prostate, liver, colon, and pancreatic cancers | [139] | |
| Trigonelline (TRG) | Coffee beans and many plants | Coffee-derived alkaloid | Prevention of nuclear translocation of Nrf2 | Pancreatic cancer cell lines | [140] | |
| Ascorbic acid (vitamin C, L-ascorbic acid, AscA, AA) | Citrus fruits | Natural vitamin | Electrophilic modification of Keap1-Cys-151 | Chronic myelogenous leukemia KCL22/SR cells | [141] | |
| Retinoic acid (RA) | Carrots, sweet potatoes, mangoes, papayas, and apricots | Metabolite of vitamin A | Prevention of nuclear translocation of Nrf2 | Breast, prostate, and lung cancer | [142] | |
| Chrysin (5,7-dihydroxy-2-phenyl-4H-chromen-4-one (CHR)) | Honey and propolis | Plant flavone | Prevention of nuclear translocation of Nrf2 | Lung, breast, liver, colon, leukemia, prostate, and melanoma | [143] | |
| Quercetin | Apples, various fruits, and vegetables | Flavonoid | High concentrations can inhibit Nrf2 to increase oxidative stress and promoting cancer cell death | Malignant tumor formation | [144] | |
| Wogonin (5,7-dihydroxy-8-methoxyflavone) | Root of Scutellaria baicalensis | Flavonoids | Nrf2 inhibitor wogonin holds good potential as an efficient natural sensitizer for anti-neoplastic resistance in human myelogenous leukemia | Myelogenous leukemia | [145] | |
| Halofuginone | Root of Dichroa febrifuga Lour. (Blue Evergreen Hydrangea) | Naturally occurring alkaloid | Enhanced the chemosensitivity of cancer cells via suppressing Nrf2 activation | Cancer, fibrosis | [146] | |
| Berberine | Coptidis Rhizoma | Isoquinoline alkaloid | BBR could reverse the lapatinib resistance in HER2-positive breast cancer cells by upregulating the level of ROS through inhibiting the Nrf2 pathway | Breast cancer cells | [147] | |
| Brucein D | Seeds of Brucea javanica | Quassinoid compound | Inhibiting Nrf2 expression via promoting the ubiquitin-proteasome-dependent degradation and downregulating its downstream genes such as HO-1, NQO1, AKR1B10 and γGCSm | Pancreatic ductal adenocarcinoma | [148] | |
| Cryptotanshinone | Root of Salvia miltiorrhiza | Diterpene quinone | Induce cell death and apoptosis in human lung carcinoma A549 (A549/DDP) cells and enhance the sensitivity to cisplatin by down-regulating the Nrf2 pathway | Human lung carcinoma A549 | [149] | |
| Ginsenoside Rd | Active constituents of ginsenosides, is a potent antitumor agent | Active ingredients of ginseng | Depletion of the expression of Nrf2 and its target genes, resulting in an enhanced sensitivity of A549 cells to cisplatin and amelioration of chemoresistance | NSCLC | [150] | |
| Triptolide | Tripterygium wilfordii | Diterpenoid | Reduced Nrf2 expression and transcriptional activity in NSCLC and liver cancer cells | Malignant tumors | [151] |
AA: arachidonic acid; HO-1: haem oxygenase 1; Keap1: Kelch-like ECH-associated protein 1; NQO1: NAD(P)H-quinone oxidoreductase 1; Nrf2: nuclear factor erythroid 2-related factor 2; NSCLC: non-small-cell lung cancer; ROS: reactive oxygen species; AREs: antioxidant response elements.
