Affiliation:
1Department of Materials and Mechanical Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama 10206, Sri Lanka
Email: gayansiriwardana@sjp.ac.lk
ORCID: https://orcid.org/0000-0003-4165-8741
Affiliation:
2Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama 10206, Sri Lanka
ORCID: https://orcid.org/0000-0002-9380-538X
Affiliation:
2Department of Biosystems Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama 10206, Sri Lanka
Email: rdassanayake@sjp.ac.lk
ORCID: https://orcid.org/0000-0002-4608-2131
Affiliation:
1Department of Materials and Mechanical Technology, Faculty of Technology, University of Sri Jayewardenepura, Homagama 10206, Sri Lanka
ORCID: https://orcid.org/0009-0008-2714-9709
Explor Med. 2024;5:17–47 DOI: https://doi.org/10.37349/emed.2024.00203
Received: September 30, 2023 Accepted: November 15, 2023 Published: February 06, 2024
Academic Editor: Nermeen A. Elkasabgy, Cairo University Faculty of Pharmacy, Egypt
The article belongs to the special issue Exploration of 3D and 4D Printing in the Biomedical and Personalized Medicine Fields: Merits and Challenges
Three-dimensional (3D) and four-dimensional (4D) printing have emerged as the next-generation fabrication technologies, covering a broad spectrum of areas, including construction, medicine, transportation, and textiles. 3D printing, also known as additive manufacturing (AM), allows the fabrication of complex structures with high precision via a layer-by-layer addition of various materials. On the other hand, 4D printing technology enables printing smart materials that can alter their shape, properties, and functions upon a stimulus, such as solvent, radiation, heat, pH, magnetism, current, pressure, and relative humidity (RH). Myriad of biomedical materials (BMMs) currently serve in many biomedical engineering fields aiding patients’ needs and expanding their life-span. 3D printing of BMMs provides geometries that are impossible via conventional processing techniques, while 4D printing yields dynamic BMMs, which are intended to be in long-term contact with biological systems owing to their time-dependent stimuli responsiveness. This review comprehensively covers the most recent technological advances in 3D and 4D printing towards fabricating BMMs for tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics. In addition, the challenges and gaps of 3D and 4D printed BMMs, along with their future outlook, are also extensively discussed. The current review also addresses the scarcity in the literature on the composition, properties, and performances of 3D and 4D printed BMMs in medical applications and their pros and cons. Moreover, the content presented would be immensely beneficial for material scientists, chemists, and engineers engaged in AM manufacturing and clinicians in the biomedical field.
Three-dimensional (3D) printing, or additive manufacturing (AM), is a novel technology that fabricates materials on a print bed layer-by-layer. AM manufactures objects with simple to complex geometries using computer-aided design (CAD) models. AM can process various materials, such as polymers, hydrogels, ceramics, glass, metals, and other composites. Several AM-based techniques with different material processing technologies, including material extrusion [1–3], vat photopolymerization (VP) [4], powder bed fusion (PBF) [5], material jetting (MJ) [6], binder jetting (BJ) [7], directed energy deposition (DED) [8], and sheet lamination are currently in use.
Material extrusion employs delivering material through a print nozzle onto a print bed via heat [fused deposition modeling (FDM)] [2] or pressure [direct ink write (DIW)] [9, 10]. FDM processes thermoplastics, and the cooling process solidifies the final object-built layer-by-layer. DIW handles photocurable polymers and hydrogels, and ultraviolet (UV) curing allows hardening of the printed material on the bed. In VP, a photocurable polymer/resin is placed in a vat, a layer of the resin is placed on the build platform and UV laser [stereolithography (SLA) and two-photon lithography] rasters the required pattern on the resin surface, enabling crosslinking and solidifying the liquid resin, subsequently curing the layers. In digital light processing (DLP), another form of VP, a projected light source, is used to cure the entire layer completely. In PBF, a laser source or high-energy electron beam fuses polymer/metal powders together. The main PBF methods are direct-metal laser sintering (DMLS), electron beam melting (EBM), selective laser sintering (SLS), selective heat sintering (SHS), and selective laser melting (SLM). In MJ, photopolymers, waxes, and plastics are jetted (deposited) onto a build platform through a nozzle via either a continuous or drop-on-demand method and the layers are cured under UV light. BJ involves spraying a liquid-type bonding agent onto the surface of metal/polymer powder, thereby bonding particles together to build the object layer by layer. Other AM techniques include DED for polymers, metals, ceramics and sheet lamination for metals [ultrasonic AM (UAM)] and papers [laminated object manufacturing (LOM)] [4]. The main AM techniques currently active in manufacturing biomedical materials (BMMs) are FDM, SLA, SLS, PBF, DED, BJ, and bioprinting [11–13].
Four-dimensional (4D) printing is considered the next-generation advancement of AM technology, adding a fourth dimension as the time-dependent shape/functional change after printing. 4D printing processes smart materials capable of changing the shape or function upon exposure to certain stimuli such as humidity, temperature, light, pH of the medium, solvent, and magnetic and electric fields [14, 15]. Shape-memory polymers (SMPs) play a key role in this context. AM technologies involved in 4D printed BMMs are mainly DIW, SLA, and multi-MJ, targeting applications in tissue engineering, drug delivery, medical devices, and diagnostics [16].
Both 3D and 4D printing technologies share similarities and differences. For instance, both materials are manufactured layer-by-layer and possess a length, width, and thickness. Moreover, both these technologies commonly use techniques such as extrusion, VP, jetting, DED, and PBF. However, one of the main differences between 3D and 4D manufacturing is the type of material used to process. Only 4D printing can change the shape, properties, and functions upon exposure to certain stimuli as opposed to 3D printing [14]. Consequently, 4D printing yields dynamic time-dependent stimuli-responsive materials, while 3D printed objects are static.
BMMs are broadly defined as biomaterials manufactured or processed to be utilized as medical devices or related components. BMMs include prostheses, reconstituted tissues, intravenous catheters, sutures, implants (prosthetic heart valves, ureteral stents, and hernia meshes), and scaffolds [15]. Currently, AM contributes to the manufacturing of BMMs mainly via polymers, ceramics, bioactive glass (BG), metals/alloys, and composites for biomedical applications such as tissue engineering, drug delivery, porous metal implants, cell-materials interactions, wear degradation, bionanotechnology, and biopharmaceuticals [17].
Even though numerous studies have been reported on AM of BMMs, only limited information is available on 3D and 4D printed BMMs, their challenges, and prospects in different medical applications. Hence, the current review fills this knowledge gap for the first time by comprehensively covering the most recent 3D and 4D printing techniques, printed BMMs, and their applications in four distinct biomedical fields: tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics. Furthermore, this review also elaborates on current challenges and future directions of 3D and 4D printing in the healthcare sector.
BMMs prepared from 3D and 4D printing are widely employed in medicine. The following section concentrates on the recent developments of 3D and 4D printed BMMs in four major biomedical applications: tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics. In Figure 1, the summary of 3D and 4D printed BMMs and their uses in the above-mentioned biomedical fields is presented.
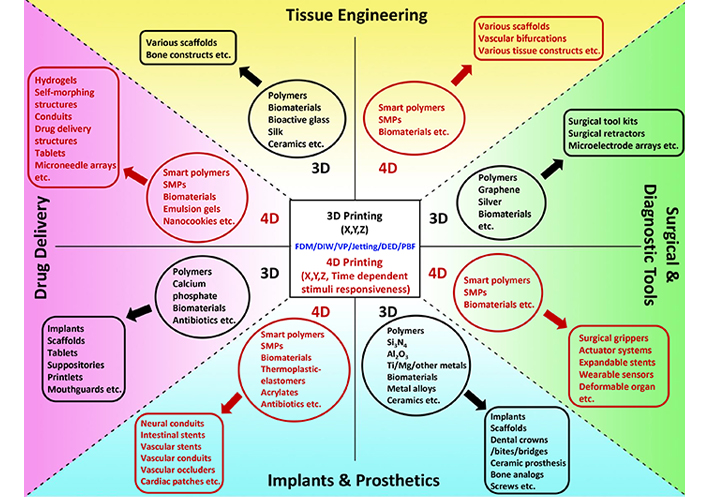
A summary of recent developments of 3D and 4D printed BMMs and their applications in tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics. Recent trends of raw materials (inside ovals) and 3D and 4D printed BMMs (inside rounded rectangles) are illustrated under each biomedical application
Tissue engineering, a discipline of biomedical engineering, uses a combination of cells, engineered materials and methods, and suitable biochemical and physiochemical factors to restore, maintain, improve, or replace various types of biological tissues. The primary criteria for a polymer to be qualified for tissue engineering applications are its high bioresorbability or biodegradability, high mechanical strength, and enhanced cell attachment ability [18]. Polylactic acid (PLA) is one such qualifier widely explored in bone tissue engineering. Gregor et al. [19] successfully fabricated PLA scaffolds with an average pore size of 350 microns and 30% porosity via FDM-based 3D printing. The authors reported high proliferation rates in osteosarcoma cells with 30% and 50% porous scaffolds while exhibiting mechanical properties to support load-bearing bone growth. Although alginate (Alg) hydrogels show porosity, biocompatibility, and solubility, they are restricted in 3D printing applications due to low mechanical strength, cell attachment, and easy degradation [20, 21]. However, a blend prepared by crosslinking Alg and gelatin (Gel) displayed high 3D printability and cytocompatibility with osteoblasts [22].
