Affiliation:
1Department of Mechanical Engineering, Dibrugarh University, Dibrugarh 786004, Assam, India
ORCID: https://orcid.org/0000-0002-0884-8693
Affiliation:
2Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh 786004, Assam, India
ORCID: https://orcid.org/0000-0002-3632-2939
Affiliation:
2Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh 786004, Assam, India
ORCID: https://orcid.org/0000-0001-7921-4658
Affiliation:
2Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh 786004, Assam, India
ORCID: https://orcid.org/0000-0003-0147-6556
Affiliation:
2Department of Pharmaceutical Sciences, Dibrugarh University, Dibrugarh 786004, Assam, India
Email: kalyakster@gmail.com
ORCID: https://orcid.org/0000-0002-1421-8962
Affiliation:
3Department of Pharmaceutics, College of Pharmacy, Najran University, Najran 11001, Saudi Arabia
ORCID: https://orcid.org/0000-0003-2979-8776
Affiliation:
4Department of Pharmacology, College of Pharmacy, Najran University, Najran 11001, Saudi Arabia
ORCID: https://orcid.org/0000-0002-5545-6422
Explor Med. 2025;6:1001374 DOI: https://doi.org/10.37349/emed.2025.1001374
Received: August 27, 2025 Accepted: October 28, 2025 Published: November 27, 2025
Academic Editor: Gaetano Isola, University of Catania, Italy
The advent of three-dimensional (3D) printing has transformed modern dentistry by introducing innovative approaches that enhance customization, precision, and efficiency in clinical and educational settings. This review provides a comprehensive analysis of recent developments and emerging trends in 3D printing applications within dentistry. It explores key domains, including Applications in Orthodontics, Applications in Crown Production, Applications in Implants and Surgical Guides, 3D Printing Applications in Dentures, and Applications in Dental Models and Educational Tools. In orthodontics, 3D printing facilitates the production of patient-specific aligners, brackets, and retainers, improving treatment accuracy and reducing turnaround times. In crown production, the integration of computer-aided design and manufacturing (CAD/CAM) with additive manufacturing allows for the fabrication of highly precise and esthetic prosthetic crowns with rapid chairside delivery. One of the most impactful uses is seen in implants and surgical guides, where 3D printing supports the creation of customized surgical templates and implant components, thus enhancing procedural outcomes and reducing surgical risks. 3D printing has revolutionized denture fabrication by enabling the production of complete and partial dentures with improved fit, material efficiency, and reduced laboratory time. In dental education, the technology is increasingly employed to produce anatomical models, simulated teeth, and other educational tools that improve student training and diagnostic planning. The novelty of this review lies in its integrative perspective linking technical advancements with practical dental applications and highlighting material innovations such as nanocomposites and biocompatible polymers. It also discussed future prospects such as AI-driven design optimization and the role of smart materials in expanding clinical applicability. By presenting a structured overview across multiple specialties, this paper offers valuable insights into how 3D printing is reshaping the future of dental care and education.
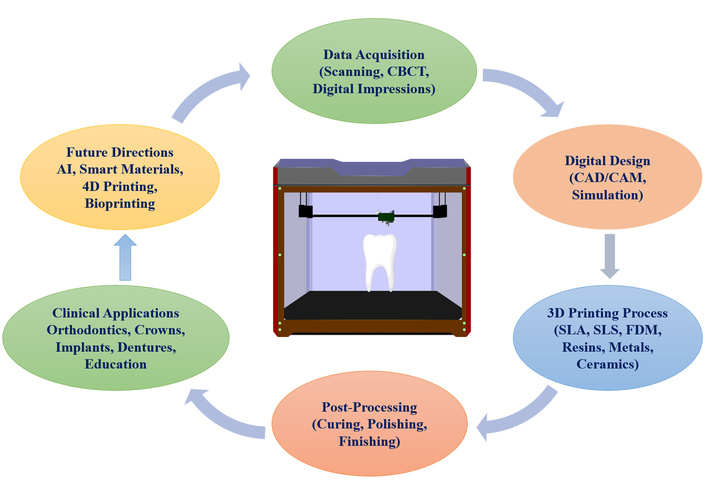
Three-dimensional (3D) printing, or additive manufacturing, has emerged as a cornerstone of digital dentistry, offering the ability to fabricate complex, customized dental structures with high precision and efficiency. This technology builds objects layer by layer from digital designs, enabling the rapid and accurate creation of prosthetic devices, orthodontic appliances, surgical tools, and anatomical models. Its integration into dental workflows has streamlined clinical procedures and revolutionized treatment planning, production timelines, and patient outcomes [1–3]. The digital workflow in dental 3D printing typically involves three main phases: data acquisition, digital design, and manufacturing. Intraoral scanners and cone-beam computed tomography (CBCT) are commonly used to capture detailed, high-resolution images of the patient’s oral cavity [4, 5]. These digital impressions are processed using computer-aided design (CAD) software to generate precise virtual models of restorations or appliances. Once finalized, the designs are sent to compatible 3D printers, which fabricate the objects using specific materials and printing technologies [1, 6]. At the core of this process are several distinct 3D printing technologies, such as vat photopolymerization (VP), including stereolithography (SLA), digital light processing (DLP), and liquid crystal display (LCD) methods, which are widely adopted for their high resolution and ability to print using photopolymer resins [1, 7]. Powder bed fusion (PBF) techniques, such as selective laser melting (SLM) and selective laser sintering (SLS), are employed primarily for creating durable metal restorations like crowns and implant components [8]. Fused deposition modelling (FDM), while less precise, remains useful for producing study models and educational replicas due to its affordability and accessibility [2, 7]. The clinical applications of 3D printing in dentistry are vast and evolving. In prosthodontics, 3D printing enables the efficient fabrication of crowns, bridges, dentures, and implant-supported restorations [9, 10]. Orthodontics benefits from custom-fitted aligners, retainers, and brackets that improve treatment accuracy and patient satisfaction [11]. 3D-printed surgical guides have become essential tools in implantology and maxillofacial surgery, facilitating minimally invasive procedures with enhanced precision [12, 13]. Furthermore, dental schools and research institutions utilize 3D-printed models for simulation-based training and clinical experimentation, while advances in bioprinting show promise for regenerating periodontal tissues and soft tissue constructs [5, 14]. A particularly transformative domain is the application of 3D printing in fixed prosthodontics. The technology allows the fabrication of highly customized crowns, bridges, inlays, and onlays with exceptional control over morphology, occlusion, and shade, reducing the need for manual adjustments [15, 16]. Both temporary and permanent restorations benefit from enhanced marginal fit, internal precision, and smooth surfaces enabled by SLA and DLP technologies [16–18]. Studies report marginal gaps below 60 μm, meeting clinical standards for adaptation [19, 20]. The trueness of 3D-printed zirconia crowns is comparable to that achieved through conventional CAD/computer-aided manufacturing (CAM) systems [21]. The integration of monolithic zirconia and modern resin materials, which offer superior aesthetics, strength, and biocompatibility, further reinforces the role of 3D printing in permanent restorative dentistry [16, 22, 23]. The ability to produce patient-specific devices with a high level of accuracy minimizes human error and enhances clinical predictability [3, 9]. The technology supports on-demand fabrication, which reduces material waste, eliminates the need for large inventories, and accelerates the treatment process [24]. Moreover, digital workflows enable clinicians to deliver same-day restorations in some cases, contributing to greater patient convenience and practice efficiency [9].
Despite these strengths, several challenges persist. Material-related limitations, such as interlayer bonding strength, esthetic properties, and long-term durability, remain under investigation [2, 25]. Researchers are continuously refining resin compositions, post-processing protocols, and surface treatment methods to improve overall performance [9, 16]. Regulatory and legal barriers, including device certification, data security, and practitioner liability, also present obstacles to widespread clinical integration [1, 26]. 3D printing has become an essential tool in personalized orthopedic surgery, enabling the creation of patient-specific anatomical models, customized implants, and surgical instruments that enhance precision and reduce recovery time. It is also valuable for fracture management, prosthetic development, and preoperative planning, ensuring treatments are tailored to individual needs. Beyond therapeutic use, it serves as an important educational resource, advancing both medical training and personalized patient care [27–29]. In bone grafting, 3D printing enables the fabrication of scaffolds with optimized porosity and mechanical strength, supporting osteogenesis and vascularization while minimizing transplant-related complications. This strategy is increasingly viewed as a cornerstone of regenerative medicine and a promising alternative to traditional bone grafts [30]. Despite these advances, several challenges persist, including material limitations such as bonding strength, esthetic properties, and long-term durability, as well as regulatory and legal barriers surrounding device certification, data security, and practitioner liability. Rapid prototyping through 3D reconstruction also plays an important role in complex trauma surgeries, providing precise visualization of anatomical relationships, aiding in implant design, and reducing intraoperative uncertainty. Its growing use highlights the potential of additive manufacturing as a critical tool in trauma surgery planning [31]. Similarly, imaging-based 3D modeling has enabled the production of accurate surgical guides and implants, with scaffold lattices designed to enhance osteointegration and reduce implant stiffness. However, high costs, long production times, and limited intraoperative adaptability remain challenges, underscoring the need for new biomimetic materials and integrated planning platforms to support routine clinical adoption [32]. Early clinical applications have already demonstrated the potential of 3D printing in orthopedics. Patient-specific instrumentation and anatomical models can improve surgical accuracy, shorten operative time, and enhance planning, particularly in complex trauma cases. Although still in early adoption, its broad applicability across surgical fields suggests a strong future for personalized orthopedic care [33]. Beyond orthopedics, dentistry has also embraced 3D printing, where techniques such as SLA, DLP, and SLS are used to fabricate accurate models, prosthetics, and restorations. Continued development of biomaterials with suitable biological and mechanical properties is key to translating these advances into widespread clinical practice [34]. Furthermore, technological frontiers such as 4D printing, where printed materials change shape or function over time in response to environmental stimuli, are being explored for future dynamic dental solutions [11, 13]. 3D printing offers unprecedented opportunities for improving the precision, efficiency, and customization of dental care. With the continued evolution of materials, digital technologies, and regulatory frameworks, it is poised to become a foundational element of restorative, surgical, and educational dental practices [15, 16]. Figure 1 depicts the overview of 3D printing in dentistry.