To prevent cancer and carcinogenesis, several natural (polyphenols, carotenoids, flavonoids, isothiocyanates, vitamins, etc.) and synthetic compounds have been identified and studied as Nrf2 activators for modulating its expression and signaling. Compounds typically belonging to phenolic and sulfur-containing groups are found to be promising chemoprotective agents. Curcumin and resveratrol (RSV) are the most extensively studied natural compounds, crucially found to inhibit many types of cancer cell proliferation [152–154]. It was reported by a group that curcumin and RSV were found to be involved in the inhibition of cell growth, which was affected by dosage [155]. The IC50 of DLD-1 cell lines is observed 71.8 μM (20.5 μM curcumin + 51.3 μM RSV), while the IC50 of CaCo-2 cell line was 66.21 μM (18.9 μM curcumin + 47.3 μM RSV) [156]. It has also been reported that curcumin significantly facilitates apoptosis in various cancer cell types in the human colon [157, 158]. Bisht and Maitra [159] have established in a nude mouse xenograft model that curcumin is found to be crucially linked to the obstruction of in vivo growth suppression in head and neck squamous cell carcinoma. Along with these, liposomal formulations of curcumin have been extensively studied in various cancers, including pancreatic, colorectal, and prostate [160–163]. Kunnumakkara et al. [164] have demonstrated that cell proliferation and metastasis are inhibited by curcumin in breast cancer cells via downregulating the Fen1 expression by activating and modulating the Nrf2 cell signaling. Many dietary vitamins, such as ascorbic acid (vitamin C), retinoic acid (vitamin A), flavonoids, phytochemicals, including cryptotanshinone, polyphenols like epigallocatechin gallate (EGCG), theaflavin, luteolin, isothiocyanates, etc., demonstrated a promising role as Nrf2 activators in chemoprevention. Among all, tea polyphenols emerged as an extensively studied natural phytochemical with enormous health benefits. It is bestowed with chemoprotective and therapeutic virtues against carcinogenesis due to tea polyphenols’ redox modulating properties [165, 166]. Bioavailability of tea polyphenols and their concentrations are also determining factors in therapeutics against tumorigenesis. Tea polyphenols modulate Nrf2 via many cross-regulators, thus crucially linking to play a vital role in preventing and reducing cancer risk across major cancer types such as ovarian cancer, breast cancer, colorectal cancer, urinary bladder cancer, prostate cancer, and stomach cancer [167, 168]. A schematic representation of Nrf2 activators is summarized in Figure 7.
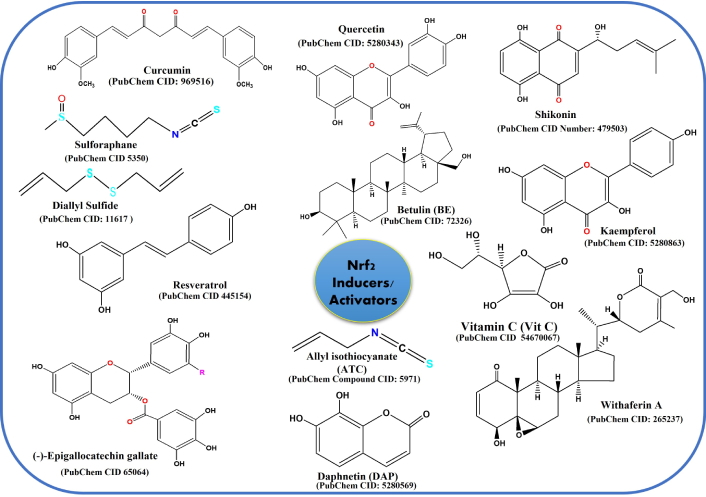
Compounds that are used as Nrf2 inducers/activators as cancer chemoprotective agents. Nrf2: nuclear factor erythroid 2-related factor 2.
