Affiliation:
1Department of Biomedical, Surgical and Dental Science, University of Milan, 20122 Milan, Italy
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0002-4151-8063
Affiliation:
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, 70124 Bari, Italy
†These authors share the first authorship.
Email: giannadipalma@tiscali.it
ORCID: https://orcid.org/0000-0002-5947-8987
Affiliation:
3College of Medicine and Dentistry, Ulster University, B4 6BN Birmingham, UK
ORCID: https://orcid.org/0000-0002-3288-490X
Affiliation:
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, 70124 Bari, Italy
ORCID: https://orcid.org/0000-0002-6366-1039
Affiliation:
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, 70124 Bari, Italy
ORCID: https://orcid.org/0000-0001-9568-9346
Affiliation:
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, 70124 Bari, Italy
ORCID: https://orcid.org/0000-0003-0104-6337
Affiliation:
2Department of Interdisciplinary Medicine, University of Bari Aldo Moro, 70124 Bari, Italy
Email: francesco.inchingolo@uniba.it
ORCID: https://orcid.org/0000-0003-3797-5883
Explor Med. 2024;5:1–16 DOI: https://doi.org/10.37349/emed.2024.00202
Received: October 23, 2023 Accepted: November 22, 2023 Published: February 06, 2024
Academic Editor: Lindsay A. Farrer, Boston University School of Medicine, USA
The article belongs to the special issue Biomaterials and Biomarkers in Dentistry: Up to Date
Aim: In regenerative dentistry, the success is influenced by the graft material, which should act as an osteoconductive scaffold. It provides a mineral substrate during resorption and induces the activity of osteoinductive cells capable of producing new bone, platelet growth factors, and cell differentiation factors that guide the differentiation of undifferentiated mesenchymal cells. Given that dentin shares many biochemical characteristics with bone tissue, it has recently attracted considerable interest as a biomaterial for bone repair. The aim of this study is to compare two grinder types to determine the optimal method for producing dentinal particles using a mechanical grinder.
Methods: A sample of 40 natural human teeth without restorations, prostheses, or root canal treatments was used and divided into two groups subjected to two different grinder speeds (high-speed and low-speed).
Results: The high-speed showed a greater dispersion (53.5% ± 9.89% of the tooth) due to the pulverisation (highly thin granules) of part of the tooth. The low-speed grinder did not pulverize the dentin and the percentage of tooth loss is 9.16% ± 2.34%.
Conclusions: The low-speed grinder allows to save a major part of the tooth and has a maximum quantity of graft material but requires more time. Further studies must be promoted to optimise the grinding procedures.
In recent decades, the field of regenerative medicine has experienced significant growth and advancement, with a notable contribution from biotechnology. Within this context, researchers have shown a keen interest in the regeneration of bone tissue, a topic particularly relevant in dentistry [1–6]. Studies have emphasized the importance of considering the size of bone defects when aiming for effective regeneration. Various types of bone defects have emerged, presenting significant challenges in clinical practice and highlighting the pressing need for materials that can facilitate bone repair [3, 7, 8].
The development of bone regeneration techniques involved both autologous grafts and xenografts, with the aim of increasing the bone tissue for implant purposes [9]. In the context of bone grafting, it is crucial for the graft material to be decontaminated and to serve as an osteoconductive scaffold. This scaffold should provide a mineral substrate during resorption, stimulate the activity of osteoinductive cells capable of generating new bone, and release growth factors such as bone morphogenetic proteins (BMPs). BMPs play a pivotal role in transforming undifferentiated mesenchymal cells into osteogenic cells (osteoblasts) [10–14].
Recently, dentin has garnered substantial attention as a potential biomaterial for bone repair. Its biochemical composition has been likened to that of bone tissue, with both consisting of approximately 61% inorganic material (hydroxyapatite crystals) and 39% biological material. The organic component, primarily composed of collagen, imparts strength and flexibility to the structure, enhancing its resistance to fractures [15]. Non-collagen proteins, representing around 10% of the total composition, include various proteins such as osteopontin (OPN), dentin sialoprotein (DSP), dentin glycoprotein (DGP), bone sialoprotein, osteocalcin, and more. Notably, these proteins are shared between dentin and bone [16–21].