Overview of 3D printing in dentistry. FDM: fused deposition modelling; 3D: three-dimensional.
The advancement of 3D printing in dentistry has been significantly driven by the development and refinement of materials specifically engineered for intraoral use. These materials must meet rigorous standards for biocompatibility, mechanical durability, esthetic performance, and printability. Currently, the main categories of materials used in dental 3D printing include polymers and composites, resins, metals, ceramics, and emerging hybrid formulations. Each category offers unique advantages that cater to the diverse requirements of restorative, surgical, and orthodontic dental procedures.
Polymers are widely used in dental 3D printing due to their favorable combination of printability, biocompatibility, and mechanical properties. Commonly employed polymers include polylactic acid (PLA), polyether ether ketone (PEEK), polyether ketone ketone (PEKK), polymethyl methacrylate (PMMA), and polycaprolactone (PCL). These materials are particularly suitable for dental models, splints, surgical guides, and some types of provisional restorations [22, 23, 35]. In addition to base polymers, research has focused on enhancing mechanical strength and biological performance through the development of polymer-based composites. These include nanodiamond-reinforced PMMA, PLA reinforced with nanohydroxyapatite or magnesium, and poly(lactic-co-glycolic acid) (PLGA) composites embedded with tricalcium phosphate and magnesium. These composite materials aim to improve durability and support regenerative applications in soft and hard tissue engineering [22].
Photopolymer resins are among the most commonly utilized materials in dental 3D printing, especially for temporary restorations, surgical guides, diagnostic models, and occlusal splints. Materials such as IBT Resin, BioMed Amber Resin, and Dental LT Clear Resin are specifically formulated to meet dental clinical demands. For example, Dental LT Clear Resin is notable for its superior tensile modulus, making it suitable for long-term intraoral appliances [36]. Resins are valued for their ease of use and ability to reproduce fine detail with smooth surface finishes [37].
Metals play a vital role in permanent prosthodontic and implant-based treatments due to their high strength, corrosion resistance, and long-term biocompatibility. Titanium alloys and cobalt-chromium (Co-Cr) are the most frequently used metals in dental 3D printing and are fabricated using techniques such as SLM and electron beam melting (EBM) [38, 39]. These methods enable the production of highly accurate frameworks for removable and fixed partial dentures, crowns, and implant abutments with excellent mechanical performance.
Ceramic materials, particularly zirconia, are increasingly used in aesthetic restorations such as crowns and bridges. These materials exhibit high flexural strength, wear resistance, and esthetic translucency, making them ideal for anterior and posterior restorations [40]. However, ceramics pose significant manufacturing challenges due to their high melting points and inherent brittleness, which complicate the printing process [41, 42]. Innovative printing techniques and sintering processes are currently under investigation to make ceramic 3D printing more clinically viable.
While 3D printing in dentistry provides clear advantages in terms of customization, precision, and efficiency, significant material challenges continue to limit its widespread clinical adoption. One of the most pressing issues lies in the brittleness of printed ceramics such as zirconia and lithium disilicate. These materials are valued for their excellent biocompatibility and natural esthetics, yet their mechanical strength remains inferior to conventionally manufactured alternatives. Surface defects introduced during additive processes exacerbate brittleness, requiring exceptionally tight control during manufacturing to achieve adequate durability [43, 44]. Beyond ceramics, resin-based materials face challenges in long-term clinical use. Although 3D-printed resins are increasingly used for provisional and permanent restorations, they often demonstrate lower flexural strength, fracture resistance, and fatigue performance compared to milled PMMA or bis-acrylic counterparts [45, 46]. Water sorption, solubility, and the anisotropic nature of printed layers further compromise their long-term stability in the oral environment, while color instability and surface roughness can negatively affect aesthetics and patient satisfaction [16, 47]. Additionally, post-processing steps such as curing, polishing, or coating are critical in determining final material performance, and inconsistencies in these procedures may lead to compromised mechanical or chemical properties [48]. Compounding these material challenges are systemic barriers: high material costs, the need for specialized training, and gaps in practitioner knowledge regarding the limitations of 3D-printed dental materials, all of which slow adoption in smaller practices and teaching environments [49, 50].
A critical evaluation of different dental 3D printing technologies reveals significant differences in accuracy, cost-effectiveness, and clinical performance. Understanding these factors is essential for selecting the most suitable technology for specific dental applications (Table 1).
Comparison of different dental 3D printing technologies.
| Technology | Accuracy | Precision | Material properties | Speed | Cost | Clinical applications | References |
|---|---|---|---|---|---|---|---|
| SLA | High accuracy, especially for full-arch models | Comparable to FDM | Varied mechanical properties; lower flexural strength and hardness | Moderate | Higher cost | Provisional restorations, dental models | [17, 45, 51, 52] |
| DLP | High accuracy, especially for full-arch models | High precision | Enhanced mechanical properties with specific resin formulations | Fast | Moderate cost | Crowns, bridges, orthodontic appliances | [17, 51, 53, 54] |
| LCD | Lower accuracy compared to DLP | Lower precision compared to DLP | Acceptable mechanical properties | Moderate | Lower cost | Dental models, orthodontic appliances | [17, 53] |
| FDM | Comparable accuracy to SLA | Comparable precision to SLA | Lower mechanical properties; higher surface roughness | Moderate | Lower cost | Training models, non-clinical applications | [17, 52] |
| MultiJet | High accuracy | High precision | Good mechanical properties | Fast | Higher cost | Inlay/Onlay restorations, dental models | [17, 51] |
| PolyJet | High accuracy | High precision | Good mechanical properties | Fast | Higher cost | Dental models, orthodontic appliances | [17, 51, 55] |
| CLIP | Lower accuracy compared to SLA and DLP | Acceptable precision | Good mechanical properties | Fast | Higher cost | Dental prostheses, surgical guides | [17, 51] |
SLA: stereolithography; FDM: fused deposition modelling; DLP: digital light processing; LCD: liquid crystal display; CLIP: continuous liquid interface production; 3D: three-dimensional.
SLA has been widely recognized for its high accuracy. Studies have shown that SLA printers achieve a mean relative error as low as 0.3% for crown height and 0.2% for crown width, highlighting their suitability for precise dental restorations [56]. However, some comparative analyses suggest that SLA may fall short in accuracy when compared with DLP and PolyJet technologies [57]. DLP, in particular, demonstrated superior precision, with studies reporting a 97% match between DLP printed models and original scans [55]. PolyJet technology has also shown exceptional accuracy, often outperforming SLA and comparable to DLP, with root mean square (RMS) deviations being the lowest in some comparative trials [51, 57, 58]. FDM remains less accurate, showing relative errors of 1.7% for crown height and 2.0% for crown width. Despite these limitations, it has been found acceptable for certain noncritical clinical applications [52, 56].
Cost is another critical factor influencing the choice of 3D printing technologies in dentistry. FDM is consistently reported as the most economical, with material costs as low as $3.12 per model, making it highly attractive for practices with budget constraints [56]. SLA, while more accurate, incurs a moderate cost of $5.18 per model, representing a balance between precision and affordability [56]. In contrast, PolyJet is the most expensive option, with a per-model cost of $7.84, restricting its widespread adoption despite superior accuracy [56]. DLP typically falls between SLA and PolyJet in cost, offering a practical compromise between financial investment and clinical accuracy [57].
From a clinical perspective, SLA, DLP, and PolyJet technologies all provide sufficient accuracy for full-arch dental model production, surgical guides, and prosthodontic restorations, contributing to highly reliable patient outcomes [51]. DLP and PolyJet, in particular, are often preferred for complex prosthodontic and orthodontic work due to their superior dimensional stability [58]. FDM, despite its lower accuracy, remains clinically useful in scenarios where cost-effectiveness is prioritized over precision, such as preliminary diagnostic models or educational purposes [52]. MultiJet’s superior accuracy compared to FDM makes it particularly suitable for detailed restorations, where dimensional precision is paramount [59].
A critical consideration in the selection of 3D printing materials is biocompatibility. Some photopolymers may release residual monomers post-printing, which can lead to cytotoxic effects. As a result, extensive post-processing procedures such as washing and UV curing are necessary to mitigate these risks and enhance clinical safety [60–62]. Additionally, the mechanical properties of these materials, such as tensile and flexural strength, must be optimized to endure functional forces in the oral environment. Recent studies have shown that incorporating silver nanoparticles (AgNPs) or cellulose nanocrystals (CNCs) into PMMA resins can significantly enhance their mechanical resilience [63]. The dental materials landscape is also evolving toward hybrid materials that merge the properties of polymers, metals, and ceramics to achieve enhanced functionality. These advanced formulations are designed to offer the best combination of print fidelity, strength, and biocompatibility for complex dental procedures [23, 37]. Furthermore, the environmental impact of 3D printing is drawing attention, with current research exploring biodegradable and eco-friendly alternatives to traditional materials to align with sustainable dentistry goals [22]. Each material type exhibits distinctive mechanical, biological, and durability characteristics that determine its suitability for specific applications.