Likewise, sulforaphane (SFN), an isothiocyanate prevalently found in cruciferous vegetables such as broccoli, is the most extensively studied natural compound crucially involved in modulating Nrf2-Keap1 signaling, thus leading to the reduction in cancer incidents [169]. The chemoprotective properties of broccoli sprouts are also studied by performing a phase II clinical study done in China, that SFN present in broccoli suppresses alpha-toxin-DNA adducts, thus resulting in protection against hepatocellular carcinoma [170]. SFN crucially aids in directly modifying Keap1, resulting in Nrf2 release and modulating nuclear translocation. Other signaling pathways which linked to the phosphorylation of Nrf2 are also activated by SFN, which leads to the Nrf2 activation [171]. RTA405, commonly known as oleanane triterpenoid, is shown to modulate and activate Nrf2 via binding to Keap1, abrogate it to facilitate Nrf2 degradation. The effect of RTA405 on Nrf2 activation via mere deletion of Keap1 has been demonstrated, and the findings indicate that the mechanism is different, which distinctly affects cancer proliferation and carcinogenesis [172]. Allicin (dillyl thiocyanate), specifically found in crushed garlic, was primarily identified earlier as antimicrobial, anti-inflammatory, and possessing other biological activities [173, 174]. Later, other studies depicted its anticancer activity and showed that it induced apoptosis via elevated translocation and promoter binding activity of Nrf2. It has been established that Nrf2-mediated allicin induced apoptosis of colon cancer cells, thus playing a vital role in checking the cancer cell growth [175–177]. A dietary polyphenol known as RSV (5-[(E)-2-(4-hydroxyphenyl)ethenyl]benzene-1,3-diol), abundantly found in white hellebore, blueberry, peanut, and grapes, with structural similarity to mammalian estrogen 17ꞵ-estradiol (E2) [117]. RSV plays a key role in the reduction of breast cancer, along with having anti-inflammatory, anti-metastatic, anti-angiogenic, and pro-apoptotic activities. RSV is found to be predominantly involved in modulating antioxidant bioactivities by regulating antioxidant gene expression via the Keap1/Nrf2 pathway and SIRT1 [178]. A study revealed that estrogen-induced breast carcinogenesis in the E2-treated rat model and MCF-10A cells is substantially obstructed by RSV [179]. RSV-mediated Nrf2 activation and its nuclear accumulation pose inhibition of carcinogenesis in E2-incubated MCF-10A cells and 7,12-dimethyl-benz(a)anthracene (DMBA)-induced mammary tumor growth in rats, along with reduced inflammatory response and downregulation of many prognostic breast cancer markers such as cyclin D1 (CCND), cytokeratin 19 (CK19), etc. [180]. A study demonstrated that synthetic 5α-reductase inhibitors such as finasteride and durasteride gained potential attention as possible prostate chemopreventive agents. Two clinical trials showed that finasteride and durasteride crucially involve and suppress the occurrence of prostate cancer formation in men [181, 182]. However, in contrast, these studies also revealed the occurrence of aggressive prostate tumors (Gleason scores 7–10) enhanced after the administration of finasteride or durasterideto the patients [183]. These studies revealed the positive side of Nrf2 activation as well as their side effects via many natural and synthetic modulators in preventing cancer cell proliferation as well as in therapeutics.
Literature is rich in reports elucidating the beneficial effects of Nrf2 activation in chemoprevention; however, numerous reports also reflect the harmful role of Nrf2 in chemoresistance [184]. Wang et al. [185] have studied the role of Nrf2 in tissue samples of lung cancer in patients from stage I to stage III. The group demonstrated that overexpression of Nrf2 elevates chemoresistance, whereas downregulation of Nrf2 reduces cancer cell proliferation, with increased sensitivity towards chemotherapeutic drugs like cisplatin, doxorubicin, etc. Therefore, they suggested that to enhance the efficacy and bioavailability of anticancer drugs to treat patients, specific Nrf2 inhibitors should be identified and designed [185]. Figure 8 depicts a few natural and synthetic Nrf2 inhibitors.
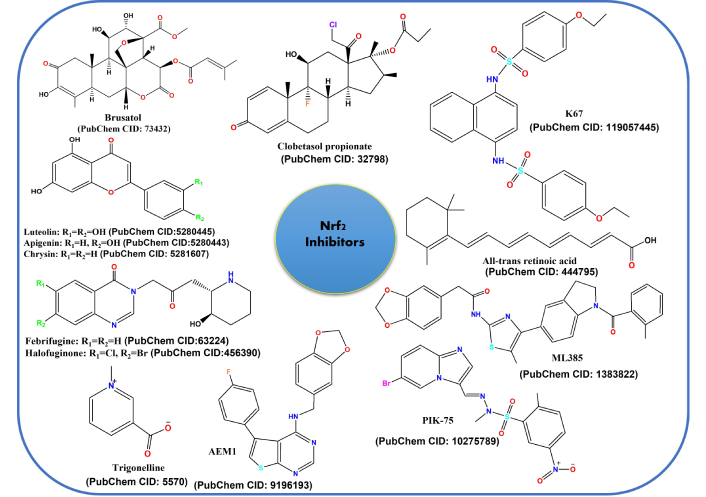
Compounds that are used as Nrf2 inhibitors for cancer chemoprotective agents. Nrf2: nuclear factor erythroid 2-related factor 2.