The concept of tooth grafting was introduced over 50 years ago by Yeomans and Urist [22, 23], who discovered the osteoinductive potential of demineralized dentin matrix (DDM). Schmidt-Schultz et al. [24] conducted pioneering research on the use of teeth as a biomaterial for grafting, isolating growth factors like insulin-like growth factor-II (IGF-II), BMP-2, and transforming growth factor (TGF) from teeth dating back thousands of years [25, 26]. More recently, Bessho et al. [27] demonstrated the presence of BMPs in the human dentin matrix, with thirteen different BMPs identified [28]. BMP-2, in particular, plays a pivotal role in promoting mesenchymal cell differentiation into osteoblasts [29, 30]. BMP-3 and BMP-7 also contribute to bone growth and osteoblast differentiation [31].
The process by which demineralized dentin stimulates bone regeneration closely mimics the mechanism of autologous bone factors [32–34]. This similarity is observed in both demineralized bone matrix (DBM) and DDM, both of which contain type I collagen and growth factors, with a particular emphasis on BMP-2 [35–37].
Key growth factors for bone regeneration are found in both bone and dentin matrices and serve as a rich source of BMPs and bioactive growth factors like TGF-β, which play vital roles in the bone repair process [38–40]. In dentin, according to Bono et al. tests [41], there are 200 pg/g of BMP-2 after treatment with acids. In bone, according to tests conducted by Wildemann et al. [42] using different extraction methods, the range is between 400 and 3,778 µg/g of BMP-2. The extraction method determines the quantity of BMP-2 present, and in the literature, tests have been carried out using non-comparable systems. However, the crucial aspect is the presence of BMP-2 and its usability. The demineralization process, which removes mineral content, has been shown to provide superior bone augmentation compared to non-demineralized dentin [43, 44]. Furthermore, the chemical composition of bone and dentin is nearly identical, featuring an inorganic component of hydroxyapatite and an organic component primarily composed of type I collagen and other secondary proteins [45]. For over 35 years, heterologous or alloplastic grafting materials have been used in bone augmentation treatments, serving primarily as mechanical scaffolds for host cells without offering osteoinductive stimuli [12, 46–49]. Recent studies have confirmed the effectiveness and safety of on-site preparation of autogenous partially DDM for clinical use in implant dentistry procedures such as socket preservation, alveolar ridge augmentation, and maxillary sinus floor augmentation [4, 39, 50, 51].
Various methods of tooth demineralization have been investigated, each yielding varying outcomes in bone tissue development. When comparing different tooth crushing systems, factors such as the degree of sterilization, system repeatability, liquid types and concentrations, degree of demineralization, granule size, residual protein content, wettability, plasticity of granules, and system ergonomics must all be taken into account [44, 52].
A recent prospective study has validated the use of teeth as grafting materials in socket preservation procedures [53]. Bianchi et al. [54] emphasized the importance of understanding how human cells react to different dentinal derivative grafting materials in various clinical scenarios. Their study evaluated human periodontal ligament fibroblasts (hPLF) exposed to different dentinal derivative particles, including mineralized dentine, deproteinized and demineralized dentine, and demineralized dentine, along with deproteinized bovine bone as a control material [55, 56]. Observations revealed the expression of proliferative markers and cytoskeletal elements involved in the adhesion process [57]. Notably, signals for vinculin and integrin were particularly strong in the sample exposed to the demineralized dentine material, confirming the biocompatibility and high conductivity and inductivity properties of dentinal derivatives, which are crucial for the regenerative process [54, 58].
Clinical and histological assessments have shown the absence of inflammation or adverse reactions following the use of teeth treated as grafting materials. Clinical tests have demonstrated seamless dentin integration during the regeneration process [59]. However, further research is needed to delve into the intricacies of the regeneration stage. Histomorphometric analysis of tooth derivative materials used as bone substitutes in socket preservation procedures indicated an average of 38% new bone formation and only 7% graft residue after just 5 months [60–63].
Grinding serves as the initial step in preparing teeth for use as graft materials, and it is imperative to develop a protocol that enables the consistent production of particles while preserving osteoinductive capabilities [64]. In the surgical application of teeth, meticulous care must be exercised to prevent the dispersion of this irreplaceable sample (the extracted tooth) during grinding processes [65, 66]. Disadvantages of improper grinding techniques include the creation of non-uniformly sized particles, often resulting in material loss, xenograft material, even though it has irregular particles, still falls within a range of sizes. In this case, within the same range, there remains little material. Hence, achieving uniform particle size, as close as possible to optimal weights, is essential. There are two primary types of systems for pulverizing solid materials: high-speed mills and low-speed grinders [26, 67].