Ceramics. Ceramic-based 3D printing materials, such as polymer-derived ceramics (PDCs), exhibit exceptionally high compressive strength, stiffness, and thermal resistance. Their mechanical performance can be further enhanced with nanofillers, including silicon nitride and alumina, which improve toughness and reduce brittleness [64]. However, their inherent brittleness limits application in dynamic or high-impact environments [65].
Metals. Metals remain the benchmark in additive manufacturing for load-bearing applications. Titanium alloys, in particular, demonstrate superior tensile and fatigue strength, corrosion resistance, and mechanical stability, making them a gold standard for orthopedic and dental applications [66, 67]. Metal filaments like stainless steel 316L also provide good structural properties but require optimized sintering to minimize porosity and dimensional shrinkage [66].
Resins. Dental and medical-grade resins enhanced with ceramic particles such as yttria-stabilized zirconia (YSZ) have shown significant improvements in flexural strength and modulus, allowing their use in restorative and prosthetic dentistry. However, compared to metals and ceramics, resins typically exhibit lower impact resistance and require reinforcement for load-bearing applications [66].
Polymers. Polymers like PMMA and PCL exhibit good flexibility and processability. Their mechanical performance can be improved with additives such as carbon nanoparticles or titanate nanofillers, which increase tensile strength and reduce the rate of structural failure during printing [68, 69].
Composites. Composite materials, such as PLA/TPU/PMF blends, combine the flexibility of polymers with the reinforcement provided by fillers, leading to enhanced tensile and compressive properties. These composites often show antibacterial features and higher mechanical integrity compared to pure polymers, making them suitable for biomedical devices and implants [70, 71].
Ceramics. Ceramic biomaterials exhibit outstanding biocompatibility due to their osteoconductive and osteoinductive properties, which promote bone regeneration and integration with host tissue. PDCs also support high cell viability and adhesion [64].
Metals. Titanium alloys are highly biocompatible and widely accepted in medical implants due to their corrosion resistance and ability to form a stable oxide layer. However, processing methods such as sintering can influence surface chemistry and, in turn, affect cell compatibility [66].
Resins. Dental resins, when enhanced with biocompatible fillers such as YSZ, demonstrate excellent tissue tolerance and are considered safe for intraoral applications. However, maintaining mechanical integrity under long-term oral conditions is an ongoing challenge [67].
Polymers. Biodegradable polymers like PCL and PLA are extensively used in tissue engineering due to their ability to degrade safely in vivo while maintaining acceptable biocompatibility. Nanofiller reinforcement can further improve both biological and mechanical properties [68, 69].
Composites. Composite systems offer combined biocompatibility from polymers and reinforcement fillers. For instance, PLA-based composites integrated with antibacterial fillers support both tissue safety and infection resistance, broadening their biomedical potential [70].
Ceramics. Ceramics are known for long-term chemical and thermal stability, making them excellent for permanent implant applications. However, their brittleness and limited fracture toughness restrict their use under cyclic loads [65].
Metals. Metals, especially titanium alloys, provide outstanding long-term mechanical stability and resistance to corrosion. Their performance over decades in orthopedic and dental implants is well documented. However, residual porosity from additive processes must be minimized to ensure fatigue resistance [66].
Resins. Resin-based parts often face challenges in long-term durability due to environmental stress cracking and moisture absorption. Reinforcement with ceramic fillers improves their resilience and extends their service life, especially in dental prosthetics [66].
Polymers. The degradation rates of polymers like PLA and PCL make them suitable for temporary scaffolds in tissue engineering. However, their long-term mechanical stability is limited. Incorporating nanofillers can slow degradation and extend functional lifetimes [68].
Composites. Composites exhibit improved long-term performance compared to pure polymers due to reinforcement with nanofillers or blended polymers. Their mechanical stability and antibacterial properties make them reliable for sustained biomedical use [70]. A comparison of commonly used dental 3D printing materials highlights variations in strength, biocompatibility, and long-term stability (see Table 2).
Comparative table of 3D printing materials properties.
| Material | Mechanical properties | Biocompatibility | Long-term performance | Reference(s) |
|---|---|---|---|---|
| Ceramics |
|
|
| [64] |
| Metals |
|
|
| [67] |
| Resins |
|
|
| [66, 67, 72, 73] |
| Polymers |
|
|
| [66, 67, 72] |
| Composites |
|
|
| [69, 74, 75] |
AgNPs: silver nanoparticles; CNCs: cellulose nanocrystals; 3D: three-dimensional.
The biological response to 3D-printed dental materials has been widely studied, with particular focus on cytotoxicity, inflammatory reactions, and osseointegration, as well as emerging in vivo findings. Cytotoxicity remains a critical concern, as some resins used in additive manufacturing exhibit early or prolonged toxic effects, while others demonstrate good biocompatibility. For instance, Graphy resin has been shown to support high cell viability, whereas LuxCreo resin demonstrated early cytotoxic responses on periodontal ligament cells [76]. Similarly, Accura® ClearVueTM resin presented mild cytotoxicity after extended exposure, and temporary restorative resins caused a significant reduction in cell viability when compared to conventional restorative materials [77]. In contrast, polycarbonate-acrylonitrile butadiene styrene (PC-ABS) thermoplastic filaments revealed no significant cytotoxicity, showing strong proliferation and osteogenic potential in human cells. However, several resins designed for orthodontic devices and surgical guides were found to be strongly cytotoxic within 4–72 hours of exposure [78].
Inflammatory reactions to these materials also differ depending on their composition. Temporary restorative resins induced a stronger proinflammatory response in gingival keratinocytes compared to conventional alternatives [77]. Studies further suggest that certain novel resins increase the secretion of cytokines such as interleukin-6 (IL-6) and prostaglandin E2 (PGE2), which are known mediators of inflammatory pathways [79]. Nevertheless, materials like PC-ABS have shown minimal impact on inflammatory response, suggesting that engineering modifications can help mitigate immune challenges [78].
A promising area of advancement lies in osseointegration, which is essential for the long-term success of dental implants. A 3D-printed Ti-6Al-4V implant with porous structures significantly promoted osteoblast growth and biomarker expression, demonstrating excellent osseointegration properties [80]. Similarly, incorporation of tantalum oxide nanoparticles into 3D-printed denture base resins enhanced osteoblast formation and bone integration [81]. Another approach using bioactive glass-modified methacrylate resin not only improved the mechanical stability of the printed material but also enabled fluoride ion release, supporting remineralization and osseointegration [82].
Despite these advances, in vivo evidence remains limited. A study evaluating 3D-printed silicone structures showed no significant increase in cytotoxicity or tissue reaction, suggesting minimal inflammatory response and acceptable biocompatibility. Similarly, the encouraging in vitro results of porous Ti-6Al-4V implants highlight their potential for clinical application, though additional in vivo validation is necessary [80].
The rapid digitization of dentistry has been significantly advanced by the development and adoption of specialized 3D modelling software. These tools play a vital role in designing, simulating, and fabricating dental restorations and appliances with unprecedented accuracy. From diagnosis and treatment planning to prosthetic manufacturing and education, 3D modelling software now underpins nearly every facet of digital dental practice. A variety of software tools have emerged, each addressing specific stages of the dental workflow. Slicer 4.10.2, for instance, has been employed to extract 3D solid models of decaying teeth from 2D DICOM images. This is particularly useful in generating detailed tooth morphologies for computational analysis and simulation of forces in decayed dentition [83]. Similarly, Autodesk Meshmixer is widely used in clinical practice for articulating maxillary and mandibular 3D arch models using scanned interocclusal records. Its affordability and open-source nature make it an accessible option for virtual planning in restorative and surgical procedures [84].
SolidWorks, a professional-grade CAD software, has been utilized in prosthodontics to develop libraries of dental implants and custom abutments. It allows integration with other CAD/CAM systems to enhance precision in designing and milling implant restorations [85]. Studies have also explored workflows between Blender and SolidWorks, enhancing the compatibility of open-source and commercial platforms for use in dental prosthetic design [86].
ModelMatch3D, a tool originally developed for forensic odontology, enables automatic surface comparison between 3D models. This technology has potential applications in dental diagnostics and surgical planning due to its rapid 3D surface matching capabilities [87].
Several open-source tools are also gaining traction among dental professionals. For example, Blue Sky Plan, ViewBox, and Blender have found increasing popularity for their versatility and cost-effectiveness in orthodontics and general dental modeling [88]. These programs are particularly favored by younger clinicians and educators for their adaptability in customizing appliances, studying occlusion, and generating simulations.
The OptikDent CAD/CAM suite, which includes integrated hardware and software for digital scanning, modeling, and manufacturing, offers a closed-loop system for dental restorations. It features an intraoral camera for 3D data acquisition and automation protocols for producing crowns and bridges [89, 90]. In orthodontics, 3D modelling software, such as Blue Sky Plan and ViewBox, is used to generate digital models of dental arches and facilitate the design of clear aligners, retainers, and other corrective devices [88, 91]. In prosthodontics, CAD platforms like SolidWorks and OptikDent support the full workflow from virtual tooth preparation to crown design and CAM fabrication [85, 90].
For surgical planning, Autodesk Meshmixer and ModelMatch3D allow clinicians to visualize bone structures and soft tissues in 3D, enabling more precise osteotomies and implant placement [84, 87]. These tools also support simulation-based learning in dental education, where students interact with virtual patients and perform virtual procedures. Platforms such as Blender and simulation-specific tools are now used for osteotomy training and prosthodontic exercises [92, 93]. The adoption of 3D modelling software has brought significant improvements in workflow efficiency, patient customization, and treatment predictability. These tools allow dental professionals to virtually plan procedures, simulate outcomes, and manufacture patient-specific restorations, reducing the number of clinical visits and errors.