Several natural compounds have been identified to possess Nrf2 inhibitory properties. Brusatol, a natural quassinoid, extracted from Brucea javanica, is crucially involved in Nrf2 inhibition by hindering the transcriptional activity of Nrf2, facilitating the elevation in sensitivity of chemotherapy in tumor and cancer cell lines. In contrast, brusatol has also exhibited morbid effects on protein translation, affecting many short-lived beneficial proteins along with Nrf2 [186, 187]. In contrast, brusatol was found to cause side effects such as hypotension, nausea and vomiting in clinical studies [188]. This is very obvious and can be explained as Nrf2 is widely expressed in normal cells, and using Nrf2 as a target for tumor therapy may have some side effects also. The serious challenges of using brusatol for personalized cancer medicine include unclear mechanism of action/pathways, non-specific targets, toxicity, and side effects like nausea, hypotension, etc. Further research is required to explore patient specific development of analogues to improve the therapeutic profile and clinical translation [189]. Few natural flavonoids like luteolin have also been found to be linked to inhibiting Nrf2 transcription, thus aiding in sensitizing the cancer cells towards anticancer drugs [190, 191]. Moreover, studies have also indicated its significant role in Nrf2 activation, raising concerns regarding the exact role of Nrf2 in carcinogenesis. Another compound, ochratoxin A, inhibits Nrf2 at distinct phases, such as Nrf2 translocation into the nucleus, Nrf2 transcription, along Nrf2-DNA binding interference, respectively [192]. Synthetic compounds, i.e., dexamethasone and clobetasol propionate, are also shown to inhibit either obstructing Nrf2 transcription or preventing Nrf2 translocation. Retinoic acid also inhibits Nrf2 via binding to the Neh7 domain of Nrf2, thus resulting in the hindrance of Nrf2 transcription [142]. In addition, numerous natural and synthetic compounds have been identified that impede the expression of Nrf2, consequently leading to cell differentiation and cell proliferation [107].
However, the role of Nrf2 activation and inhibition is controversial in carcinogenesis; a comprehensive investigation is thus required to gain more insight about the exact signaling pathways involved in carcinogenesis.
The role of Nrf2 is paradoxically skeptical in cancer prevention as well as cancer promotion, as its involvement in signaling of multiple genes [193]. Cancer cells hijack Nrf2 signaling, which triggers Nrf2 modulators to accelerate tumor progression and metastasis observed in a mouse model [194–196]. Mounting evidence revealed that cancer cells exploit Nrf2 signaling for tumor proliferation and aggressive growth [197, 198]. With the advent of modern advances in genomics, proteomics, and metabolomics and the availability of large clinical genetic databases, more precise treatment with greater efficacy can be facilitated, which is the main basis of precision cancer medicine. Nrf2 has been found to be a potential biomarker in precision cancer medicine because of genetic alterations in this transcription factor and poor prognosis in cancer [199, 200]. Myriad mutations are observed in Nrf2 involved in multiple cancers; therefore, targeting Nrf2 mutations by using nuclease-based genome editing technology is a significant therapy to combat cancer. In this therapy, DNA-double strand breaks (DSBs) are introduced by nucleases at a genomic locus of interest, in the presence of single strand oligonucleotides (ss-ODN) to the targeted region is activated to seal the breaks. DSBs followed by non-homologous end joining (NHEJ) could be introduced to eliminate amplified Nrf2 in cancer [201, 202].