To the best of our knowledge, this study represents the first in the literature to comprehensively analyze the two grinder types and establish a procedure for producing dentinal particulates through mechanical grinding.
The aim of this study is to evaluate which grinding system is more effective in terms of reduced substance loss and homogeneity (similar particle sizes) of the produced particulate matter.
In the effort of trying to understand which was the system that guaranteed the best performance, two different grinding systems (Figure 1) were evaluated, one at high-speed (testing two different grinders), similar to a coffee grinder, and one at low-speed, with concentric conical blades comparing the amount of material produced. The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethical Committee for Biomedical Research of Chieti and Pescara (Protocol Number 1869/21.03.2019).
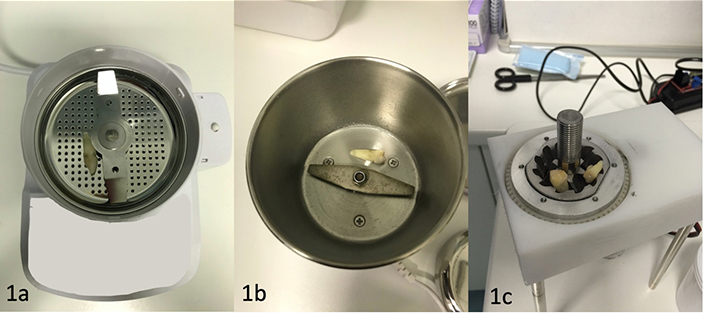
The high-speed blades are composed of a rotating hammer moving at high-speed along its axis, all contained within a metal cylinder with a lid that contains the fragments propelled into the surrounding space by the impacts with the high-speed hammer. The low-speed blades consist of two concentric conical blades that drag the material to be fragmented downwards. The granule sizes are determined by the distance between the two blades, and the resulting granules fall downward due to gravity. (a) and (b) two high-speed grinders; (c) the low high-speed grinder
A literature review was conducted using the following keywords: “high-speed grinder” or “low-speed grinder” and “tooth.” The results from the last 10 years are as follows:
PubMed: 0 results, Web of Science (WOS): 50 results, Scopus: 14 results. All the articles found are not relevant to dentistry.
The reasons for this could be attributed to the development of a new research field in regenerative dentistry that has gained momentum in recent years, focusing on the use of teeth as graft materials.
The study was conducted by dividing the subjects into two groups. Group 1 used the high-speed grinder, while group 2 used the low-speed grinder. Each group was provided with 20 natural teeth (without restorations, prosthetics, or root canal treatments) to be reduced into particulate matter.
The difference between high-speed and low-speed is in the revolutions per minute (RPM). Basically, it is how many times in a minute the burrs rotate. The speed is the measurement of one complete revolution of the blades. Most high-speed grinders have a hammermill that spins anywhere between 1,100 RPM to 1,200 RPM. These machines are typically assisted with some sort of feeding system. On the other hand, low-speed shredders spin at 30 RPM to 40 RPM and are often built without an assisted feeding mechanism. Concentric blades do not require a sieve due to their design. Low-speed concentric blades fragment the tooth, pushing the residues downward. The granule sizes are determined by the distance between the blades.
Low-speed: These are at a lower RPM for two reasons. They usually have smaller burrs, and most are conical shaped. The lower RPM grinders usually have less heat buildup. Low-speed shredder blades are conical concentrics with sharp edges. They have an accumulation zone of the material to be ground called the upper chamber and a lower one. The distance between the blades in the lower chamber determines the size of the granulate.
High-speed: These usually have flat burr sets, and beefier motors. The flat burrs provide a more consistent grind and the higher RPM grind the teeth faster. High-speed grinders are direct drive, which means fewer gears to wear or break, and are quieter. The blades of the high-speed shredder are shaped like a specular hammer, rotating on a central pin placed inside a sealed drum. The tooth is placed inside the drum and only after rapid rotation of the hammer, the granulate is produced that then must be filtered to the right size of the granules [68, 69].
A tooth was triturated, and then analyzed under a microscope (ESEM Zeiss EVO50, Carl Zeiss, Milan, Italy) linked to a secondary electron detector for energy dispersive X-ray spectroscopy (EDS) analysis was used to analyze the surface morphology of sample particles to reveal the shape of the produced granules (Figure 2), filtered through calibrated sieves (Figure 3). A sieve analysis (or gradation test) is a practice or procedure used to assess the particle size distribution (also known as gradation) of a granular material by passing it through a series of sieves with progressively smaller mesh sizes and weighing the amount of material caught by each sieve as a fraction of the total mass [70, 71].