However, several challenges persist. Many advanced platforms come with a steep learning curve, requiring dedicated training for effective use. Additionally, licensing and subscription costs can limit access, particularly for smaller clinics or educational institutions [88]. Figure 2 gives a rough idea of the different techniques used in dentistry related to 3D printing.
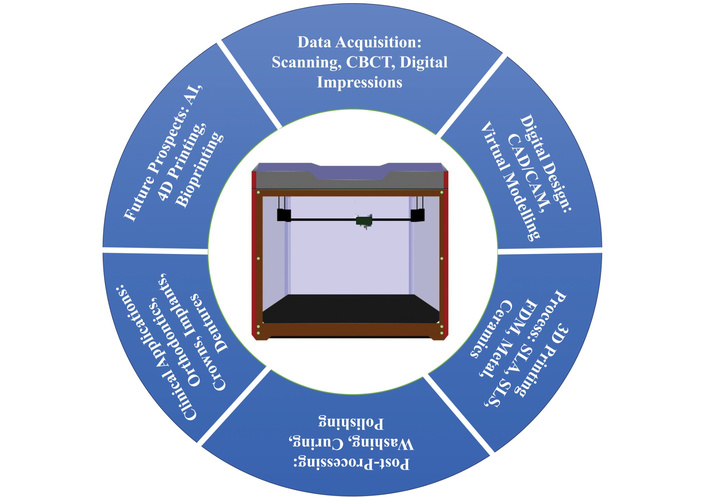
Techniques used in dentistry related to 3D printing. CBCT: cone-beam computed tomography; CAD/CAM: computer-aided design and manufacturing; SLA: stereolithography; SLS: selective laser sintering; FDM: fused deposition modelling; 3D: three-dimensional.
One of the primary benefits of 3D printing is its superior precision and accuracy, which directly translates to better-fitting appliances. Through high-resolution scanning and printing processes, orthodontic devices such as aligners, retainers, brackets, and expanders can be fabricated to conform exactly to a patient’s dental anatomy. This leads to enhanced comfort, improved performance, and fewer required adjustments during treatment [94–96]. Unlike conventional methods, clinicians can design fully customized orthodontic appliances based on intraoral scans and CAD/CAM models, enabling individualized solutions even for complex cases [11, 97, 98]. In-office manufacturing of appliances allows clinicians to bypass external laboratories, thus reducing both turnaround time and associated costs. It also makes same-day delivery of appliances feasible, which is especially advantageous for urgent orthodontic interventions [98, 99]. This streamlined process reduces the number of patient visits required for fitting and adjustment, optimizing chair time and improving clinical throughput [94, 96]. Emerging smart materials, such as shape-memory polymers, are beginning to enable “4D printing” applications, where appliances dynamically adapt over time to physiological changes [9, 11, 100].
Digital design tools and virtual configurators also play a pivotal role in the customization process. Through CAD/CAM platforms, clinicians can visualize and simulate treatment outcomes before printing the appliance. This enhances precision, improves communication between orthodontists and dental technicians, and enables patient-specific modifications during the planning phase [98, 101]. Such systems also allow for the efficient creation of appliances like distalizers, lingual brackets, and indirect bonding trays with unparalleled control over design features [97].
In terms of clinical versatility, 3D printing enables the production of a wide variety of orthodontic devices, from diagnostic models and clear aligners to indirect bonding trays and guided-surgery templates, on demand. This eliminates the need for maintaining bulky inventories and reduces material waste, contributing to cost-effective and environmentally sustainable orthodontic practice [9, 102].
AI is playing an increasingly transformative role in 3D dental printing by advancing automated design optimization, error detection, and workflow efficiency. In terms of design optimization, AI-driven generative design algorithms enable the creation of innovative and highly functional dental structures by analyzing large datasets and generating optimized solutions that combine both function and aesthetics [103, 104]. Automated design tools such as 3Shape Automate and Medit Splints further streamline this process by automatically generating customized dental appliances, including occlusal devices and lingual bracket trays, based on anatomical data, thereby reducing manual intervention and potential errors [105]. Moreover, AI enhances personalization by integrating patient-specific data from CBCT scans and intraoral impressions, allowing for the fabrication of dental prosthetics and implants that are more accurate and better fitting [106]. Equally important is the role of AI in error detection and quality assurance. AI-enabled systems facilitate real-time monitoring of the 3D printing process, enabling dynamic adjustments that minimize material waste and ensure consistent quality [103, 104]. Through machine learning (ML) and deep learning approaches, these systems can detect micro-defects or deviations during the printing process, significantly reducing the risk of compromised restorations [107]. Additionally, predictive maintenance supported by AI allows early identification of machine failures and reduces downtime, which is vital for clinical reliability in dentistry [108].
Beyond accuracy, AI is improving workflow automation and efficiency. By handling critical steps such as parameter setting, support structure optimization, and design iteration reduction, AI shortens the production cycle and enhances precision [109]. ML integration also improves the adaptability of additive manufacturing systems to complex geometries, ensuring greater accuracy and surface quality in dental prosthetics. Despite these advances, challenges remain, particularly in integrating heterogeneous datasets, ensuring standardized workflows, and addressing regulatory and ethical concerns related to patient data privacy [103, 110].
Despite these advances, certain challenges remain, particularly in areas where current methods fall short of addressing complex, real-world conditions. One of the most pressing is material longevity. Many 3D-printed resins, while suitable for short-term use, may not yet offer the durability required for long-term orthodontic interventions. Moreover, setting up in-office 3D printing requires investments in hardware, staff training, and compliance with regulatory standards regarding sterilization, biocompatibility, and quality control [50, 99]. Table 3: summary of key studies on 3D printing in orthodontics (primary focus, applications, major findings, and identified challenges).
Summary of key studies on 3D printing in orthodontics.
| Study focus | Applications | Key findings | Challenges | Reference(s) |
|---|---|---|---|---|
| 3D-printed accessories in orthodontics | Customized intraoral devices, auxiliary devices | Enhanced customization, precision, and workflow improvements; potential for 4D memory shape materials | Durability, biocompatibility, and long-term clinical performance | [11] |
| General applications in orthodontics | Dental models, clear aligners, orthodontic brackets, transfer trays, and removable appliances | High accuracy, efficiency, simple operation, and personalization | Material limitations, cost | [11, 12] |
| Scoping review on 3D printing in dentistry | Aligners, diagnostic models | Improved patient comfort, treatment precision | Material durability, high costs, and post-processing requirements | [50] |
| Orthodontic appliances manufactured using 3D printers | Brackets, archwires, nasoalveolar molding devices, surgical splints, removable appliances, expansion appliances, clear aligners, retainers, auxiliary attachments, working models | Increased production of customizable appliances, digital clinical workflow | Printing accuracy, material properties | [97] |
| Mechanical properties of 3D-printed material | Occlusal splints | Promising alternative to traditional materials, higher maximum bending stress | Compression and tensile strength are lower than traditional materials | [50, 55] |
| Comparison of 3D printing and CNC milling | Clear aligners | Better fitting and rapid teeth movement with CNC milling | Staircase effect in 3D printing, stress distribution | [50] |
| Digital workflow for palatal TADs | Guided insertion of palatal TADs | Reduced chair time, complete customization | Deviations in clinical steps, cumulative effect of deviations | [102] |
3D: three-dimensional; CNC: cellulose nanocrystal; TADs: temporary anchorage devices.
3D printing has revolutionized the dental industry by enabling the efficient and precise production of dental crowns, a core component of restorative dentistry. Among its most practical uses is the fabrication of temporary crowns, often manufactured using fused FDM with biocompatible polymers such as PLA. These crowns provide an affordable and quick solution for provisional restorations, although they may exhibit inferior surface smoothness and limited translucency compared to conventional methods [111].
In contrast, permanent crowns are increasingly being fabricated using advanced techniques like SLA and DLP, which offer exceptional precision and surface detail. These methods also facilitate chairside production, shortening clinical timelines [54, 112, 113]. However, the durability and long-term biocompatibility of the resins used remain an ongoing area of investigation. Additionally, metal crowns and frameworks can be produced using additive manufacturing processes such as laser PBF (L-PBF). This approach ensures excellent marginal fit and high mechanical strength when combined with post-processing steps like heat treatment and polishing [114].
A major advantage of 3D-printed crowns lies in their superior accuracy and fit. Recent comparative studies demonstrate that 3D printing yields more precise marginal and internal fits than traditional subtractive methods like milling, thereby reducing the likelihood of crown failure or patient discomfort [115, 116]. Customization is another critical benefit. Because 3D printing is inherently data-driven and compatible with CAD/CAM workflows, dental crowns can be individually tailored to suit each patient’s anatomical and occlusal requirements, improving both aesthetics and function [15, 117].
The technology also enhances workflow efficiency. Rapid prototyping accelerates the process of crown production and minimizes delays in dental treatments, which is advantageous for both clinicians and patients [54, 112]. Despite these benefits, several challenges persist. Material constraints, particularly the mechanical strength and biocompatibility of some printable resins, prevent full equivalence with ceramics like zirconia [62, 63]. Another limitation concerns surface texture, where printed crowns, especially those produced by lower-resolution printers, may display rough finishes that affect their clinical performance [20, 111].