Recently, Kim et al. [23] prepared a bioink by combining Alg and silk fibroin (SF) protein to fabricate hydrogel scaffolds using DIW and visible light irradiation (Figure 2A). Due to the increase of cell compatibility through SF, these scaffolds supported the proliferation of fibroblasts with improved cytocompatibility than conventional Alg bioinks, making Alg/SF a promising material for tissue engineering.
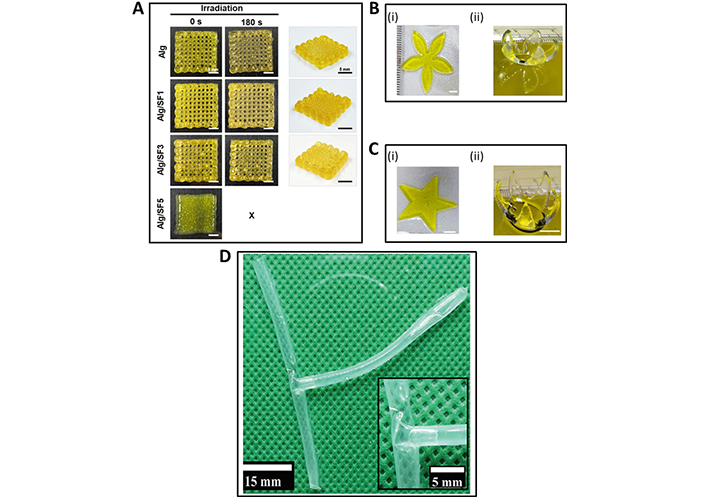
Recently developed 3D and 4D printed BMMs for tissue engineering. (A) Alg/SF scaffolds 3D printed using DIW and visible light irradiation; (B) and (C) DLP-based 4D bioprinted [Gel methacrylate (GelMA)/poly(ethylene glycol) dimethacrylate (PEGDM)] petal and star-shaped tissue scaffolds, respectively. (Bi), (Ci) printed structures and (Bii), (Cii) curved structures after absorbing deionized water; (D) 4D printed crosslinked Alg dialdehyde (ADA)/Gel after swelling in pure water to form a tubular T-junction and the inset of (D) is the magnified view at the junction area
Note. (A) Adapted with permission from “Silk fibroin enhances cytocompatibilty and dimensional stability of alginate hydrogels for light-based three-dimensional bioprinting,” by Kim E, Seok JM, Bae SB, Park SA, Park WH. Biomacromolecules. 2021;22:1921–31 (https://pubs.acs.org/doi/10.1021/acs.biomac.1c00034). © 2021 American Chemical Society; (B) and (C) adapted with permission from “Visible light-based 4D-bioprinted tissue scaffold,” by Gugulothu SB, Chatterjee K. ACS Macro Lett. 2023;12:494–502 (https://pubs.acs.org/doi/epdf/10.1021/acsmacrolett.3c00036). © 2023 American Chemical Society; (D) adapted from “4D biofabrication of T-shaped vascular bifurcation,” by Kitana W, Apsite I, Hazur J, Boccaccini AR, Ionov L. Adv Mater Technol. 2023;8:2200429 (https://onlinelibrary.wiley.com/doi/full/10.1002/admt.202200429). CC BY.
In another effort, Lafuente-Merchan et al. [24] employed biopolymers such as Alg, nanofibrillated cellulose (NC), and hyaluronic acid to fabricate NC-Alg-hyaluronic acid scaffolds via extrusion-based AM. Mesenchymal stromal cells were used for cell viability analysis. Adding hyaluronic acid improved the scaffold properties, biocompatibility, and cell viability compared with NC-Alg scaffolds. Chameettachal et al. [25] demonstrated a DIW-3D printed enzymatic crosslinked silk-G bioink as a suitable material for 3D bioprinting of cartilage constructs.
SLM-based 3D printed hydrogel scaffolds containing platelet-rich plasma (PRP)-GelMA have also been studied [26]. Bioactive ceramics, another class of materials used in bone tissue engineering, have been successfully 3D printed to form complex bioceramic parts with dense and porous multifunctional structures [27]. While BJ and SLA have frequently been used to process bioceramics, various AM techniques, such as FDM, DIW, DED, and SLS, have been employed [28–30]. Ceramic-polymer composites have also been successfully 3D printed using polymers like polycaprolactone (PCL), PLA, or polylactide glycolic acid (PLGA) with calcium phosphate (CaP) [31, 32]. The incorporation of polymers improves the processability and flexibility of the fabricated composites during 3D printing. BGs, especially the highly abundant 45S5 composition (45 SiO2, 24.5 CaO, 24.5 Na2O, and 6 P2O5—in wt%), other melt-derived formulations, and sol-gel derived BGs have also been explored for 3D printing of scaffolds for bone tissue engineering. Recent research by Ma and co-workers [33] exhibited 3D printing of 45S5 BG-based scaffolds using SLA-based AM.
Using the composites prepared with 45S5 BG and tricalcium phosphate (TCP), Bose et al. [34] demonstrated successful 3D printing of scaffolds via BJ. 45S5 BG-based scaffolds have also been 3D printed via the SLS AM technique [35] and DIW [36]. Several studies have also been reported on the successful 3D printing of sol-gel-derived BGs. For instance, Wu and co-workers [37] described DIW 3D printed scaffolds of sol-gel-derived mesoporous BGs combined with polyvinyl alcohol (PVA). The study by Dai et al. [38] illustrated DIW 3D printed Gel/SF scaffolds incorporating sol-gel derived 1 wt% of Cu-doped BG particles for bone defect repair applications. Moreover, polymer-BG composites have also been successfully 3D printed. FDM technique has been successful with BG-based composites prepared from PCL [39], PLA [40], poly(hydroxybutyrate-co-hydroxyvalerate) (known as PHBV) [41], and polyolefin binders [42] while DIW has been mostly used with silk [43].
Contribution from 4D printing towards tissue engineering has also been evidenced in recent literature [44, 45]. Live cells containing bioinks have been 4D printed, enabling the fabrication of functional tissues for successful organ transplantation and efficiently repairing damaged tissues [16]. Gugulothu and Chatterjee [46] demonstrated a successful 4D printed bioink consisting of a blend of GelMA and PEGDM with a photoinitiator and a photoabsorber via DLP technique. This 4D bioprinted material acts as a shape-morphing and cell-laden hydrogel for tissue engineering applications by supporting cell viability and proliferation while altering its shape upon hydration, a cell-friendly stimulus (Figure 2B and C) [46]. Díaz-Payno and co-workers [47] recently prepared an extrusion-based 4D printed smart multi-material system using two hydrogel-based materials, hyaluronan and Alg. This scaffold self-bends upon differential swelling between the two zones, mimicking the natural cartilage structure. Ding et al. [48] showed a DIW-based 4D bioprinting to produce a shape-morphing cell condensate-laden bilayer system. The technology facilitates the creation of tissue constructs with precise control over cellular organization and distribution and would pave the way for future research on scaffold-free tissue regeneration.
Using ADA and Gel, Kitana et al. [49] exhibited DIW-based 3D printed tubular structures that self-transformed into a T-junction after immersing in water, hence showing 4D printing behaviour. The transformation of the 4D printed crosslinked ADA/Gel, into a tubular T-junction after swelling in pure water is depicted in Figure 2D [49]. Human endothelial cells seeded on the T-junction showed outstanding growth properties and excellent cell viability. This finding could pave the way for future vascularized tissues for the survival and function of larger engineered organs. Furthermore, 4D printing has become promising for fabricating patient-specific, functional organs that can be adapted and integrated within the recipient’s body [50].
