Affiliation:
1Laboratorio de Oncología Molecular, Programa Institucional de Formación de Investigadores, Escuela Superior de Medicina del Instituto Politécnico Nacional, México City 11340, México
ORCID: https://orcid.org/0009-0000-0475-2363
Affiliation:
1Laboratorio de Oncología Molecular, Programa Institucional de Formación de Investigadores, Escuela Superior de Medicina del Instituto Politécnico Nacional, México City 11340, México
ORCID: https://orcid.org/0009-0009-6902-5680
Affiliation:
2Laboratorio de Oncología Molecular, Sección de Posgrado e Investigación, Escuela Superior de Medicina del Instituto Politécnico Nacional, México City 11340, México
Email: neherrera@gmail.com
ORCID: https://orcid.org/0000-0003-0646-6955
Explor Immunol. 2025;5:1003205 DOI: https://doi.org/10.37349/ei.2025.1003205
Received: February 10, 2025 Accepted: June 19, 2025 Published: August 05, 2025
Academic Editor: Calogero Caruso, University of Palermo, Italy
Cancer remains one of the leading causes of morbidity and mortality globally, driven by genetic alterations, uncontrolled cell proliferation, and metabolic reprogramming. The tumor microenvironment (TME) is a highly dynamic and heterogeneous system composed of tumor cells, immune cells, stromal cells, and extracellular matrix (ECM) components, which influence cancer progression. Tumor-associated macrophages (TAMs), especially those polarized into the M2 phenotype, play a critical role in modulating this environment. M2 macrophages promote tumor progression through mechanisms such as immune suppression, angiogenesis, and metastasis. This polarization is heavily influenced by the altered metabolic landscape of tumors, where the Warburg effect leads to excessive lactate production, which in turn drives M2 polarization through G protein-coupled receptor 132 (GPR132). M2 macrophages secrete cytokines like IL-10, transforming growth factor β (TGF-β), and vascular endothelial growth factor (VEGF), which contribute to immune escape, tumor growth, and metastasis. The metabolic shifts within TAMs, especially the transition from oxidative phosphorylation to glycolysis, further support the pro-tumoral functions of these cells. This review explores the intricate relationship between M2 macrophage polarization bias, tumor metabolism, and the resulting impact on cancer progression, highlighting the potential of targeting these pathways for therapeutic strategies. The findings suggest that M2 macrophage polarization could serve as a key prognostic factor for cancer outcomes and provide a basis for future research into therapeutic interventions that target macrophage polarization and the tumor metabolic milieu.
Projections for 2025 indicate that the United States will have about 2,041,910 new cancer cases and 618,120 cancer-related deaths [1]. Estimates indicate that about one in five men or women will develop cancer in their lifetime, while roughly one in nine men and one in twelve women will die from cancer [2].
Cancer is a group of genetic diseases resulting from the accumulation of alterations in the genome of the cells [3]. Cancer can originate from any cell in the body [4]. Alterations in the cell’s genome produce high cell proliferation and the development of tumors, benign or malignant [5]. Malignant tumors grow uncontrollably and metastasize to other parts of the body, different from the origin of the tumor [6]. Tumors disseminate to distant sites through direct, lymphatic, and hematogenous spread [7, 8].
As mutations keep accumulating, cancer develops certain key features denominated “hallmarks of cancer” (Figure 1). As a result of this incessant mutation, tumors are formed of cancer cell populations with different genotypes and phenotypes [9]. This heterogeneity and the cancer cell’s adaptability dictate the progression, dissemination, and treatment of the tumor [5, 9].
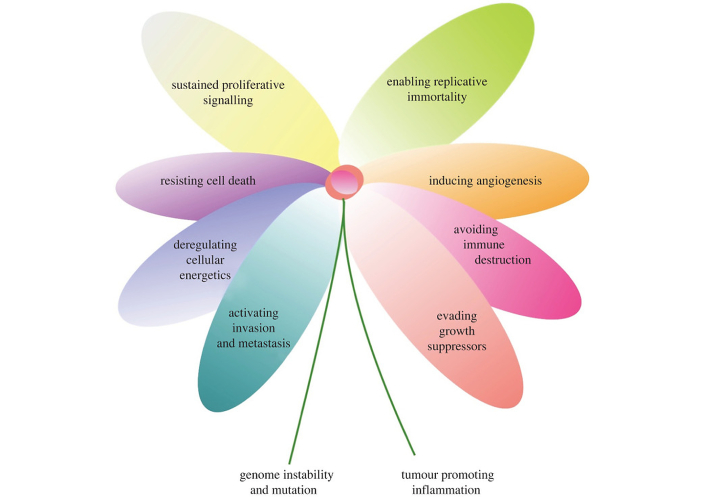
The hallmarks of cancer. The image shows the main features of cancer, including its capacity to maintain proliferative signaling and promote the tumor’s survival and dissemination. Remarkably, one feature is the deregulation of cellular energetics, which translates into the opportunistic way of cancer cells to acquire nutrients. Reprinted from Senga et al. [5]. © 2021 The Authors. CC BY 4.0
Otto Warburg defined the “Warburg effect” as an irreversible damage to cell respiration and an increase in the cell fermentation [10]. Malignant metabolism is a term that refers to cancer cells’ metabolism and altered aerobic, glycolysis also known as “Warburg effect” [11].