Recently, the clustered regularly interspaced short palindromic repeats (CRISPR)-associated protein (Cas) 9 system has emerged as a powerful tool to achieve the desired alteration in the Nrf2 gene, including elimination and correction of point mutations [203–205]. CRISPR-Cas9 technology is exploited by researchers worldwide to manipulate oncogenes and the Nrf2 gene in several cancer types and reflects a very promising therapeutic potential. A report has established that CRISPR-directed gene editing, mediating the disabling of the Nrf2 gene in A549 lung carcinoma cells suppresses cell proliferation and enhances the sensitivity of cancer cells towards chemotherapy, which contributes to clinical application [206]. The US FDA moved a step ahead and approved CRISPR therapy in sickle cell disease, which brings hope for developing CRISPR-based technology to combat cancer via activating Nrf2 mutations [207]. In a very recent report by Hasani et al. [207], it was found that the in vitro anticancer effects of anticancer drug paclitaxel are enhanced via targeting Nrf2 in gastric cancer cells. Despite the unresolved riddle about the role of Nrf2 as tumour suppressor and tumour activator, it is apparent that Nrf2 is actively linked to the maintenance of malignant cells and thus can be targeted by combinatorial therapies as well as multimodality therapy [208–210].
Unquestionably, for many decades, a strong body of evidence has consistently demonstrated and established the association of ROS imbalance in severe disorders such as neurodegenerative diseases, cancer, diabetes, cardiovascular diseases, etc. ROS pose a threat to biomolecules (DNA, protein, and lipids) and have detrimental effects, such as DNA base oxidation, protein carbonylation, and LP in cellular membranes. ROS-mediated LP products such as acrolein, MDA, 4-HNE, and isoprostane have long half-lives and are involved as secondary messengers in various signaling processes, thus leading to the progression of various diseases. Elevated levels of secondary metabolic products are used as biomarkers of carcinogenesis and are reflected in various cancers like breast cancer, skin cancer, prostate cancer, hepatocellular cancer, colorectal cancer, etc. The cellular antioxidant system activates its defense system at the onset of an OS condition. Nrf2 transcription factor, a master regulator of the antioxidant genes, is thus activated in response to cellular stress conditions, resulting in abrogation of the deleterious effects of ROS. Although dietary supplements cannot mitigate the detrimental effects of ROS, they can very well attenuate carcinogenesis by activating Nrf2. Accumulated evidence has suggested that activation and modulation of Nrf2 play a substantial role in preventing the overproduction of ROS.
Over the years, targeting the Nrf2-mediated signaling pathways via natural and synthetic compounds has emerged as a potential therapeutic approach to treat cancer. The role of Nrf2 is controversial in carcinogenesis as it displays a dual role in the prevention as well as progression of cancer. The intricate mechanism of Nrf2 transcriptional activity is still infancy. Up/Down regulation of Nrf2 via activators and inhibitors has been extensively investigated and documented to design systematic strategies to combat cancer. Natural products like polyphenols, flavonoids, vitamins, sulfur compounds, etc., are found to play a key role in the modulation of Nrf2 via overexpression or suppression of transcriptional activity, offering a potential avenue for cancer prevention and treatment. Nrf2 overactivation poses a threat to cancer cell proliferation and cell differentiation and displays resistance to chemotherapy and radiotherapy drugs. Therefore, Nrf2 signaling pathway should be targeted according to the needs of the individual tumor profile. Recently, nanocarriers like liposomes, nanoparticles, metal-organic frameworks, and cell-inspired biomaterials have been implemented to impede cancer cell proliferation via antioxidant supplementation. Nano-drug delivery systems (NDDSs) emerged as a promising therapeutic approach for targeted delivery via encapsulation of Nrf2 modulators in nanosized structures using polymeric nanoparticles, liposomes, polymeric micelles, and solid lipid nanoparticles (SLNs) [210]. Bioavailability, reduced toxicity, dose concentration, and timing are equally important aspects for the treatment, which can be overcome by using NDDSs.