Drug delivery refers to a broader scientific field involving various approaches, formulations, manufacturing techniques, storage systems, and technologies that transport a pharmaceutical compound to a specific target site to obtain a desired therapeutic effect. Polymers such as PLGA, PCL, and other materials like CaP ceramics, BGs, bioactive ceramics, and ceramic-polymer pastes have been explored in this field. BJ AM technology has been widely utilized to fabricate BMMs in this field. VP, PBF, and material extrusion AM techniques have also been used to 3D print relevant scaffold structures. During drug delivery, porous scaffold structures are first 3D printed from the relevant material, followed by the loading of the drug. This strategy avoids the degradation of drugs upon high-temperature processing. There has been extensive research on processing, mechanical property measurements, and biocompatibility evaluations in vitro and in vivo of many CaP ceramic scaffolds.
Ceramic-polymer composites have been 3D printed successfully. Adding polymers such as PCL, PLA, and PLGA into CaP ceramics improves the processability and flexibility of the printed part [31, 32]. The BJ AM technique enables the delivery of heat-labile molecules such as growth factors and antibiotics by fabricating low-temperature CaP-based scaffolds [28]. Controlling pharmacokinetics is essential in drug delivery applications. Inzana et al. [51] described that a PLGA-based post-printing coating of CaP scaffolds achieved first-order drug release kinetics over 14 days. Drug/growth factor-loaded composite CaP scaffolds could be successful via extrusion-based AM under low temperatures and mild post-processing conditions. Mineralized slurry or paste compositions, extrudable under physiological temperature, become ideal for this approach [28]. Martínez-Vázquez et al. [52] described successful DIW-based 3D printing of porous silicon-doped hydroxyapatite (HASi) and Gel composite scaffolds for delivering vancomycin antibiotics. Mild scaffold fabrication conditions maintained the antibiotic’s antimicrobial activity in standard in vitro assays. In a similar DIW-based approach, Akkineni et al. [53] demonstrated the fabrication of vascular endothelial growth factor (VEGF) or bovine serum albumin (BSA) loaded CaP-based scaffolds. The mechanical properties of these scaffolds were comparable to those of trabecular bone and showed biocompatibility with mesenchymal stem cells for up to 21 days. Poldervaart et al. [54] exhibited a rare in vivo effort to 3D print composite macroporous Alg scaffolds via extrusion, laden with Gel microparticles (GMPs) and mesenchymal stem cells. However, concentrations greater than 3% w/v Alg could not be extruded due to the high viscosity factor of the printing ink.
On the other hand, high-temperature scaffold fabrication or processing enables CaP-based ceramics to achieve improved mechanical properties. However, high-temperature processing lacks uniform printability of cells and bioactive molecules. As a precaution, additional post-processing routes can be taken, such as the incorporation of bio-factors and cells onto the printed structures, including surface adsorption or surface modifications, regardless of the 3D printing technology [28]. Moreover, doping CaP with silicon improved both the bioactivity and mechanical properties of these scaffolds. Combined with bone morphogenetic protein (BMP) in the form of recombinant human BMP-2 (rhBMP-2), these scaffolds enabled bone ingrowth, osseointegration, and vascularization. Ishack et al. [55] fabricated biphasic CaP [15% hydroxyapatite (HA) and 85% β-TCP] scaffolds via extrusion-based AM. After loading with either BMP-2 or dipyridamole and implanting into a mouse calvarial defect, they promoted bone regeneration eight weeks post-operatively. Koski et al. [56] demonstrated the use of naturally sourced gelatinized starch as a natural binder system with HA ceramic to obtain extrusion-based solid-freeform fabricator (SFF) scaffolds. These scaffolds showed improved compressive strength and in vitro biocompatibility with osteoblast cells without crosslinking or post-processing.
4D printing is applied in numerous advanced drug delivery systems to improve efficiency in treatment outcomes. Controlled release of drugs, patient-specific dosing, and targeted delivery are the main advantages of these 4D printed structures compared to conventional systems [57]. Researchers can fabricate devices that release drugs at a precise rate over a predetermined period by utilizing the ability of the 4D printed scaffolds/structures to respond to specific stimuli like temperature and pH changes [58].
Tran et al. [59] devised 4D printed smart hydrogel systems that respond to thermal, magnetic, electrical, photo, pH, and water stimuli. For example, a pH-responsive hydrogel capsule releases its content gradually in an acidic environment of the stomach. The authors reported that this controlled drug-releasing strategy is highly beneficial for minimal side effects and improved therapeutic efficacy. Moreover, AM techniques such as SLA, DLP, two-photon photopolymerization (2PP), and extrusion have successfully fabricated these hydrogels. Cancer treatment is another area where 4D printed personalized drug-eluting implants have recently become a highly versatile technique. Upon responding to external stimuli like pH changes or biomarkers, these implants could release chemotherapeutic agents at a controlled rate, enabling precise and timely distribution of drugs to specific sites, minimizing side effects and enhancing the overall efficiency of the treatment [60]. Makvandi et al. [61] prepared a 4D printed microneedle patch for personalized pain management. This BMM could release analgesic drugs in response to inflammation/pain signals. Importantly, 4D printing approaches ensure effective and efficient drug delivery with minimal side effects.
Some of the recent 3D and 4D printed BMMs targeted for drug delivery applications are depicted in Figure 3. These include PLA/PVA-based FDM-3D printed mouthguard (Figure 3A–D) loaded with food-grade flavor vanillic acid (VA) and clobetasol propionate (CBS) model drug [62], polymer/carbon-based magnetoelectric responsive porous nanocookie conduit 4D printed via DLP (Figure 3E) [63], and ethyl cellulose/hydroxypropyl methylcellulose (HPMC)/polyvinyl pyrrolidone (PVP)/cellulose acetate-based controlled drug release shell (Figure 3F and G) 3D printed via pressure-assisted microsyringes (PAM) technology [64].
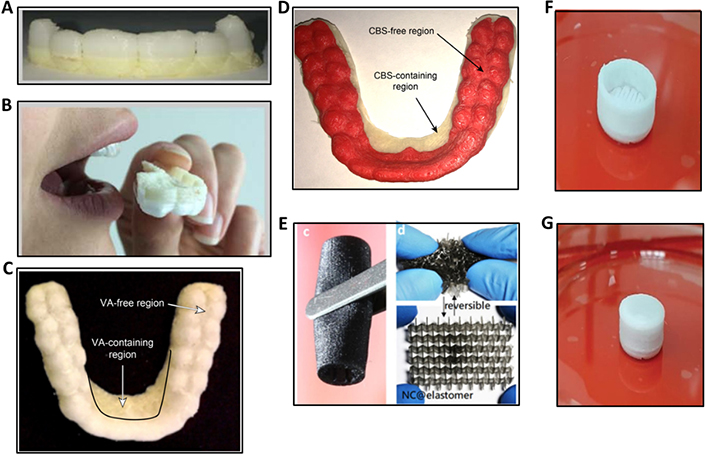
Recent 3D and 4D printing advancements in drug delivery. (A) PLA/PVA-based mouthguard in its FDM-3D printed form; (B) evaluations in humans; (C) VA food flavor-loaded and free regions; (D) CBS model drug-loaded and free regions; (Ec) DLP-4D printed nanocookie conduit showing its printed form and elastic properties (Ed); (F) PAM-3D printed controlled drug release shell without cap; (G) prepared for drug delivery applications
Note. (A)–(D) Adapted from “3D printing of a wearable personalized oral delivery device: a first-in-human study,” by Liang K, Carmone S, Brambilla D, Leroux J. Sci Adv. 2018;4:eaat2544 (https://www.science.org/doi/10.1126/sciadv.aat2544). CC BY-NC; (E) adapted from “4D printing of stretchable nanocookie@conduit material hosting biocues and magnetoelectric stimulation for neurite sprouting,” by Fang JH, Hsu HH, Hsu RS, Peng CK, Lu YJ, Chen YY, et al. NPG Asia Mater. 2020;12:61 (https://www.nature.com/articles/s41427-020-00244-1) CC BY; (F) and (G) adapted from “Optimization of semisolid extrusion (pressure-assisted microsyringe)-based 3D printing process for advanced drug delivery application,” by Mohammed AA, Algahtani MS, Ahmad MZ, Ahmad J. Ann 3D Print Med. 2021;2:100008 (https://www.sciencedirect.com/science/article/pii/S2666964121000035). CC BY.