Looking ahead, future directions in 3D-printed crown development focus on advanced materials and optimization of print parameters. Novel formulations such as nanodiamond-reinforced PMMA or PLA composites with nanohydroxyapatite are under exploration for enhanced strength and biocompatibility [22, 118]. Simultaneously, improvements in shape optimization algorithms and resin chemistry are expected to refine the aesthetic and mechanical performance of printed crowns, enabling broader clinical adoption [113].
In conclusion, 3D printing is becoming a transformative tool in the fabrication of dental crowns, offering benefits in terms of precision, efficiency, and personalization. While certain technical and material limitations must still be addressed, ongoing research continues to push the boundaries of what is clinically possible through digital dentistry. Table 4 provides a summary of selected studies on 3D printing in dental crown production, including their focus, printing technologies, materials, and key findings.
Summary of studies on 3D printing in dental crown production.
| Study focus | 3D printing technology | Materials used | Key findings | Reference(s) |
|---|---|---|---|---|
| Overview of 3D printing in dental prostheses | Not specified | Cobalt-chromium, zirconia | Clinically acceptable results for interim restorations; further research needed for zirconia crowns. | [119] |
| Additive manufacturing of dental restorations | Lithography-based ceramic manufacturing (LCM) | Ceramics | High accuracy and surface quality; suitable for staining and glazing. | [18] |
| Clinical application of 3D-printed temporary crowns | FDM | PLA | Temporary crowns were maintained without issues, rough surface texture, and translucency limitations. | [111] |
| Evaluation of 3D trueness of zirconia crowns | 3D printing vs. CAD/CAM milling | Zirconia | 3D-printed crowns met trueness requirements; suitable for ceramic restorations. | [21] |
| Development of 3D-printed crown resin materials | Not specified | Resin with zirconia glass (ZG) and glass silica (GS) microfillers | Improved mechanical properties; increased surface roughness; further optimization needed. | [120] |
| Case report on occlusal rehabilitation with 3D-printed crowns | Not specified | Not specified | High precision; minimal occlusal corrections needed; cost-effective and predictable treatments. | [15] |
| Accuracy of resin-based fixed dental restorations | DLP and SLA | Polymer-based materials | Clinically acceptable trueness; significant variability observed. | [21, 121] |
| Technological landscape of 3D-printed provisional crowns and bridges | Vat photopolymerization (SLA, DLP) | Resin-based materials | High mechanical characteristics; challenges with material durability and interlayer bonding. | [16] |
3D: three-dimensional; FDM: fused deposition modelling; PLA: polylactic acid; CAD/CAM: computer-aided design and manufacturing; DLP: digital light processing; SLA: stereolithography.
One of the most prevalent applications of 3D printing in oral healthcare is the production of surgical guides. These guides are meticulously designed to assist clinicians in the accurate placement of dental implants, ensuring that the drilling and insertion are performed at the ideal angle, depth, and position. The use of biocompatible surgical resins enhances the reliability of these guides during procedures, leading to fewer complications and improved prosthetic alignment [122–124].
Beyond guides, 3D printing also enables the production of a variety of custom implants, such as root-analog implants, subperiosteal structures, and maxillofacial reconstructive implants. These are typically produced using SLS or direct metal laser sintering (DMLS) techniques, allowing the use of materials like titanium and Co-Cr to fabricate durable, anatomically contoured implants [10, 22, 125].
Moreover, 3D printing is widely used in the fabrication of prosthetic restorations and orthodontic appliances, including temporary and permanent crowns, bridges, retainers, and aligners. This customization promotes better clinical outcomes by tailoring the prosthetic to the patient’s unique oral structure [10, 13, 22]. Figure 3 gives an idea of the detailed process of 3D printing.
Technological progress has led to a broad spectrum of printable materials suitable for clinical use. These include polymers, ceramics, and metal alloys. Notably, materials like nanodiamond-reinforced PMMA and PEEK reinforced with titanium or hydroxyapatite are under active development, showing promise for their enhanced mechanical properties and biocompatibility [22, 126]. These innovations allow for not only improved osseointegration and long-term functionality but also aesthetic outcomes in anterior restorations. The range of additive manufacturing techniques, such as SLA, FDM, and laser melting, also contributes to greater precision in printing, allowing for finer resolution and better adaptation to anatomical details [125, 127]. The major benefits of using 3D printing in this domain lie in customization, accuracy, and time efficiency. Surgical guides and implants can be fabricated rapidly and tailored precisely to the individual patient’s anatomy, which improves surgical precision and reduces intraoperative time [128, 129]. Additionally, 3D-printed devices enhance patient satisfaction due to their superior fit and minimally invasive nature. However, there are limitations. The cost of equipment and materials remains a barrier for widespread clinical adoption. Furthermore, there are regulatory challenges associated with ensuring the safety and efficacy of patient-specific devices, especially those produced in-office without centralized quality control [124, 128]. Clinical validation through long-term studies is also required to establish performance benchmarks and durability standards. Looking ahead, the convergence of AI and ML with 3D printing workflows has the potential to fundamentally transform dental and maxillofacial practices. By leveraging large datasets from clinical imaging, patient records, and prior manufacturing outcomes, AI-driven algorithms can autonomously optimize design parameters, predict structural weaknesses, and detect errors before fabrication begins. This automation not only streamlines the production pipeline but also minimizes manual intervention, thereby enhancing consistency, reducing turnaround time, and lowering the risk of human error [130]. Table 5 presents a summary of studies on the application of 3D printing in implants and surgical guides, highlighting their focus, clinical applications, and key findings.
Summary of studies on 3D printing in implants and surgical guides.
| Study focus | Application | Key findings | Reference |
|---|---|---|---|
| 3D printing in dentistry and MFS | Custom design and print surgical drill guides, implants, and other dental appliances | Highlights the progress in 3D/4D printing technologies in dentistry, including the use of various materials for printing | [13] |
| Mechanical behavior of 3D-printed dental resin | Fabrication of surgical guides for implant placement | The stability and performance of the surgical guide resin were proven through tensile and three-point bending tests | [122] |
| Compensation for guide hole shrinkage | Dental implant surgical guides | Technique to adjust the guide hole size using software to compensate for resin shrinkage during 3D printing | [131] |
| 3D printing in modern dental practices | Printing of surgical guides, custom-made meshes, and implants | Use of laser sintering and laser melting techniques for printing surgical guides and customized implants | [10] |
| Materials in 3D printing for dental applications | Crowns, bridges, surgical guides, and implants | Exploration of various materials used in 3D printing, including polymers and composites | [22] |
| Rapid prototyping (RP) in dental implants | Dental implants and surgical guides | Evaluation of RP/imaging/CAD/CAM techniques in making implants for dental reconstruction | [127] |
| Impact of 3D printing in oral and maxillofacial surgery (OMFS) | Dental implants and surgical guides | Improved precision, predictability, and reduced operation times in OMFS | [129] |
| 3D printing for surgical planning and implantable devices | Surgical guides in implant dentistry | Strong recommendation for using 3D-printed surgical guides to facilitate planning and reduce operative risks | [123] |
| Scoping review on 3D printing in dental implantology | Customized dental implants, surgical implant guides, and implant-supported prostheses | Proven technology for producing surgical implant guides with the best accuracy using the MultiJet printer | [132] |
| Advancements in 3D printing in surgical dentistry | Dental implantology, prosthodontics, MFS | Comprehensive review of 3D printing techniques, materials, and clinical applications in dental practices | [128] |
3D: three-dimensional; CAD/CAM: computer-aided design and manufacturing.
The integration of 3D printing into denture fabrication has brought transformative changes to prosthodontics, allowing for faster, more precise, and cost-effective solutions compared to traditional workflows. One of the primary advantages of 3D printing lies in its capacity for precision and personalization. Through digital scanning and modelling, clinicians can design and fabricate denture bases that closely match a patient’s oral anatomy, improving fit, retention, and comfort [133–135]. This customization ensures not only greater comfort but also functional outcomes, which are critical for patients requiring long-term prosthetic care.
In terms of efficiency, 3D printing dramatically reduces production time by bypassing labor-intensive processes such as flasking, packing, and curing that are associated with conventional denture fabrication. As a result, clinicians can deliver complete or interim dentures faster and at a lower cost, improving patient satisfaction and practice workflows [128, 134]. Furthermore, additive manufacturing technologies facilitate batch production and easy replication, which is particularly advantageous for large clinics and dental labs [136].
Material development is also central to the success of 3D-printed dentures. A variety of new polymeric resins have been engineered to improve properties like mechanical strength, biocompatibility, and color stability. For instance, resins used in SLA and DLP have shown promising results in producing highly detailed and durable dentures [135, 136]. PMMA remains the most commonly used material, but limitations such as brittleness and lack of fracture resistance have prompted researchers to explore alternatives such as PEEK and ABS, both of which offer superior toughness and wear resistance [126, 136].
3D printing has proven effective in fabricating a variety of denture types. Complete dentures created through additive manufacturing exhibit comparable or even superior accuracy in base adaptation compared to conventionally processed ones. However, concerns such as fracture resistance, dimensional stability, and color retention under oral conditions remain areas requiring further investigation [113, 137]. In removable prosthodontics, the technology is used for interim partial or full dentures, as well as maxillofacial prostheses that require complex geometries and delicate anatomical considerations [10, 37, 134].
Another growing application is in the fabrication of temporary prosthetic restorations, particularly during the osseointegration phase of implant therapy. These 3D-printed temporaries not only maintain esthetics and function but also allow for adjustments and iterative improvements during the healing process [46]. Meanwhile, cutting-edge research into resin nanocomposites has shown that incorporating fillers such as titanium dioxide or zirconia nanoparticles into denture base materials can enhance their physical, chemical, and antimicrobial properties, although these enhancements introduce new complexities in material processing [138].