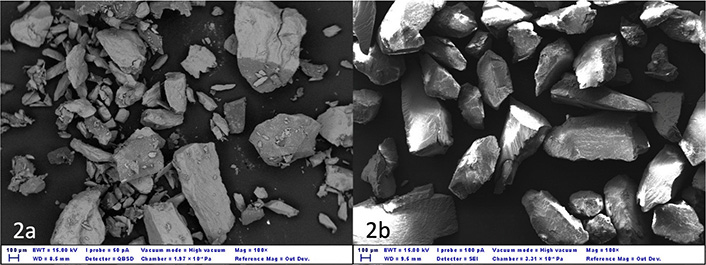
Morphology and particle size of teeth subjected to different grinding treatments. (a) Granules are produced with the high-speed grinder. Different sized granules can be seen, many of which are very small; (b) granules are produced with a low-speed grinder, similar in size and similar in shape. EWT: energy-weighted transmission; WD: working distance; I Probe: current probe; QBSD: quantitative backscatter detector; SEI: secondary electron imaging; Mag: magnification; Out Dev.: output device
The procedure to sieve is: the vertical throwing action is combined with a small circular motion, resulting in sample dispersion throughout the whole sieve surface. The particles are vertically propelled (thrown upwards). When they fall back, they do free rotations in the air and interact with the holes in the sieve mesh. Particles that are smaller than the openings pass past the sieve. They are tossed if they are bigger. The spinning motion while hung increases the likelihood that the particles will have a different orientation to the mesh when they fall back, and so may pass through the mesh.
Finally, granules with a size between 0.5 mm and 1 mm were weighed.
Tests were carried out to understand the percentage dispersion in terms of the dry weight of a ground tooth and then filtered through a sieve (Figure 3), to keep only the granules with a size between 0.5 mm and 1 mm. The granules were produced using a grinder (low- and high-speed) and then filtered by size using metal sieves (Figure 3, Filtra, Seneco S.r.l., Milan, Italy) and divided into three groups: i) group A: particles from 200 μm to 900 μm; ii) group B: particles < 200 μm; iii) group C: particles > 900 μm. Only granules from group A were used for the tests.
The two mesh sieves have dimensions of 850 μm for the upper and 425 μm for the lower sieve. The tooth was weighed using the Tanita super precision Mini weighing scale (TANITA, Arlington Heights, IL, USA), before grinding and after grinding.
A sample of 40 natural human teeth (Figure 4) was used without restorations, prostheses, or root canal treatments. In the case of vital teeth vascular and nervous tissues should not be removed, whereas residues of fillings or tartar need to be removed, as well as the periodontal tissue, since periodontal tissue is more challenging to eliminate during preparation.
In the case of the low-speed grinder, the teeth were sectioned due to problems of insertion into the space between the blades and this resulted in a weight loss caused by the cuts.
However, it was decided not to weigh the teeth after sectioning because this represents one of the limitations of this system and it must be fully evaluated. The whole teeth were inserted into the high-speed grinder.
The granules filtered through a sieve were then inserted into a device (Mastersizer 3000, Malvern Panalytical Ltd, Malvern, UK) allowing the quantification of both dry and wet granules through laser diffraction, to understand if the granules’ dimensions were in line with the requisite.
The laser diffraction test indicated that the average particle size varied between 406 μm and 815 μm with peaks up to 1,110 μm.
The results of the two different grinding systems have been recorded in the following table (Table 1). The results were determined by calculating the average for the 20 teeth ground using each grinding system and showing the two types of grinding and the corresponding tooth weight before grinding and after grinding. The weight difference is shown in the percentage of tooth loss.