The focus of recent research on the 3D printing of biomedical devices from porous scaffolds has been shifted slightly towards structures like surgical tools. A surgical tool or instrument is a medical device for performing specific actions or carrying out desired effects during surgery, including the modification of biological tissues. Surgical tools can be prepared with AM techniques such as MJ with thermoplastics and thermosets and BJ and PBF-based techniques like SLS and SLM with metals, ceramics, polymers, and glasses [65]. Francis and co-workers [66] investigated using 3D printed surgical tools to develop a reliable and rapid high-level disinfection process for austere environments to diminish supply chain issues. George et al. [67] developed an SLS-based AM of a surgical tool kit, including hemostats, needle drivers, scalpel handles, retractors, and forceps, using virgin and recycled Dura-Form EX plastic powder. These approaches establish AM facilities for fabricating medical tools in places like surgical hospitals in combat zones, spacecraft or third-world environments. Rankin et al. [68] illustrated successful FDM-based 3D printing of an army-navy surgical retractor using PLA. This tool met the required mechanical properties inside an operating room. In another study, Wu and co-workers [69] displayed an FDM-3D printed silver nanoparticle-polyacrylamide (AgNP-Pam)/HPMC-based superporous hydrogel for wound dressing applications (Figure 4A). The large pores in 3D printed templates could buffer the swelling tendency of these dressings, thus diminishing the detachment from wounds. Further, in vivo studies proved that these dressings could heal the infected wounds, restraining scar tissue formation [69].
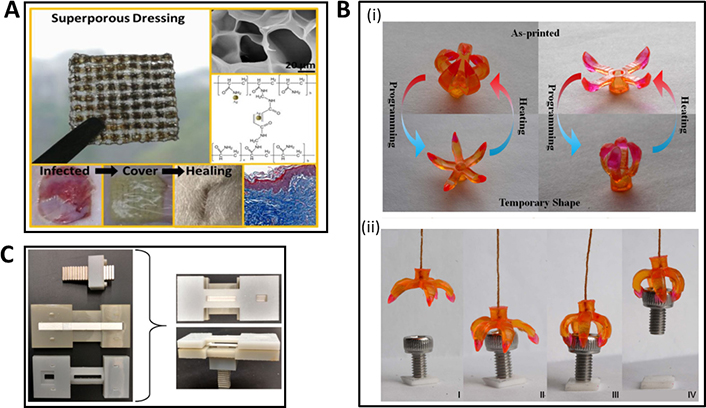
Recent contributions in 3D and 4D printing towards surgical/diagnostic tools. (A) FDM-based 3D printed AgNP-Pam/HPMC superporous hydrogel as a wound dressing; (B) 4D printed surgical gripper system: (i) illustrating the transition from the printed shape to the temporary shape, (ii) sequential snapshots of gripping of an object; (C) 3D printed device for magnetic focus lateral flow sensor for detecting cervical cancer biomarkers
Note. (A) Adapted with permission from “Combination of the silver-ethylene interaction and 3D printing to develop antibacterial superporous hydrogels for wound management,” by Wu Z, Hong Y. ACS Appl Mater Interfaces. 2019;11:33734–47 (https://pubs.acs.org/doi/10.1021/acsami.9b14090). © 2019 American Chemical Society; (B) adapted from “Multimaterial 4D printing with tailorable shape memory polymers,” by Ge Q, Sakhaei AH, Lee H, Dunn CK, Fang NX, Dunn ML. Sci Rep. 2016;6:31110 (https://www.nature.com/articles/srep31110). CC BY; (C) adapted with permission from “Magnetic focus lateral flow sensor for detection of cervical cancer biomarkers,” by Ren W, Mohammed SI, Wereley ST, Irudayaraj J. Anal Chem. 2019;91:2876–84 (https://pubs.acs.org/doi/full/10.1021/acs.analchem.8b04848). © 2019 American Chemical Society.
4D printing technology can fabricate smart surgical tools adaptable to environmental changes. These are functionally tailored to provide precision and control during complex surgical procedures. More interestingly, such smart tools can alter their shape and stiffness in response to specific external stimuli. These properties provide them with several advantages, such as conforming to different surgical scenarios and anatomical structures, mitigating the risk of damage to surrounding tissues, improving surgical outcomes, and lowering patient recovery times [70]. Using high-resolution projection microstereolithography (PμSL) and multiple shape memory polymers, Ge and co-workers [71] successfully 4D printed a surgical gripper system that changed its shape and stiffness responding to stimuli within the human body (Figure 4B). These properties could enable this gripper system to steer through tight spaces, grasp, and control fragile tissues while minimizing the risk of damage during surgical work. Bodaghi et al. [72] demonstrated a 4D printed actuator system utilizing fibres of shape memory polymers. This responsive surgical tool acted as a self-expanded stent, changing its diameter to a specific need. This could be utilized successfully during endovascular procedures, ensuring optimal blood flow and adapting to time-based fluctuations in vessel diameter or pressure. Han and co-workers [73] investigated another fascinating smart system, a DLP-based 4D printed microneedle array with backward-facing curved barbs biomimicking porcupine quills-like structures. These barbs provided a more secure and stable connection with the particular tissue, 18 times stronger than the barbless microneedle. This smart microneedle array-based approach would be beneficial in future transdermal drug delivery systems and or in wound closer applications. Zhou et al. [74] described a 4D printed wound closure device that adapted its shape and stiffness in response to wound contours. The device supported reduced scarring and efficient healing with a precise and gentle closure mechanism.
Medical diagnosis determines which disease or condition is responsible for a set of symptoms/signs. The devices that are utilized in this detection process are referred to as diagnostic tools, which include equipment such as stethoscope, blood pressure monitors, pulse oximeters, electrocardiographs (ECGs), electroencephalography (EEGs), ultrasonography (US), X-ray machines, and biosensors. Recent literature illustrates medical diagnostic tools fabricated via FDM, DIW, SLA, SLM, and SLS-based AM techniques.
Gaal et al. [75] exhibited 3D printed integrated, transparent, and sealed microchannel system via FDM using PLA. Different materials like paper, glass, wire, and polymers could be integrated within a microchannel. Then, an e-tongue sensor was 3D printed to detect basic tastes below the human threshold. In another study by Manzanares Palenzuela and co-workers [76], highly sensitive graphene-based rings and disc-shaped electrodes were 3D printed via FDM. Different redox probes were used to detect the electrochemical performance. The incorporation of PLA increased the electroactivity. López Marzo et al. [77] discussed an FDM-based 3D printed enzymatic biosensor for H2O2 detection. Biosensor performance was enhanced by applying gold nanoparticles (AuNPs) to facilitate heterogeneous electron transfer. Ren and co-workers [78] 3D printed a thermoplastic frame or a device using FDM to support a magnetic focus lateral flow sensor (Figure 4C) detecting and diagnosing cervical cancer biomarkers. Cardoso et al. [79] developed another grapheme-PLA (G-PLA) based amperometric biosensor for glucose detection in biofluids. This FDM-based 3D printed biosensor could also be modified to detect nitric and uric acid for saliva and urine analysis. 3D printed models have also been used to characterize the anatomical structure of the fractures and lesions as a complete pre-surgery evaluation [80]. Aerosol jet printing (AJP), a form of a DIW, uses a directed aerosol stream depositing a polymer on a substrate [81]. Past research evidences 3D fabrication of diagnostic tools via AJP. Yang and co-workers [82] developed silver microelectrode arrays (MEA) via AJP. The sensor successfully detected H2O2 and glucose levels, illustrating the potential of AJP to fabricate MEAs for applications like touch sensing, biosensing, and strain sensing.
Numerous studies have been reported on the 3D printing of diagnostic tools via SLA. For instance, Kuo et al. [83] developed a microfluidic device via SLA using low molecular weight poly(ethylene glycol) diacrylate (PEGDA) at sub-millimeter resolution. They fabricated an active micro-mixer containing pneumatic micro-valves and micro-channels with high resolution. These complex microfluidic devices would serve in various diagnosis fields, such as patch-clamp chips, biosensors, organ-on-a-chip, and tumor-on-a-chip. Narayanan et al. [84] investigated a dual-mode electrochemical biosensor via SLA to diagnose glucose and H2O2. The structure was developed by coating with AuNPs and colloidal platinum as a function-support matrix. Simultaneous detection of both glucose and H2O2 could be beneficial in potential real-time applications in clinical, biological, and environmental fields.