Despite its advantages, several challenges hinder the full adoption of 3D printing in clinical practice. Achieving optimal occlusion, masticatory function, and long-term esthetic performance still poses difficulties. Furthermore, the lack of widespread regulatory guidelines for printed dental prostheses and the steep learning curve associated with digital workflows limit their integration into everyday practice [26, 137]. However, with continued innovations in material science, digital software, and clinician training, these limitations are expected to diminish in the near future. Table 6 summarizes studies on the use of 3D printing in denture fabrication, including the main research focus, types of materials employed, key findings, and reported challenges.
Summary of studies on 3D-printed dentures.
| Study focus | Materials used | Key findings | Challenges/Considerations | Reference |
|---|---|---|---|---|
| Color stability of 3D-printed resins | Hard PMMA-based resin, soft urethane-based resin, traditional heat-polymerized PMMA resin | 3D-printed hard PMMA resin showed similar color stability to traditional resin; soft resin had more discoloration | Further improvements are needed for dental use | [135] |
| Polymeric materials in 3D-printed dentures | PMMA, PEEK, ABS, PLA | PMMA is popular but has brittleness issues; PEEK has excellent mechanical properties; ABS and PLA have potential but need further research | Need for improved mechanical properties and biocompatibility | [136] |
| Clinical application of 3D-printed complete dentures | Various 3D-printed materials | Technical aspects are well-studied; clinical aspects like fitting and masticatory function need more research | Challenges in achieving precise occlusion and aesthetic results | [137] |
| Influence of glaze on 3D-printed denture bases | Yller Cosmos Denture resin, Makertech Labs PriZma Bio Denture resin | Glaze application increased hardness and decreased roughness; no significant effect of printer type on biofilm formation | Need for further study on long-term effects | [139] |
| Nanoparticles in 3D-printed denture resins | PMMA with nanoparticles (zirconia, titania) | Nanoparticles improve mechanical properties significantly | High cost and complex fabrication processes | [140] |
| Fracture resistance of 3D-printed denture teeth | Dentca 3D printing denture teeth resin | 3D-printed teeth showed adequate fracture resistance compared to conventional teeth | Need for further research on long-term durability | [141] |
| Accuracy of 3D-printed complete dentures | FDM and SLA techniques | SLA printing showed better accuracy in tooth positioning compared to FDM | Combining 3D printing with traditional methods can be effective | [142] |
| Surface characteristics and biocompatibility of 3D-printed DBR | 3D-printed DBR, packed, milled | 3D-printed DBR had the lowest surface roughness and biofilm formation; non-cytotoxic | Need for further research on long-term clinical performance | [143] |
| Sterilization effects on 3D-printed denture materials | Denture 3D+, Denturetec, Optiprint Laviva, Rapid Simplified | Ethylene oxide sterilization is recommended for most materials; conventional acrylic had higher impact strength | Sterilization methods can affect material properties | [144] |
PMMA: polymethyl methacrylate; PEEK: polyether ether ketone; ABS: acrylonitrile butadiene styrene; PLA: polylactic acid; FDM: fused deposition modelling; SLA: stereolithography; DBR: dentin bone replacement; 3D: three-dimensional.
The advent of 3D printing has revolutionized both clinical and academic fields of dentistry by enabling the creation of highly detailed, patient-specific dental models and educational tools. This transformative technology allows for the production of anatomically accurate replicas that are crucial for diagnosis, treatment planning, and hands-on training. The incorporation of 3D printing into dental workflows has significantly improved precision, reduced fabrication time, and enhanced learning experiences for dental students and professionals alike.
In clinical dentistry, 3D-printed models are widely used for surgical planning, orthodontics, endodontics, and the fabrication of customized dental prostheses. These models provide clinicians with a tangible representation of the patient’s oral anatomy, allowing for more accurate assessments and enhanced communication with patients and surgical teams [117]. For instance, surgical guides fabricated using 3D printing enable dentists to pre-plan implant placements, leading to increased surgical precision and shorter operation times [145, 146]. Furthermore, 3D printing is instrumental in producing custom-fitted orthodontic aligners and periodontal guides, which facilitate targeted and efficient treatment protocols [146].
Beyond clinical applications, 3D printing plays a critical role in dental education. It enables the fabrication of realistic simulation models that mimic the tactile feedback of natural teeth. These models are used to train students in procedures such as pulpotomies, cavity preparations, and crown placements, significantly enhancing their psychomotor skills before transitioning to clinical environments [147, 148]. Notably, 3D-printed educational tools provide consistency and availability that extracted human teeth cannot guarantee, thus offering an equitable and ethical alternative for hands-on training [147]. Studies also show that students perceive 3D-printed models as more engaging and effective for learning than traditional serial models, especially in pediatric dentistry training sessions [149].
The advantages of using 3D printing in dental models and education are multifaceted. It enables unparalleled customization based on patient-specific data from intraoral scanners or CBCT, ensuring tailored treatment outcomes and high fidelity to real-life anatomy [26, 117]. The cost-effectiveness and speed of production further contribute to its growing adoption in academic and private dental practices [150]. Additionally, this technology enhances the educational experience by bridging the gap between theoretical knowledge and clinical practice.
Despite its many benefits, there are several challenges to the widespread integration of 3D printing in dental education and practice. The limited availability of biocompatible and durable materials can compromise the realism and longevity of models [26]. Moreover, inaccuracies in digital scanning or design software may lead to suboptimal printing outcomes, potentially affecting the validity of clinical simulations [145]. Regulatory and ethical considerations surrounding the digital handling of patient data and quality assurance of printed devices also require attention [26].
Looking ahead, the integration of AI and ML into 3D printing workflows may further enhance the accuracy, speed, and utility of printed models. Additionally, as new dental biomaterials are developed, the quality and realism of educational tools are expected to improve. The application of 3D printing in pediatric dentistry is also gaining traction, offering opportunities for child-friendly models that facilitate both diagnosis and behavior management [151]. Table 7 provides a summary of studies on 3D printing applications in dental education and training, outlining the type of application, specific details, findings, and corresponding studies.
Summary of studies on 3D printing applications in dental education and training.
| Application | Details | Findings | Reference |
|---|---|---|---|
| Paediatric dentistry training | Developed and evaluated a 3D printed model for paediatric dentistry training. Compared it to traditional models. | 3D models were seen as a good idea and provided a more realistic experience. Students appreciated the simulation of caries. | [149] |
| Dental education & clinical dentistry | Evaluated various 3D printing technologies and their applications in dental education and clinical procedures. | 3D printing aids in the customized production of dental implants, surgical guides, and anatomic models. It enhances preclinical skills in various dental disciplines. | [117] |
| Prosthetic treatment simulations | Created individualized models for prosthetic treatment simulations based on intraoral scans. | Students found 3D-printed models effective for preparation exercises and beneficial in bridging the gap between simulation and real patient situations. | [152] |
| Educational tools across dental specialties | Overview of 3D printing technologies in different dental specialties, including education and training. | 3D printing stimulates the training of dental skills and has the potential to improve oral health care in education. | [146] |
| Pediatric dentistry | Comprehensive review of 3D printing applications in pediatric dental practices. | 3D printing improves clinical outcomes and enhances dental students’ educational experiences. | [151] |
| Pre- and post-graduate education | Reviewed studies using 3D-printed teeth models in dental education. | 3D models are effective in acquiring hands-on skills and eliminating cross-infection risks. | [153] |
| Dental education | Summarized the role and effectiveness of 3D-printed teeth in dental education. | 3D-printed teeth improve students’ confidence, clinical skills, and learning experiences. | [147] |
| Surgical skills training | Evaluated inexpensive 3D models for training surgical skills. | Models were suitable for surgical education, with no significant differences between ABS and PLA materials. | [154] |
| Endodontic training | Fabricated 3D tooth models to improve endodontic management. | Clear resin models were better for shaping, obturation, and as educational tools. | [155] |
| Surgical training | Developed 3D models and surgical guides for sinus lift surgery training. | Models and guides were useful for training and surgical planning. | [156] |
| Endodontic ledge management | Created a tooth model for ledge management practice. | Positive feedback from students and experts, with high success rates in bypassing and correcting root canal ledges. | [42] |
| Endodontic training | Developed a biomimetic root canal model for training. | Models provided good tactile sensation and suitable radiological behavior, similar to natural dental roots. | [157] |
3D: three-dimensional; PLA: polylactic acid; ABS: acrylonitrile butadiene styrene.
The integration of 3D printing into dental practice, particularly in smaller clinics and teaching centers, requires significant financial investment and careful consideration of long-term benefits. The most immediate challenge is the high initial investment in equipment. Purchasing a dental-grade 3D printer can be prohibitively expensive for small practices, as the cost varies depending on the type of machine and its production quality [49, 158]. Beyond the equipment, material expenses further increase the financial burden. Resin- and metal-based materials required for 3D printing are costly, and comparative studies highlight that material cost differs by printing technique; for example, FDM is more economical at $3.12 per model, while SLA can cost $5.18 per model [52]. In addition to purchase and material costs, operational expenses must be considered. The adoption of 3D printing demands extensive staff training to ensure effective use, which can be time-consuming and expensive [49, 159]. Ongoing maintenance and repairs of equipment, as well as potential failures during printing, add to recurring expenses. Despite these challenges, there are significant opportunities for cost savings over time. Additive manufacturing minimizes waste, reduces inventory needs, and supports on-demand production, ultimately decreasing operational inefficiencies [9, 158]. In prosthodontics, the use of digital workflows has been shown to cut both material and chairside time, translating into economic benefits for clinics [160].