Average tooth weight before and after test. Results of the two different grinding systems
| Type of test | Average tooth weight before test ± SD (g) | Average tooth weight after test ± SD (g) | Lost tooth weight ± SD (%) |
|---|---|---|---|
| High-speed | 1.20 ± 0.53 | 0.56 ± 0.29 | 53.50 ± 9.89 |
| Low-speed | 1.44 ± 0.62 | 1.32 ± 0.58 | 9.16 ± 2.34 |
SD: standard deviation
In recent years, several researchers have investigated the use of particles derived from autologous teeth as bone grafting material. In the study by Kim et al. [72], once the soft tissues, tartar, and foreign materials were removed, the tooth elements were divided and crushed, obtaining particles between 0.5 mm and 1.0 mm in size. The particles of the crushed teeth were immersed in distilled water and hydrogen oxide solution, dehydrated with ethyl alcohol solution, and degreased with ethyl ether solution. Then, after the freeze-drying procedure, they were sterilized with ethylene gas and packaged [72]. Jun et al. [73] also created the material in the form of a powder with particles of 0.5–1.0 mm, while, in another study, sample teeth were pulverized into powder, with each particle having a diameter of 0.4–0.8 mm [74, 75].
Murata et al. [76] described that the teeth were crashed in liquid nitrogen, washed in 1 mol/L sodium chloride, and demineralized in HCl solution at pH 2.0. The tooth particles were thoroughly rinsed in cold distilled water before being lyophilized into 0.4 mm and 0.8 mm particles [76]. Nampo et al. [77] obtained the graft material by removing the crown portions of the extracted teeth with scissors and trimming the root portions of the remaining teeth as close to 500 mm as possible. The trimmed tooth was then mixed with a measured amount of β-tricalcium phosphate [77]. Finally, in the study by Kim et al. [78], the particle size ranged from 0.2 mm to 1.2 mm.
Various studies have investigated the effect of anorganic bovine bone matrix (ABBM) particle size on bone healing in order to define the perfect dimension of granules for the optimal use in regeneration surgery (Table 2). Histological and radiographic studies were carried out to understand if bone repairing could be influenced by the particle size [72–74, 76–86].
Graft granules’ dimensions reported by different studies. The table indicates what is the size of the granules commonly considered optimal in regeneration and then has a reference
| Authors | Granules’ dimensions (mm) |
|---|---|
| Kim et al., 2014 [72] | 0.5–1.0 |
| Kim et al., 2013 [74] | 0.4–0.8 |
| Murata et al., 2005 [76] | 0.4–0.8 |
| Nampo et al., 2010 [77] | 0.5 |
| Kim et al., 2010 [78] | 0.2–1.2 |
| Jun et al., 2014 [73] | 0.5–1.0 |
| Binderman et al., 2014 [85] | 0.3–1.2 |
| Dozza et al., 2017 [79] | 0.5–1.0 |
| Testori et al., 2013 [81] | 1.0–2.0 |
| Klüppel et al., 2013 [86] | 0.2–0.4 |
In the study by Klüppel et al. [86], 18 male New Zealand rabbits were employed, and four cavities were drilled and filled with varying particle sizes of ABBM. The first cavity had small particles (under 450 µm), the second cavity included medium particles (450 to 749 µm), and the third cavity contained giant particles (750 to 1,000 µm). Particulated autogenous bone was used to fill the fourth cavity (control group). The animals were suppressed for 15, 30, and 60 days following surgery. Before the decalcification process and histological assessment, radiographs of the cranial vault were taken. The authors concluded that ABBM particle size affects the bone healing process: Smaller particles resorb faster and induce more bone neoformation than bigger particles [86].
Shapoff et al. [83] studied freeze dried bone allograft (FDBA), tiny particles (100–300 µm) combined with bone marrow, and big particles (1,000–2,000 µm) mixed with bone marrow in six rhesus monkeys. The findings indicated that the tiny particles produced more bone. The authors indicate the possible superiority of the smaller graft for some reasons: The increase in surface area, the release of a large amount of calcium salts by hydrolytic enzymes, and the increase in the number of pores all encourage bone formation [83, 87].
Different results were reported in the study by Testori et al. [81]. The authors compared vital bone formation after maxillary sinus augmentation using two different particle sizes of ABBM, finding a statistically significant increase in vital bone formation in the larger particle grafts [81].
Kon et al. [88] employed twenty-four rabbit cranial bones to evaluate the augmentation process of two distinct autogenous bone graft particle sizes: large bone (1–2 mm) and small bone (150–400 µm) particles were used. Autogenous bone is thought to be the gold standard for bone augmentation in the clinical setting. Nevertheless, the capacity to enhance may vary depending on particle size. By 8 weeks, the small bone had shrunk to 51.3% and 51.0% of its initial volume and height, respectively, while the large bone had maintained its volume. Finally, they proposed to use big autologous bone particles [88].