SLM has contributed significantly to the medical and dental fields. Studies by Vandenbroucke and co-workers [85] showed that biocompatible metal alloys, Ti‐6Al‐4V and cobalt-chromium-molybdenum (Co‐Cr‐Mo), yield SLM-based 3D printed parts used as dental prostheses. These parts met the strength, stiffness, corrosion behaviour, and process precision standards for medical and dental applications. Kwon et al. [86] exhibited SLS-based low-temperature fabrication of copper nanoparticle thin films onto a polymer substrate, yielding a flexible, conductive, and transparent material. This could be applied to flexible touch electronic panels.
4D printing has recently demonstrated immense potential in developing advanced diagnostic tools for the medical field. Owing to the ability to respond to external stimuli, 4D printed diagnostic tools offer more sensitivity, accuracy, and patient-specificity, leading to improved patient care. For instance, Kumar et al. [87] introduced a wearable smart sensor made from thermoplastic polyurethane (TPU) 4D printed on fabric via FDM. Vital signals such as heart rate, blood pressure, and body temperature were monitored by the sensor owing to the stimuli-responsive nature of the TPU. The authors reported that this discovery would pave the way for future smart sensors with real-time monitoring and early detection of potential health issues. Guerra and co-workers [88] demonstrated 4D printed diagnostic tools: solid-cured tissue-engineered implants made from photo-polymerizable resins. Embedded integrated microfluidic channels in the implants would change shape or color by binding to biomarkers specific to cancer or other infections, enabling rapid and easy visualization of the diagnosis. Generating anthropomorphic phantoms via 4D printing marks a revolution in medical imaging. These act as physical models used to calibrate and validate imaging equipment for radiotherapy. Colvill and co-workers [89] succeeded in 4D printing a deformable lung, including respiratory tract and liver phantom. This helps assess the accuracy of computed tomography (CT) and magnetic resonance (MR) imaging in radiotherapy planning. The 4D printed phantom enables clinicians to optimize treatment plans by responding to organ motion during respiration. Consequently, improved patient outcomes and reduced radiation exposure could be achieved.
Medical implants are devices placed in or on the body surface. The most common implants are prosthetics intended to replace a damaged or missing body part. Apart from prosthetics, other implants deliver medicines to internal organs and tissues, support internal structures and monitor body functions. Implants can be made from biological materials such as bones, tissues, skin, metal, plastic, ceramic, and other composite materials.
AM is one of the most used techniques for manufacturing medical implants. 3D printing is preferred over conventional implant manufacturing methods mainly due to the ease of manufacturing a customized implant with enhanced compatibility and clinical results. 3D printed implants are popularly used in reconstructions of the spine, shoulder, and hip and for facial surgery and dental implants [90]. Patient-specific implants and prostheses are fabricated using a wide range of medical-grade metallic, ceramic, polymer, and composite materials [91]. Metallic biomaterials are widely used in the medical field due to their superior mechanical properties and long lifetime. 3D printed metallic implantable medical devices are commonly made with alloys of Ti, Co-Cr-Mo, and stainless steel (SS) [92] using DED and PBF AM techniques [93]. Commercially pure Ti and its alloys (Ti-6Al-4V, Ti-6Al-7Nb, Ti-5Al-6Nb, and Ti-13Nb-13Zr) are the commonly applied bone implants due to low density, lightweight, and suitable tribological and mechanical properties [93]. Ti-based alloys possess the highest biocompatibility than any other metallic content, but they are still considered bioinert materials compared to bioceramics [94, 95]. Recently, many research attempts have focused on improving the quality of Ti implants, aiming for enhanced biocompatibility, osseointegration, and antimicrobial properties. Many studies have attempted to improve biocompatibility by surface modifying the 3D printed Ti implants. Some of the surface modifications on Ti scaffolds include the application of a homogeneous layer of microporous TiO2 and calcium-phosphate [96], genetically modified elastin-like recombinamers (ELRs) containing specific cell adhesive (RGD) and osteoinductive (SNA15) moieties [97], coating of aspirin (ASP)/PLGA [98], titania nanotubes via electrochemical anodization and bioactivation through HA coating [99], and chimeric peptides [100]. Studies on adding antimicrobial properties were carried out by surface coating of the Ti implants with gallium nitrate [101], vancomycin hydrochloride [102], flavonoid quercitrin [103], chitosan (CS)-modified MoS2 coating loaded with AgNPs [104], and calcium titanate [105], to prevent bacterial adhesion and proliferation on the surface. Today, biodegradable metallic implants such as magnesium alloys are gaining popularity as promising alternatives for metallic permanent prostheses. These biodegradable magnesium implants degrade gradually over time, matching the healing rate of surrounding bones and transferring the load back to the healing bone. Due to transparency towards X-rays, Mg-based implants do not interfere with radiographic techniques, allowing efficient monitoring of the implant. Further, Mg-based implants do not need to be surgically removed, preventing risks associated with additional surgical procedures [106, 107]. Despite these beneficial properties, Mg-based implants are also associated with unfavorable characteristics, such as granular tissue formation around implants and rapid degradation of the implant before the bone heals, preventing their wide applicability. Recently, numerous attempts have been made to avoid implant degradation by surface modifications [107].
Ceramics are also used for making medical implants and can be categorized into bioactive ceramics (bioglass, HA wollastonite, phosphates) or bioinert ceramics (alumina, zirconia and titania) and composite materials [108]. 3D printed bioceramic scaffolds such as akermanite (Ca2MgSi2O7, AKT), HA, β-TCP, and BGs have been employed in creating multifunctional implants for osteosarcoma treatments. These implants may function as bone substitutes, filling the space and facilitating attachment, proliferation, and differentiation of bone cells, promoting bone regeneration. These scaffolds can be further functionalized by adding anti-tumor functional agents, nanoparticles, and even engineered microbes to display additional functions [109–111]. Ceramic biomaterials such as CaP, halloysite, alumina and zirconia contain many applications in dentistry. HA is one of the optimum ceramic materials for dental implants due to its excellent biocompatibility [112]. However, due to high elasticity modulus, HA is brittle and often used as a coating associated with other materials [113]. Zirconia is another bioactive ceramic material commonly used for dental applications due to higher biocompatibility, suitable mechanical and tribological behavior, less dental plaque production, and resistance to staining [114]. Zirconia has been used extensively for the 3D printing of dental implants and prostheses, and a recent review by Branco et al. [115] summarized the recent advances of 3D printed zirconia-based dental materials.
Polymers are a diverse group of natural or synthetic materials with favorable mechanical and physicochemical properties for applications in the medical field. Easy processing, low cost of production, compatibility with multiple 3D printing techniques, and the possibility of modifications are some of the advantages associated with polymers. 3D printing techniques such as FDM, SLA, SLS, and DIW are commonly used 3D printing methods for polymers [111, 116]. Synthetic polymers used in medical applications can be categorized into biodegradable and non-biodegradable polymers. Among the non-biodegradable polymers such as polymethyl methacrylate (PMMA), polyether ether ketone (PEEK), and polyether ketone ketone (PEKK) have all been applied in the preparation of medical implants via AM [116–118]. PMMA is a commonly used polymer for orthopedic and bone grafting implants, with specific applications for fixing orthopedic prosthetics in the shoulders, knees, and hips. However, PMMA-based bone cement has many disadvantages. Its limited interactions with the bone and non-biodegradability have prevented it from extensive usage as an implant material [119]. In a recent study by Chen et al. [120], an embedded 3D printing methodology combined with a special post-curing technique showed the potential to enhance the future fabrication of patient-specific, complex, and functional PMMA-based implants. PEEK is another leading high-performance thermoplastic organic polymer commonly used to produce medical tools, implants and prostheses via AM [121]. PEEK possesses many favorable characteristics, such as good mechanical properties, temperature stability, high wear resistance, low coefficient of friction, high processing capability and excellent biocompatibility making them suitable for a plethora of medical applications [121, 122]. One of the main advantages lies in its modulus of elasticity being similar to that of human bone, making it a suitable candidate for cranial, orthopedic, trauma and spinal implants via AM-based techniques [123]. However, the bioinertness of PEEK hinders the bone attachment to the implant surface, resulting in poor osseointegration. Recently, there have been many attempts to improve the bioactivity of PEEK by incorporating HA and using other binder agents, such as PLGA, to load the PEEK surface with other beneficial compounds to provide favorable features [123]. Among biodegradable synthetic polymers, polyglycolide acid (PGA), PLA, PLGA, and PCL have been used in the manufacturing of medical implants [119]. Both PGA and PLA are commonly used for biodegradable screws, nails, and plates to fix orthopedics. However, the wide application of these biodegradable polymers is limited due to the rapid degradation properties of PGA and intrinsic brittleness, poor toughness, and a slow degradation rate of PLA [119, 124]. de Oliveira and co-workers [125] successfully 3D printed a PLA-based interference screw via the FDM technique. This device has shown an excellent tendon-to-bone fixation comparable to its Ti counterpart, with promising results.