The integration of 3D printing into modern dentistry brings remarkable possibilities for patient-specific care, yet it also introduces complex regulatory, ethical, and legal challenges. These challenges stem from the evolving nature of additive manufacturing and its convergence with clinical medicine.
The regulatory approval of 3D printing materials in dentistry varies across different global jurisdictions, reflecting the evolving landscape of digital healthcare innovations. In the United States, the Food and Drug Administration (FDA) regulates 3D-printed medical devices under its existing medical device framework, with growing attention toward developing specific guidelines for point-of-care (PoC) 3D printing due to its rapid adoption in hospitals and dental institutions [161]. While no separate regulatory pathway exists exclusively for dentistry, the FDA has demonstrated its openness to additive manufacturing technologies, notably approving Spritam®, the first 3D-printed pharmaceutical product in 2015 [162, 163]. However, challenges persist, particularly with respect to the variability of custom-made dental prostheses and orthodontic appliances, which complicate compliance with regulatory standards of manufacturing quality assurance and reproducibility [164, 165]. In the European Union (EU), regulatory oversight is governed by the Medical Devices Regulation (MDR 2017/745), which currently does not provide a dedicated framework for 3D-printed dental devices but instead categorizes them under custom-made medical devices [166]. This approach reflects the fact that the MDR was drafted before the widespread clinical integration of 3D printing technologies, and therefore, future amendments may be necessary to address specific aspects such as the validation of digital workflows and reproducibility of patient-specific prosthodontic and orthodontic devices [166]. In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) plays a central role in regulating medical devices, but current literature does not provide explicit details regarding its stance on dentistry-specific 3D printing approvals. Given Japan’s established framework for evaluating biocompatibility and safety in medical implants, it is reasonable to infer that similar standards would apply to dental devices, though detailed guidance remains limited in published reports. Likewise, in India, the Central Drugs Standard Control Organization (CDSCO) regulates medical devices under the Medical Devices Rules (2017). While no direct evidence exists regarding approvals of 3D-printed dental materials, recent expansions of CDSCO oversight into dental implants and orthodontic appliances suggest that such products would need clearance under these regulations before clinical adoption. From a general regulatory perspective, across all regions, certain common challenges emerge. For instance, the biocompatibility of resin-based dental materials remains a primary concern, as studies highlight potential issues such as the release of residual monomers and toxic by-products during post-processing [60]. Additionally, the mechanical properties of printable dental resins, such as flexural strength and wear resistance, must be carefully validated to ensure long-term clinical success in prosthodontics and orthodontics [36, 167, 168]. Despite these challenges, clinical applications of 3D printing in dentistry are expanding rapidly, ranging from occlusal splints and prosthodontic frameworks to implant components and dentures, underscoring the urgent need for tailored regulatory pathways that can ensure safety while supporting innovation [169–171]. Among the most important international benchmarks are those issued by the International Organization for Standardization (ISO). For instance, ISO 20795 establishes detailed requirements for denture base polymers, thereby ensuring that these materials maintain safety, biocompatibility, and functional integrity when used in clinical practice [172]. Similarly, ISO 6872 provides standards for dental ceramics, particularly those used in crowns and bridges, with an emphasis on their mechanical properties, durability, and overall biocompatibility [173]. These standards are widely referenced in dental research and clinical manufacturing to guarantee consistency across global practice. With the rapid advancement of digital manufacturing in healthcare, ISO/ASTM 52900 has become particularly significant. This joint standard outlines terminology and categorization for seven types of additive manufacturing processes, providing a unifying framework for interoperability and quality assurance in 3D and emerging 4D printing applications in dentistry [174–176]. The adoption of this standard ensures that materials and workflows in dental additive manufacturing comply with internationally recognized quality criteria, which is critical for scaling up clinical applications such as custom prosthetics, surgical guides, and orthodontic appliances. In addition to ISO frameworks, the American Dental Association (ADA) Seal of Acceptance remains a cornerstone of regulatory approval in the United States. This seal is awarded only after rigorous evaluation, serving as a mark of safety and efficacy for dental products, including restorative devices, composites, and preventive care items. Importantly, many dental practices and patients in the United States rely on the ADA Seal as an indicator of trustworthiness, and it is often viewed as a prerequisite for widespread clinical adoption [177, 178].
The ethical landscape of dental 3D printing is also shifting. Bioprinting, where living tissues or biologically active components are fabricated, introduces ethical dilemmas related to patient autonomy, long-term safety, and the moral status of engineered biological materials. Clinicians must ensure that patients are adequately informed about the nature and risks of 3D-printed restorations or implants, particularly when novel or experimental materials are used. Another key ethical issue is data protection, as patient-specific anatomical data is required for designing personalized dental appliances. The secure handling, storage, and sharing of this data are paramount, especially under regulations like the General Data Protection Regulation (GDPR). Ethical equity is also in question; 3D printing can potentially widen the healthcare access gap if high implementation costs and the need for technical expertise restrict availability to wealthier institutions or regions [1, 26].
Legally, one of the most contentious areas is intellectual property (IP). In dental 3D printing, IP law must address digital blueprints, reverse engineering risks, and shared design repositories. There is growing concern about whether CAD files should be protected under copyright, patent, or trade secret laws, and how shared-use models can avoid infringing on proprietary designs. Product liability is another emerging frontier. In traditional manufacturing, liability rests clearly with the producer. But with 3D-printed dental devices, the responsibility can be fragmented across the software provider, printer manufacturer, dental practitioner, and possibly even the patient. This complicates attribution in the event of device malfunction, material degradation, or clinical harm. The current legal frameworks may not sufficiently reflect these new risk distributions. Furthermore, there are concerns about whether legal systems are adequately equipped to regulate cross-border digital designs and locally produced dental components [179, 180]. Legal considerations also extend into the field of IP, where ownership and enforcement become increasingly complex with the proliferation of 3D-printed dental devices. The digital nature of design files and the ease of replication raise concerns about unauthorized use, counterfeiting, and infringement of patented processes [180]. This is particularly significant in the dental sector, where custom-made devices blur the boundaries between proprietary design and patient-specific adaptation. Furthermore, IP intersects with competition and consumer protection laws, requiring that innovation in 3D printing respect existing patents while ensuring fair access for patients and consumers [180]. Another significant concern involves data protection. Since dental 3D printing relies heavily on digital workflows, including intraoral scanning, CAD design, and the transfer of patient-specific data to printers, issues of privacy, confidentiality, and cybersecurity become paramount. Current EU data protection legislation, such as the GDPR, offers a robust framework, yet scholars argue that additional adaptation may be necessary to address the unique vulnerabilities posed by emerging 3D printing workflows [179].
While 3D printing has demonstrated broad applications in orthopedics and dentistry, its clinical impact is best illustrated through quantitative outcomes. Several studies have reported measurable reductions in operative and treatment times when patient-specific models and guides are employed. Customized surgical guides have been shown to shorten operative duration by up to 20–30%, thereby reducing anesthesia exposure and intraoperative blood loss [32, 33]. Similarly, complication rates in complex fracture management decrease when 3D-printed models are used for preoperative planning, as they allow surgeons to anticipate reduction pathways and implant placement with greater precision [31]. In restorative dentistry, 3D-printed crowns and prostheses have achieved higher accuracy of fit compared to conventionally fabricated counterparts. Clinical trials indicate improved marginal adaptation and reduced chairside adjustment times, leading to enhanced patient comfort and long-term stability of restorations [34]. Furthermore, patient-specific implants and grafts produced with additive manufacturing demonstrate higher early success rates due to better anatomical conformity and improved osteointegration, contributing to faster recovery and fewer postoperative complications [29, 30].
The evolution of dental 3D printing is set to transform clinical practice, education, and patient care, offering increasingly precise, customized, and efficient solutions. This transformative technology continues to expand across multiple areas of dentistry, fuelled by rapid advancements in materials science, digital integration, and bioprinting research. A key prospect lies in enhanced precision and customization. 3D printing enables the creation of complex geometries tailored to individual patient anatomy, facilitating the production of dental prostheses, surgical templates, orthodontic appliances, and training models with remarkable accuracy [9, 12, 50, 181]. As design software and printing technologies improve, even greater personalization and improved clinical outcomes are anticipated. Figure 4 gives challenges in 3D printing and some solutions.
Equally important are innovations in printable materials, which are broadening the spectrum of clinical applications. Newer photopolymer and composite resins have demonstrated improved strength, aesthetics, biocompatibility, and wear resistance, qualities essential for long-term intraoral use [9, 22, 37]. The ongoing exploration of bioactive, antibacterial, and regenerative materials offers exciting avenues for prosthodontic and periodontal therapies.
Integration with digital workflows is another significant frontier. Combining CAD/CAM systems with 3D printing not only reduces manual labor and material waste but also allows for same-day restorations and improved treatment planning accuracy [150]. This streamlined approach enhances workflow efficiency in both clinical and laboratory settings.
Specialized fields such as prosthodontics are already benefiting greatly. Crowns, bridges, and implant-supported restorations can be printed quickly and with minimal material waste, leading to better patient comfort and faster turnaround [50, 181, 182]. Similarly, orthodontics has seen significant improvements through the use of printed clear aligners and digital planning tools, although challenges such as the durability of thermoplastic materials still require attention [50, 183]. In endodontics, 3D-printed surgical guides and tooth models are enhancing treatment planning and clinical training, particularly in complex cases requiring apicoectomy or root canal navigation [184]. Meanwhile, pediatric dentistry is utilizing 3D printing for space maintainers, behavior management tools, and teaching aids, offering opportunities to improve both care and dental education [151].