Future perspectives should be more focused on tailoring combination therapies, personalized medicines, individual tumors, and genetic profiling, repurposing of drugs, etc., to solve the complexities of debilitating cancers. The mysteries of carcinogenesis need to be uncovered for the rational design of therapeutic approaches. An absolute understanding of the mechanistic intricacies of Nrf2 signaling, direct as well as indirect targeting of Nrf2 signaling pathways, should be explored, which would enable the design of more molecules to modulate the cell signaling that will be instrumental in specifically disrupting the pathway that can halt or slow down cancer cell proliferation. Integration of all the conventional and modern strategies can result in the treatment and eradication of cancer without compromising the individual’s health.
•OH: hydroxyl radical
1O2: singlet oxygen
4-HNE: 4-hydroxy-2-nonenal
AA: arachidonic acid
ABCF2: ATP-binding cassette subfamily F member 2
AD: Alzheimer’s disease
ALS: amyotrophic lateral sclerosis
AREs: antioxidant response elements
Bcl-2: B-cell lymphoma 2
Bcl-XL: B-cell lymphoma-extra-large
Cas: clustered regularly interspaced short palindromic repeats-associated protein
CAT: catalase
CCND: cyclin D1
CDDCA4: cluster of differentiation 4
CK19: cytokeratin 19
CNC: Cap “n” Collar
COX: cyclooxygenase
CRISPR: clustered regularly interspaced short palindromic repeats
CXCL8: C-X-C motif chemokine ligand 8
CYP3A4: cytochrome P450 family 3 subfamily A member 4
DMBA: 7,12-dimethyl-benz(a)anthracene
DSBs: double strand breaks
E2: 17ꞵ-estradiol
EGCG: epigallocatechin gallate
F2-IsoPs: F2-isoprostanes
FAK: focal adhesion kinase
G6PD: glucose-6-phosphate dehydrogenase
GCLC: glutamate-cysteine ligase catalytic subunit
GSTA2: glutathione s-transferase alpha 2
GSTP1: glutathione S-transferase pi 1
H2O2: hydrogen peroxide
HD: Huntington’s disease
HETE: hydroxyeicosatetraenoic acid
HIF-1: hypoxia-inducible factor 1
HO-1: haem oxygenase 1
IL-11: interleukin-11
INOS: inducible nitric oxide synthase
Keap1: Kelch-like ECH-associated protein 1
KRT16: keratin 16
LA: linoleic acid
LOX: lipoxygenase
LP: lipid peroxidation
MAPK: mitogen-activated protein kinase
MDA: malondialdehyde
MEL: murine erythroleukemic
MLC: myosin light chain
MMP7: matrix metalloproteinase-7
MRP5: multidrug resistance protein 5
NDDSs: nano-drug delivery systems
NOX: NADPH oxidases
NQO1: NAD(P)H-quinone oxidoreductase 1
Nrf2: nuclear factor erythroid 2-related factor 2
NSCLC: non-small-cell lung cancer
O2−: superoxide anion
OS: oxidative stress
PD: Parkinson’s disease
PGE2: prostaglandin E2
PLO•: lipid hydroxy radical
PLOO•: lipid peroxyl radical
PUFAs: polyunsaturated fatty acids
RHOA: Ras homolog gene family member A
RNS: reactive nitrogen species
ROCK: Rho-associated coiled-coil containing kinase
ROO•: peroxyl radicals
ROS: reactive oxygen species
RSV: resveratrol
SFN: sulforaphane
SNP: single nucleotide polymorphism
SOD: superoxide dismutase
TALDO1: transaldolase 1
TKT: transketolase
VEGFA: vascular endothelial growth factor A
Dr. Shrikant Kukreti acknowledges the Institute of Eminence, University of Delhi [IoE/2024-25/12/FRP].
AS: Investigation, Writing—original draft, Writing—review & editing. RK: Writing—review & editing. LS: Writing—review & editing. SK: Conceptualization, Investigation, Supervision, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not Applicable
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1123
Download: 13
Times Cited: 0
Santiago Gelerstein-Claro ... Ramón Rodrigo