4D printing technology has contributed remarkably to fabricating smart implants and prosthetics. Its ability to respond to specific stimuli, such as temperature, moisture, light, and magnetic fields, has yielded customizable and adaptable implants and prosthetics. Khorsandi et al. [126] covered recent contributions of 4D printing towards dentistry and maxillofacial surgery in fabricating relevant implants. With the ability to perfectly fit into the oral structure of the patient and also to adapt changes in the jawbone over time, these implants offer several advantages, such as optimal functionality, reduced discomfort, and improved patient satisfaction. 4D printing contributions towards orthopedics surgery should also be acknowledged. Customizable patient-specific implants fabricated via 4D printing ensure a precise fit and diminish complications. Zamborsky et al. [127] illustrated the applicability of 4D printing in manufacturing blood vessels, tissues, intelligent bandages, and efficient wound healing via 4D printed latticework. With the ability of these 4D implants to be adjusted to the body changes of patients with time and advancements in artificial intelligence (AI) technologies such as robotics, satisfied recovery and repair, a key goal of precise orthopedics could be achieved [128]. Lin et al. [129] demonstrated successful FDM-based 4D printing of biomimetic intestinal stents using shape memory biocomposites. The design was based on wavy biomimetic networks mimicking the nonlinear stress-strain nature of biological tissues. High flexibility, facilitation of reduced irritation of the intestinal wall, biodegradability, and near-body-temperature (NBT) triggered nature of these 4D printed stents are considered next-generation intelligent implants.
Zhou et al. [130] introduced 4D printed shape-memory vascular stents of βCD-g-PCL, altering their shapes in response to fluctuations in blood flow or vessel diameter. These properties not only supported affected blood vessels but also minimized complications and additional surgical interventions for the stent, enhancing the efficiency of the treatment. Previous literature also showed the successful fabrication of 4D printed spinal implants that could gradually alter the shape supporting the spine as it heals. This could potentially minimize complications and support efficient recoveries [131, 132].
Some of the recent 3D and 4D printed BMMs tested for implants and prosthetics, reported in the literature are showed in Figure 5. The ones illustrated represent a variety of BMMs including Ti-6Al-4V-based porous channel dental implants 3D printed via DMLS (Figure 5A) [133], cross-linked PLA-based thermomagnetic responsive vascular stent 4D printed via DIW (Figure 5B) [134], PCL/acrylates-based thermo responsive vascular conduit 4D printed via DIW (Figure 5C) [135], FDM-3D printed acrylonitrile butadiene styrene (ABS)-based human skull and PEEK based porous implant applied on the skull (Figure 5D) [136], and FDM-3D printed PLA/antibiotic based interference fixation screws (Figure 5E) [137].
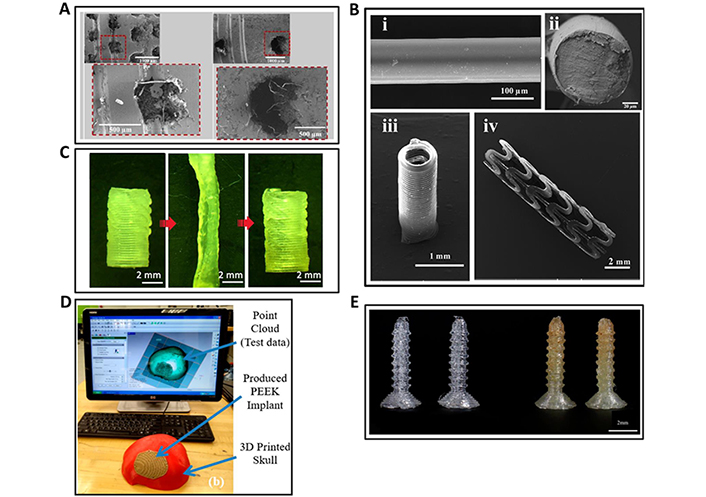
3D and 4D printed BMMs for implants and prosthetics. (A) Scanning electron microscopic (SEM) images of DMLS-based 3D printed Ti-6Al-4V porous channel dental implants; (B) SEM images of DIW-based 4D printed circular stent using crosslinked PLA; (C) DIW-based 4D printed smart vascular conduit changing its shape by thermal stimulation, initial shape (left), temporary shape (middle), recovered initial shape (right); (D) FDM-based 3D printed human skull (red portion) and the porous implant (brown portion surrounded by red skull) using ABS and PEEK, respectively; (E) FDM-based 3D printed interference fixation screws using PLA (left) and PLA-gentamicin (GS) antibiotic (right)
Note. (A) Adapted with permission from “3D printing of Ti-6Al-4V-based porous-channel dental implants: computational, biomechanical, and cytocompatibility analyses,” by Chakraborty A, Das A, Datta P, Majumder S, Barui A, Roychowdhury A. ACS Appl Bio Mater. 2023;6:4178–89 (https://pubs.acs.org/doi/10.1021/acsabm.3c00403). © 2023 American Chemical Society; (B) adapted with permission from “Direct-write fabrication of 4D active shape-changing structures based on a shape memory polymer and its nanocomposite,” by Wei H, Zhang Q, Yao Y, Liu L, Liu Y, Leng J. ACS Appl Mater Interfaces. 2017;9:876–83 (https://pubs.acs.org/doi/10.1021/acsami.6b12824). © 2016 American Chemical Society; (C) adapted with permission from “3D printing of highly stretchable, shape-memory, and self-healing elastomer toward novel 4D printing,” by Kuang X, Chen K, Dunn CK, Wu J, Li VCF, Qi HJ. ACS Appl Mater Interfaces. 2018;10:7381–8 (https://pubs.acs.org/doi/10.1021/acsami.7b18265) © 2018 American Chemical Society; (D) adapted from “Polyether-ether-ketone (PEEK) and its 3D-printed quantitate assessment in cranial reconstruction,” by Moiduddin K, Mian SH, Elseufy SM, Alkhalefah H, Ramalingam S, Sayeed A. J Funct Biomater. 2023;14:429 (https://www.mdpi.com/2079-4983/14/8/429). CC BY; (E) adapted from “3D printing custom bioactive and absorbable surgical screws, pins, and bone plates for localized drug delivery,” by Tappa K, Jammalamadaka U, Weisman JA, Ballard DH, Wolford DD, Pascual-Garrido C, et al. J Funct Biomater. 2019;10:17 (https://www.mdpi.com/2079-4983/10/2/17) CC BY.
The above discussed most recent 3D and 4D printing techniques, materials, and printed BMMs for applications in tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics are summarized in Tables 1 and 2.