The future of dental 3D printing is closely linked to its integration with bioprinting and regenerative medicine, opening pathways for patient-specific constructs in both hard and soft tissue regeneration. In hard tissue applications, bioprinted scaffolds have shown potential for alveolar bone, dentin, and periodontal ligament restoration, with studies demonstrating enhanced mechanical compatibility and cellular integration [70, 185, 186]. Soft tissue engineering is also advancing, with research focusing on gingival and oral mucosa grafts that can achieve both functional and aesthetic reconstruction [5]. Moreover, the incorporation of stem cell technologies and bioactive bioinks provides additional opportunities to enhance biological performance and precision in tissue regeneration [187, 188]. Further, the future of 3D printing in dentistry can be divided into short-, medium-, and long-term prospects.
Short-term prospects: In the near future, the most immediate advancements are expected in the field of material improvements. Researchers are actively working to overcome the current limitations of photopolymer resins, focusing on enhancing their mechanical strength, esthetics, color stability, and long-term durability [16, 170, 189, 190]. Developments include flexible resins for splints and dentures, multi-material printing for hybrid restorations, and the integration of nanoparticles or fillers to improve biocompatibility and wear resistance. These material upgrades will allow printed devices to rival or even surpass traditionally milled alternatives. Parallel to material innovation, the integration of AI in dental design workflows is gaining momentum. AI-driven planning and modeling can reduce design errors, streamline customization, and deliver prostheses, aligners, or surgical guides with greater precision. In rural and resource-limited settings, AI can also simplify workflows, reducing the dependence on highly specialized personnel while still maintaining accuracy and efficiency [158, 189, 191]. In the short term, these combined advancements will likely make 3D-printed devices more reliable, aesthetically pleasing, and clinically accessible.
Medium-term prospects: Looking ahead, wider adoption of bioprinting for soft tissues is anticipated. Bioprinting with bioinks containing stem cells, biomaterials, and growth factors is already showing promise for regenerating periodontal structures, gingival tissues, and craniofacial defects [5, 192]. As techniques for cell viability, vascularization, and structural fidelity improve, dental practitioners may begin applying bioprinting to regenerate soft oral tissues on a broader clinical scale. Such advances would address current limitations in periodontal therapies and implantology by supporting biological integration and more natural healing outcomes. In the medium term, it is realistic to envision bioprinted grafts and scaffolds becoming part of standard dental care [193, 194].
Long-term prospects: In the long term, the most ambitious prospect is the regeneration of whole teeth. While early research has demonstrated potential in regenerating dental pulp and tooth germ structures, the ultimate challenge lies in replicating the full multi-tissue complexity of a tooth, including dentin, enamel, periodontal ligament, and alveolar bone [195, 196].
Nevertheless, challenges persist, particularly with respect to cost and accessibility. The initial investment in 3D printers, material costs, and post-processing requirements can be prohibitive for smaller practices or institutions [49, 50, 184]. Additionally, the material limitations concerning strength, longevity, and intraoral safety continue to hinder widespread clinical deployment [22, 29]. The regulatory landscape and professional training needs are also critical considerations. Clinicians must not only understand how to operate and maintain 3D printing systems but also navigate the associated regulatory and ethical frameworks to ensure patient safety and legal compliance [1, 49, 197]. Looking forward, emerging trends such as bioprinting and tissue engineering hold the potential to regenerate dental pulp, bone, and periodontal structures, paving the way for biologically integrated restorations [26, 198]. Furthermore, AI is anticipated to complement 3D printing by optimizing design, detecting errors, and automating various stages of fabrication.
Although the applications of 3D printing in dentistry are expanding rapidly, existing studies often provide descriptive findings without sufficiently addressing conflicting results or methodological limitations. For instance, when comparing VP techniques, SLA has frequently been associated with superior surface smoothness and marginal adaptation, whereas DLP offers faster printing speeds and cost advantages. However, reports on trueness and durability vary significantly: some studies suggest that 3D-printed zirconia crowns demonstrate accuracy comparable to CAD/CAM-milled restorations, while others note issues with interlayer bonding, wear resistance, and long-term stability [121]. Similarly, orthodontic research highlights the benefits of 3D-printed aligners and retainers in customization and turnaround time, yet concerns remain about the longevity and intraoral safety of photopolymer resins used for these appliances. Material performance is another area where results diverge. While reinforced PMMA and nanocomposite resins have demonstrated improved mechanical properties in laboratory testing, other investigations report limited fracture resistance, color stability, and biocompatibility over time. Such variability highlights the absence of standardized testing frameworks and complicates clinical decision-making. Taken together, these inconsistencies reveal two key research gaps. First, there is a lack of rigorous head-to-head clinical comparisons between printing methods such as SLA, DLP, and FDM under uniform clinical protocols, particularly with respect to marginal fit, accuracy, and long-term mechanical performance. Second, most published evidence is based on in vitro studies or short-term evaluations, whereas longitudinal clinical trials are scarce. Future research should therefore prioritize standardized evaluation methods, long-term clinical verification, and cost-effectiveness analyses to establish evidence-based guidelines for the integration of 3D-printed devices in dentistry [20].
The integration of 3D printing into modern dentistry represents not just a technological upgrade but a paradigm shift in the way oral healthcare is conceived, delivered, and taught. This review has underscored the wide-ranging applications of additive manufacturing across the dental field, spanning orthodontics, crown and bridge fabrication, implantology, surgical guide development, denture production, and education. The ability to design and fabricate highly customized, precise, and biocompatible components has redefined standards of care, improving clinical efficiency, enhancing patient outcomes, and raising overall satisfaction with dental treatments. In orthodontics, 3D printing has accelerated the production of aligners and surgical aids, enabling a level of personalization that supports minimally invasive and patient-specific interventions. In restorative dentistry, additive manufacturing delivers unmatched accuracy in crown and bridge fabrication, drastically reducing chairside time, laboratory dependency, and human error. The most transformative impact, however, has been in implantology and surgical planning. Patient-specific implants and surgical guides created through 3D printing facilitate safer, faster, and more predictable outcomes, making complex interventions more accessible. Similarly, denture fabrication has benefited from advances in printable polymers and composites, producing prosthetics that achieve superior functionality, esthetics, and patient comfort. Beyond direct clinical applications, the incorporation of 3D-printed models and simulation tools into dental education has transformed training, offering reproducible, ethical, and cost-effective alternatives to extracted teeth. These educational applications not only enhance student learning but also ensure more standardized skill development across institutions. Despite this remarkable progress, significant challenges remain. Limitations in material performance, variability in printer accuracy, and the lack of standardized clinical protocols continue to constrain wider adoption. Regulatory and legal hurdles, including device classification, quality assurance, and liability issues, add further complexity to clinical translation. Moreover, practitioner training remains a critical bottleneck, as the effective use of 3D printing technologies demands both technical competence and digital literacy. Looking to the future, the convergence of 3D printing with AI and ML is expected to drive the next wave of innovation. AI-assisted design could automate treatment planning, optimize material selection, and enhance the accuracy of printed devices, reducing variability and broadening clinical access. Similarly, the emergence of 4D printing, in which printed materials are engineered to respond dynamically to environmental or physiological stimuli, holds the potential to create adaptive restorations, smart orthodontic devices, and responsive implants that actively support healing. In parallel, advances in bioprinting and regenerative dentistry are opening possibilities for fabricating personalized scaffolds, periodontal tissues, and even whole tooth structures, signaling a future where restorative dentistry converges with regenerative medicine. 3D printing has established itself as a cornerstone of contemporary dentistry, bridging technological innovation with patient-centered care. While challenges of regulation, materials, and training remain, the trajectory of research and clinical practice suggests a future where dental care is more precise, efficient, adaptive, and regenerative. By harnessing the synergies between 3D printing, AI, 4D printing, and bioprinting, dentistry is poised to move beyond restoration toward true biological regeneration, redefining the scope of oral healthcare in the decades to come.
3D: three-dimensional
ADA: American Dental Association
CAD: computer-aided design
CAM: computer-aided manufacturing
CBCT: cone-beam computed tomography
CDSCO: Central Drugs Standard Control Organization
Co-Cr: cobalt-chromium
DLP: digital light processing
EU: European Union
FDA: Food and Drug Administration
FDM: fused deposition modelling
GDPR: General Data Protection Regulation
IP: intellectual property
ISO: International Organization for Standardization
MDR: Medical Devices Regulation
ML: machine learning
PBF: powder bed fusion
PC-ABS: polycarbonate-acrylonitrile butadiene styrene
PCL: polycaprolactone
PDCs: polymer-derived ceramics
PEEK: polyether ether ketone
PLA: polylactic acid
PMMA: polymethyl methacrylate
SLA: stereolithography
SLM: selective laser melting
SLS: selective laser sintering
VP: vat photopolymerization
YSZ: yttria-stabilized zirconia
PPB: Conceptualization, Supervision, Validation. JJS: Methodology, Investigation. MS: Data curation, Formal analysis, Visualization. AD: Resources, Investigation, Data curation. KP: Project administration, Writing—original draft, Supervision. MZA: Writing—review & editing, Validation. BAAW: Validation, Formal analysis, Writing—review & editing. All authors have read and approved the submitted version.
Authors declare no conflicts of interest.
This study did not involve human participants or animal experiments directly. Therefore, ethical approval was not required.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4714
Download: 76
Times Cited: 0