In another work demineralized FDBA (DFDBA) was processed and crushed into two sizes: 250–500 µm and 850–1,000 µm. Ten individuals with intrabony defects were chosen at random and, for each defect, soft and hard tissue measurements were taken. The bone defect fill was computed as a depth reduction from a given position: For the tiny particle group, it was 1.32 mm, while for the big particle group, it was 1.66 mm. This difference did not result as being statistically significant [89].
The osteoconductive capacity of deproteinized bone particles of two distinct diameters (300–500 and 850–1,000 mm) in rabbits was compared in the study of Xu et al. [90]. The deproteinized bone was made from white rabbit limbs. The cortical bone was soaked in water for 10 h before being immersed in 1 mol/L HCl. Finally, the bone was sintered in an electric furnace for 3.5 h at 600°C and 3.5 h at 1,100°C. In a bone mill, the bone was crushed into two particle groups: big particles (850–1,000 µm) and small particles (300–500 µm). Finally, particles were used to perform the sinus lift. Small particle groups produced superior outcomes [90].
In sheep femoral condyles, larger particles of silicate-substituted calcium phosphate (diameters of 250–500 mm or 1,000–2,000 mm, respectively) tended to preserve the volume of early bone formation better than smaller particles (90–120 mm) [91, 92]. Larger particles tended to be retained in newly produced bone tissue, owing to the longer time required for dissolution or remodeling [84, 93]. After successful engraftment, an autogenous bone block graft demonstrated a lower bone resorption rate than the particulates [94].
Koga et al. [95] compared DDM, partial DDM, and mineralized dentinal matrix, using three different sizes of graft particles for each group. Defects in the sheep’s cortical bone were realized by inserting scaffolds of different sizes (large, 1–2 mm; small, < 0.5 mm; medium, 0.5–1.0 mm); subsequently clean, pulverized human teeth were divided into three groups according to sizes. The best result was obtained from dimensions ranging between 0.5 mm and 1.0 mm. Smaller particles are too quickly resorbed to ensure sufficient space retention over time and to allow for bone formation. It is therefore obvious that the best performances are obtained when the dimensions of the granules are homogeneous and they are between 0.5 mm and 1.0 mm [95].
Dozza et al. [79] analyzed the DBM collagen-based biomaterial. A cortical sheep bone was ground and three different granule graft sizes were inserted and analyzed: small (< 0.5 mm), medium (0.5–1.0 mm), and large (1–2 mm). The authors concluded that medium particles were the best condition for cytocompatibility and recommended the use of an average size of 0.5 mm to 1.0 mm [79].
Some authors stated that osteoclast-like multinucleated giant cells appear to prefer small particles (< 1 mm) in both autogenous bone and bone substitutes, such as bovine mineralized bone [88, 96].
Larger bone replacement particles, on the other hand, can provide a greater quantity of bone augmentation. Larger autogenous bone particles (diameter, 1–2 mm) produced a greater augmented bone volume than smaller particles in a vertical bone augmentation model in rabbit calvaria utilizing polytetrafluoroethylene chambers (diameter, 150–400 mm) [97].
DDM resorbs faster than mineralized dentin, producing the best osteoconduction results, but very small parts may result in rapid graft resorption and failure to preserve volume [63, 72].
Dimensions under 400 μm are reabsorbed from osteoclasts in a short time. Conversely, dimensions over 1,000 μm are impossible to be reabsorbed. For this reason, granules produced by the two different speed grinders are sieved and the granule dispersion is analyzed using a master sizer 3000, laser diffraction particle size analyzer (Mastersizer 3000, Malvern Panalytical Ltd, Malvern, UK). This first analysis, despite the limitations due to the number of elements analyzed and the tools used, made it possible to establish that the grinding at low-speed, resulted in a more homogeneous grinding and was more efficient, allowing a greater percentage of the tooth to be available for use.
These findings suggest that bigger particles (1 mm) have stronger mechanical resistance as a mass for space-making than smaller particles (0.4 mm) and that space-making capability is more critical for early bone formation than the balance of bone resorption and formation. Nevertheless, dentin differs from the other graft materials in several ways. One of the most significant aspects is that no volume is lost during the preparation since it is unable to add (extract) additional material, and this process is based on the use of a removed tooth, thus it makes no sense to remove another tooth for graft usage. This element, which should be a limitation, should instead push to evaluate every single part of the preparation in order to avoid losing any component of the removed and useful teeth for producing grafting material.