Recent developments in 3D printing of BMMs for biomedical applications
| 3D printing technique | Materials and printed BMMs | Reference(s) |
| Tissue engineering | ||
| FDM | PLA–scaffolds | [19] |
| DIW | Alg/Gel–scaffolds | [22] |
| Alg/SF protein–scaffolds | [23] | |
| Extrusion | Alg/NC, hyaluronic acid–scaffolds | [24] |
| FDM | PCL–scaffolds | [32] |
| BJ | 45S5 BG/TCP–scaffolds | [34] |
| SLS | 45S5 BG–scaffolds | [35] |
| DIW | 45S5 BG–scaffolds | [36] |
| BGs/PVA–scaffolds | [37] | |
| Cu-doped BG-based composite scaffolds | [38] | |
| FDM | BG/PCL–scaffolds | [39] |
| BG/PLA–scaffolds | [40] | |
| 45S5 BG/PHBV–resorbable scaffolds | [41] | |
| Ag-doped BG-based ceramic scaffolds | [42] | |
| DIW | SF/Gel/BG–bone constructs | [43] |
| FDM | PCL/nano-HA–composite scaffolds | [138] |
| Drug delivery | ||
| BJ | CaP/silica-based nanocomposite implant | [139] |
| CaP/TCP/HA/dextrin-based scaffolds | [140] | |
| PBF | CaP/PHBV-based nanocomposite scaffolds | [141] |
| PAM | Levetiracetam/PVP-vinyl acetate copolymer (PVAc)–tablets | [142] |
| Ethylcellulose/HPMC/PVP/cellulose acetate–controlled release shell | [64] | |
| FDM | Haloperidol/Kollidon® VA64/Kollicoat® IR/Affinsiol™ 15 cP/HPMC acetate succinate (HPMCAS)–tablet | [143] |
| Clotrimazole/TPUs–intravaginal ring | [144] | |
| Isoniazid (INZ)/rifampicin (RFC)/hydroxypropyl cellulose (HPC)/hypromellose acetate succinate (HPMC-AS)–bilayer tablet | [145] | |
| Semi-solid extrusion | Levetiracetam/HPC–tablets | [146] |
| Tacrolimus/Gelucire 44/14/Gelucire 48/16–suppositories | [147] | |
| Lamotrigine/Gel/HPMC–drug formulations | [148] | |
| SLS | Lopinavir/Kollicoat®/Candurin® NXT Ruby Red–printlets | [149] |
| Clindamycin palmitate/microcrystalline cellulose (MCC)/lactose monohydrate (LMH)–tablets | [150] | |
| SLA | Lidocaine hydrochloride/Elastic Resin–bladder devices | [151] |
| Hydrochlorothiazide/amlodipine/atenolol/irbesartan with PEGDA/diphenyl(2, 4, 6-trimethyl-benzoyl) phosphine oxide (TPO)–antihypertensive polyprintlet | [152] | |
| Direct powder extrusion (DPE) | Tramadol/HPC/polyethylene oxide (PEO)–opioid medicines | [153] |
| Itraconazole/HPC–UL, SSL, SL, and L (different HPC grades/compositions)–drug products | [154] | |
| FDM | PLA/PVA-based mouthguard | [62] |
| Surgical and diagnostic tools | ||
| SLS | Virgin and recycled Dura-Form EX plastic powder–surgical tool kit | [67] |
| FDM | PLA-based Army-Navy surgical retractor | [68] |
| PLA-based microchannel system | [75] | |
| Graphene/PLA–ring- and disc-shaped electrodes | [76] | |
| Graphene-based enzymatic biosensor | [77] | |
| Graphene/PLA–electrode | [79] | |
| DIW-AJP | Ag–microelectrode arrays | [82] |
| SLA | PEGDA–biomicrofluidic devices | [83] |
| SLS | Cu nanoparticles/polyethylene-naphthalate (PEN)–flexible touch panel | [86] |
| FDM | PLA/PAM/HPMC–hydrogel wound dressings | [69] |
| FDM | Thermoplastic–frame for magnetic focus lateral flow sensor detecting cervical cancer biomarkers | [78] |
| Implants and prosthetics | ||
| DED | Si3N4/Al2O3/HA/Ti6Al4V–composites | [92] |
| DIW | Ti/Pluronic F-127–scaffolds | [97] |
| SLM | Ti-6Al-4V-based implants | [98] |
| SLA | Composites-based dental crowns | [155] |
| SLA | Antimicrobial HA-based dental bite | [156] |
| DLP | Polymer-based dental crowns and bridges | [157] |
| DLP | Zirconia-based dental ceramic prostheses | [158] |
| MJ | Biocompatible photopolymer-based interim dental crowns | [159] |
| FDM | PEKK-based bone analogs | [117] |
| FDM | PMMA/PEEK–cranial implant | [118] |
| PMMA–medical implants | [120] | |
| PLA–interference screw | [125] | |
| DMLS | Ti-6Al-4V–porous channel dental implant | [133] |
| FDM | ABS–human skull; PEEK–porous implant | [136] |
| FDM | PLA/GS–interference fixation screws | [137] |
Recent developments in 4D printing of BMMs for biomedical applications
| 4D printing technique | Stimuli | Materials and printed BMMs | Reference(s) |
|---|---|---|---|
| Tissue engineering | |||
| DLP | Hydration | GelMA/PEGDM–tissue scaffold | [46] |
| Extrusion | Solvent | Hyaluronan/Alg–bilayered scaffold | [47] |
| DIW | Shear strain | Oxidized and methacrylated Alg (OMA)/GelMA–cell condensate-laden bilayer system | [48] |
| DIW | Solvent | ADA-Gel-based T-shaped vascular bifurcation | [49] |
| DIW | Solvent | Methacrylated Alg (AA-MA) and methacrylated hyaluronic acid–vascular tissue | [160] |
| Solvent, near-infrared (NIR) light, and temperature | Alg/polydopamine–tissue scaffolds | [161] | |
| Inkjet | Solvent | GelMA/Gel-carboxylated-methacrylate bilayer | [162] |
| SLA | Temperature | Soybean oil epoxidized acrylate (SOEA)–cardiac tissue | [163] |
| Poly(methyl methacrylate)–neural tissue | [164] | ||
| FDM | Magnetism | PCL/iron doped HA–bone tissue | [165, 166] |
| Temperature | PLA/PCL/SOEA–muscle tissue | [167] | |
| Solvent | AA-MA/PCL–muscle tissue | [168] | |
| Temperature | SOEA–muscle tissue | [169] | |
| DIW and inkjet printing | Magnetism | Agarose/collagen type I-based cartilage tissue | [170] |
| Extrusion-based printing | Temperature | Polyurethane (PU) Commercial polymers–tissue scaffolds | [171] |
| DLP | Solvent | PEG(700)DA–tissue scaffolds | [172] |
| DIW | pH | PEG-based microgel scaffolds | [173] |
| DLP | Temperature | PCL diacrylate (PCLDA)-based bilayer membrane | [174] |
| Drug delivery | |||
| Extrusion | Humidity and temperature | PU and polyethylene–dual stimuli self-morphing structures | [175] |
| Alg-Ca2+ coordination | Pluronic F127 diacrylate macromer (F127DA)/Alg–shape memory hydrogels | [176] | |
| DLP | Magnetoelectricity | 4-hydroxybutyl acrylate (4-HBA)/urethane-polyethylene glycol-polypropylene glycol (PU-EO-PO) monomer/electromagnetized carbon porous nanocookies–conduit material | [63] |
| FDM | Temperature/fluid | PVA-based expandable drug delivery structures | [177] |
| Water | PVA and glycerol-based intravesical drug delivery device | [178] | |
| DIW | Temperature/pH/enzyme | Pickering emulsion gels BSA methacryloyl (MA)/poly(N-isopropylacrylamide)-P(NIPAAm) (thermo-sensitive ink) BSA-MA/poly[2-dimethylaminoethyl methacrylate]-P(DMAEMA) (pH-sensitive ink) BSA-MA + F127 (enzyme sensitive ink)–hydrogels | [179] |
| DLP-PμSL | Solvent/light | PEGDA–microneedle array | [73] |
| DIW | pH | Alg fibres-based porous scaffolds | [180] |
| FDM | pH | PVP/methacrylic acid co-polymer-based tablets | [181] |
| HPMC-AS-based tablets | [182] | ||
| Surgical and diagnostic tools | |||
| PμSL | Temperature | SMPs-based surgical gripper system | [71] |
| Jetting | Temperature | SMPs-based actuator system/self-expanded stent | [72] |
| FDM | Load | PU/fabric–wearable smart sensor | [87] |
| FDM | Motion | TPU–deformable lung | [89] |
| Implants and prosthetics | |||
| FDM | Temperature | Poly(ethylene glycol)/shape memory PLA (SMPLA)–biomimetic intestinal stents | [129] |
| DIW | Temperature | βCD-g-PCL–vascular stent | [130] |
| DIW | Temperature | PCL/acrylates-based vascular conduit | [135] |
| FDM | Temperature | Thermoplastic copolyester elastomer–vascular stent | [183] |
| PLA-based vascular stent | [184, 185] | ||
| Thermo-magnetism | PLA-based magnetic nanocomposites–vascular occluder | [186] | |
| DIW | Thermo-magnetism | Fe3O4/PLA/dichloromethane/benzophenone–vascular stent | [134] |
| SLA | Internal stress | GelMA/PEGDA–cardiac patch | [187] |
| SOEA/graphene–neural conduit | [188] | ||
| SLA | Temperature | PCL/isocyanato ethyl methacrylate–tracheal stent | [189] |
| DLP and DIW | NIR light and temperature | Bisphenol A diglycidyl ether, poly(propylene glycol) bis(2-aminopropyl) ether, and decylamine–cardiac patch | [190] |
| DIW | Fe3+ ions, sodium lactate/UV | Acrylamide-acrylic acid/cellulose nanocrystal–bilayer hydrogel stent | [191] |
| FDM | Magnetism | Fe2O3/shape memory PLA–occluders | [192] |
3D printing of biomaterials has revolutionized the biomedical sector with the ability to print precise, highly reproducible, and customized medical materials for numerous clinical applications. Despite modern advances, the limited availability of suitable 3D printable material and the need for a universal processing technique hinder the application of 3D printing in the medical sector. The applicability of some 3D bioprinted materials, such as medical implants, is often challenged due to low mechanical strength, biocompatibility, wear resistance, and sustainability [193]. A static 3D printed material becomes incompatible with more dynamic biological systems [194]. The unresponsive, static nature of 3D printed materials has motivated researchers to explore smart materials. Hence, the idea of 4D printing was conceptualized. Although 4D printing is a promising technique, it is still in the infancy level, with many challenges and opportunities for development.