As a result, several researchers have investigated the tooth’s weight and typical volume [98]. A total of 205 removed teeth were weighed and measured with a millimeter-level syringe using a professional digital micro scale. The average weight varied from 0.68 g to 1.88 g, while the average volume was 0.38 mL to 0.96 mL. The volume is sufficient to accomplish the majority of the graft operation, but it is impossible to add more material in the same procedure if production is low. The findings indicated that the material produced from teeth might be adequate to be used as bone grafting material. However, the varying grinding from different machines may alter the volume available for regeneration [98].
The evaluations of the authors are exactly the point and purpose of this study. The only usable engineering solutions for the fragmentation of a solid structure transforming it into granular are: The first is high-speed by means of flat blades that rotate inside a capsule container and the structure to be fragmented is placed inside the capsule itself, the second is a concentric conical blades that rotate on the same axis and at low-speed where the solid structure must be inserted in the space between the conical blades.
The test anlyzed three different data:
average tooth weight before the test: Each tooth was weighed to be able to compare the weight after the treatment;
average tooth weight after the test: Each pulverized tooth was weighed after the treatment to be able to compare with the same tooth weight before the treatment;
lost tooth weight (%): The difference between before and after the treatment was measured in the percentage of weight loss.
After sieving, the two different speed grinders produced the same particulate. The high-speed showed a greater dispersion. The dispersion was due to the pulverization (highly thin granules) of part of the tooth. The shape and the dimension of the granules were very different, so to obtain optima granules for the regeneration surgery a lot of parts of the tooth could be lost (Figure 2a). The percentage of the mean tooth lost with this high-speed grinder is 53.50% ± 9.89% of the tooth load.
The low-speed grinder does not pulverize the dentin and creates a regular dimension of the tooth granules. The percentage of teeth loss is 9.16% ± 2.34%.
The hypothesis is that low-speed grinding is more effective, even though it requires a longer tooth preparation. The working hypothesis has thus been confirmed by the obtained data. This investigation conducted a comparative analysis between a low-speed grinder and a high-speed grinder, yielding disparate outcomes. Despite both methods yielding similar particle sizes, a marginal dispersion of tooth material was observed in the low-speed grinder group. This observation underscores the clinical and ethical imperative to preserve a substantial portion of the tooth, ensuring the maximal quantity of graft material. It is crucial to recognize that the tooth, being a non-commercial graft material, is irreplaceable, and procurement of additional graft material is not feasible if the production proves insufficient. Consequently, optimization strategies are imperative to maximize the volume derived from a single extracted tooth. While the low-speed grinder aligns with this imperative by minimizing material dispersion, it concurrently extends the duration of the procedural timeline. To address this trade-off, future endeavors should focus on the development of an innovative system capable of amalgamating the expeditious nature of high-speed grinding with the meticulous preservation of volume inherent in low-speed procedures. This proposed system would not only uphold the ethical mandate to conserve tooth material but also enhance procedural efficiency. Anticipating the evolution of dental technology, further investigations are warranted to explore and refine methodologies that balance speed and volume preservation. The advancement of such technologies is pivotal in advancing the field of dental grafting, ensuring both clinical efficacy and ethical responsibility in the utilization of precious biological resources. Consequently, the promotion of additional studies is imperative to propel the realization of these technological advancements and their integration into clinical practice.
ABBM: anorganic bovine bone matrix
BMPs: bone morphogenetic proteins
DDM: demineralized dentin matrix
RPM: revolutions per minute
EM, GD, ADI, and FI: Conceptualization, Methodology, Writing—original draft, Writing—review & editing. AP: Conceptualization, Methodology, Writing—original draft. FV and AMI: Conceptualization, Methodology, Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
The authors declare no conflict of interest.
Institutional Review Board Statement: The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethical Committee for Biomedical Research of Chieti and Pescara (Protocol Number 1869/21.03.2019).
Informed consent was obtained from all subjects involved in the study.
Not applicable.
Data from the present manuscript will be made available upon reasonable request, and the corresponding author will provide the data upon request.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Simona Santonocito ... Gaetano Isola
Saeed Asgary, Laleh Alim Marvasti
Renzo Guarnieri ... Luca Testarelli
Domenico Baldi ... Jacopo Colombo
Salwa Mekled ... Geraldine Weinstein