One of the major challenges of 4D printing includes the limitation of suitable stimuli-responsive materials. Moreover, many 3D printable materials also show poor stimuli responsiveness, making them undesirable for 4D printing. The materials used for the 4D printing of BMMs should also be biocompatible and biodegradable with acceptable mechanical properties. Although biomaterials, including metals, polymers, and ceramics, can be used as smart materials, only smart polymers are currently successful in 4D printing [194].
Currently, there are limitations in providing contactless stimulations to 4D printed materials in vivo, and many available contactless stimuli are incompatible in the cellular environment. Thermal stimulus is commonly used for achieving shape-changing properties. The lack of understanding of unconventional novel stimuli and the unknown behavior of the printed material upon repeated exposure to a stimulus hinder the exact prediction of responses. Further, the unpredictable nature and complexity of the stimuli present in biological systems challenge the optimum performance of the printed materials in vivo. The lack of 4D printable materials capable of reversible transformation is another issue that requires future studies. Overall, 3D and 4D printing still produce simple designs; hence, developing, standardizing, and regulating highly complex structures could take time, skill, and effort.
Novel five-dimensional (5D) and six-dimensional (6D) printing technologies have recently been developed using the knowledge of 3D and 4D printing. 5D printing targets multidimensional objects printed using five axes. Those five axes are x, y, z, and two additional rotational axes that represent the movement of the printing head and the print bed at a specified angle. The main objective of 5D printing is to manufacture products with enhanced mechanical properties using less material. 5D printing has excellent potential in producing curved, complex structures of medical devices, including artificial bones and complex implants with curved surfaces and improved mechanical properties [193, 195].
6D printing was first introduced in 2021, incorporating 4D and 5D printing techniques [196]. Similar to 4D printing, 6D printed materials can change shape, properties, or functions in the presence of an environmental stimulus. Moreover, the production process in 6D printing is identical to 5D printing. Interestingly, 6D printed objects are more complex and flexible, with superior mechanical properties and sensitivity [193, 196]. Future research should be based on the development of novel materials for 4D and 6D printing, where a desirable change is achieved as a response to the stimulus.
All 3D, 4D, 5D, and 6D AM techniques show enormous potential for biomedical applications in the future. Smart-printed materials with tailored structures and improved mechanical properties can be achieved when progressing from 3D to 6D printing. However, the cost of the printing device also increases with higher-order printing setups [193]. Therefore, the user needs to make educated decisions related to the selection of printing technique for each scenario.
Safety, biocompatibility, precision, and functional effectiveness are some of the main parameters to consider when AM materials are employed in biomedical applications. When customizing 3D and 4D printed materials for clinical usage, a multidisciplinary expert panel should conduct a comprehensive analysis covering all aspects. Effective preoperative planning directly influences the outcome of the surgical applications of 3D printed implants. Therefore, combining modern imaging and simulation techniques is imperative for conducting successful clinical procedures using AM materials. In future AM, emphasis should also be given to contactless manipulation stimuli that can cause changes in the materials without physical contact [193].
Fabricating 4D printed multi-stimuli responsive materials could possibly improve the performances of biomedical applications. For example, these materials would adjust their function depending on the body temperature, pH, and other biological factors, yielding efficient treatments and also could reduce the high cost of 5D and 6D printing [179]. Biomimetic 3D printed materials display better compatibility, hence mimicking the structural and functional performance of natural body parts [197]. Incorporating self-healing properties into 4D printed materials would repair themselves in response to damages, benefitting implants or prosthetics without needing replacements or complex surgeries [198]. Moreover, integrating sensors and electronic components within 4D printed devices would bring several advantages, such as real-time monitoring of the device performance and gathering and storing valuable health-related information.
Further, research should be conducted to enhance the biocompatibility of 3D printed materials by incorporating biocompatible materials and surface coatings [199]. Developing biohybrid systems by combining living cells with 4D printed structures endows biomimetic features and functionalities by supporting cell proliferation and enhancing cell functions in regenerative medicine.
Although 3D printing emerged as a novel technique more than 30 years ago, its clinical application became popularized within the last 10 years. Therefore, technical, regulatory, quality control, and licensing guidelines still need to be fully developed. Due to the current popularity of 3D printing in clinical applications globally, it is urgently required to introduce standards and include them in the quality control frameworks [95]. Many organizations, including the International Organization for Standardization (ISO), the International Medical Device Regulatory Forum (IMDRF), and the International Electrotechnical Commission (IEC), are currently attempting to establish global standards for products and procedures associated with AM [93].
The recently introduced cutting-edge technology is 3D and 4D printing of energy storage batteries [200–203]. For example, recent research efforts have succeeded in the 4D printing of polydimethylsiloxane (PDMS)-based batteries controlled by external magnetic fields [203]. Interestingly, tunable mechanical properties could also be achieved by incorporating different filler combinations such as carbon particles, ceramic, and metal. These additively manufactured programmable PDMS-based composites would pave the way for future implant batteries and ceramic-based medical implants [203].
3D and 4D printing technologies have opened up a new era in manufacturing smart constructs and devices for the biomedical field. This review has summarized the recent advances in 3D and 4D printing technologies to fabricate BMMs for tissue engineering, drug delivery, surgical and diagnostic tools, and implants and prosthetics applications. The paper has also compared major similarities and differences between 3D and 4D printing and their challenges in this domain. The review has also explored several exciting 3D and 4D printing prospects in developing advanced smart materials, biohybrids, 5D and 6D printing technologies, and bioelectronic devices. Finally, 3D and 4D printed BMMs exhibit immense potential in future biomedical and bioengineering applications, ultimately empowering medical diagnosis and treatments and invigorating more efficient and sustainable human healthcare.
3D: three-dimensional
4D: four-dimensional
5D: five-dimensional
6D: six-dimensional
ABS: acrylonitrile butadiene styrene
ADA: alginate dialdehyde
AgNP-Pam: silver nanoparticle-polyacrylamide
AJP: aerosol jet printing
Alg: alginate
AM: additive manufacturing
BG: bioactive glass
BJ: binder jetting
BMMs: biomedical materials
BMP: bone morphogenetic protein
BSA: bovine serum albumin
CaP: calcium phosphate
DED: directed energy deposition
DIW: direct ink write
DLP: digital light processing
DMLS: direct-metal laser sintering
FDM: fused deposition modeling
Gel: gelatin
GelMA: gelatin methacrylate
HA: hydroxyapatite
HPMC: hydroxypropyl methylcellulose
MJ: material jetting
NC: nanofibrillated cellulose
PAM: pressure-assisted microsyringes
PBF: powder bed fusion
PCL: polycaprolactone
PEEK: polyether ether ketone
PEGDA: poly(ethylene glycol) diacrylate
PEGDM: poly(ethylene glycol) dimethacrylate
PGA: polyglycolide acid
PHBV: poly(hydroxybutyrate-co-hydroxyvalerate)
PLA: polylactic acid
PLGA: polylactide glycolic acid
PMMA: polymethyl methacrylate
PVA: polyvinyl alcohol
PVP: polyvinyl pyrrolidone
PμSL: projection microstereolithography
SF: silk fibroin
SLA: stereolithography
SLM: selective laser melting
SLS: selective laser sintering
SMPs: shape-memory polymers
TCP: tricalcium phosphate
TPU: thermoplastic polyurethane
UV: ultraviolet
VP: vat photopolymerization
The authors acknowledge the financial support provided for this work by the Research Council, University of Sri Jayewardenepura, Nugegoda, Sri Lanka.
GAA: Conceptualization, Writing—original draft, Writing—review & editing. SSA and RSD: Writing—original draft, Writing—review & editing. AW: Writing—review & editing.
The authors declare no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work received support from the University Research Grant [ASP/01/RE/TEC/2022/74] provided by the Research Council, University of Sri Jayewardenepura, Nugegoda, Sri Lanka. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Pankaj Sharma, Vinay Jain
Anushikha Ghosh ... Abhik Mallick
Dasharath Ramavath ... Sudheer Reddy Devana
Kalyani Pathak ... Barbie Borthakur