The Warburg effect provides the cancer cell with more than just metabolic advantages; it also supports rapid proliferation [10, 12]. An altered metabolism is part of a “hallmark of cancer”, providing a sustaining proliferative signaling and deregulating cellular energetics, which accelerates malignant progression [5, 12]. Hypoxia-inducible factor 1 alpha (HIF-1α) drives the Warburg effect by upregulating the enzymes necessary for aerobic glycolysis, such as hexokinase (HK), lactate dehydrogenase A (LDHA), transporters like glucose transporters (GLUTs) and monocarboxylate transporters (MCTs) (Figure 2) [12–15]. In cancer cells, glucose metabolism is diverted through the glycolytic pathway, which converts glucose to pyruvate and subsequently to lactate, even under aerobic conditions [12, 15]. Key isoforms of HK2, pyruvate kinase M2 (PKM2), and LDHA are overexpressed, enabling rapid ATP generation [14]. Critically, the terminal step—conversion of pyruvate to lactate by LDHA—not only sustains tumor growth but also acidifies the microenvironment through lactate export via MCTs [16]. As a result, cancer cells develop an opportunistic way to acquire nutrients [13]. Furthermore, the Warburg effect is a prevalent feature in aggressive cancers [17, 18].
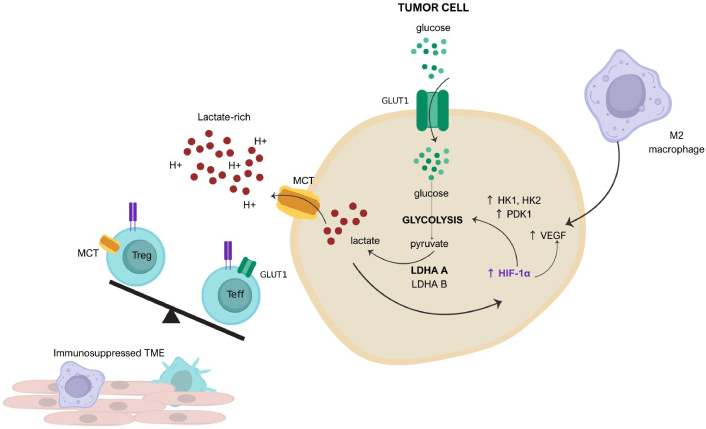
The role of tumor metabolism, the immune microenvironment, and hypoxia. Tumors deplete the microenvironment of glucose due to a high expression of glucose transporter 1 (GLUT1) transporters. Malignant cells abundantly produce lactate as a consequence of aerobic glycolysis, and lactate is released into the tumor microenvironment through monocarboxylate transporters (MCTs), resulting in the acidity of the microenvironment. Lactate activates hypoxia-inducible factor 1 alpha (HIF-1α), which promotes glucose metabolism by upregulating hexokinase (HK) and pyruvate dehydrogenase kinase 1 (PDK1). HIF-1α promotes vascular endothelial growth factor (VEGF) production, thereby promoting neo-vessel formation and metastasis. Lactate-rich, glucose-low, and hypoxic environments provoke the retention of T regulatory cells (Treg), reinforcing an immunosuppressed microenvironment. LDHA: lactate dehydrogenase A. Adapted from Schreier et al. [15]. © 2023 The Authors. CC BY 4.0
Other oncogenic drivers of metabolic programming are Myc proto-oncogene, nuclear factor erythroid 2-related factor 2 (Nrf2), and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-ĸB) [19, 20]. Myc is a transcriptional regulator that reprograms tumor cell metabolism to meet the demands of rapid proliferation [19]. Myc enhances glycolysis by upregulating GLUTs and key aerobic glycolysis enzymes (HK2, LDHA, PKM2) while promoting glutaminolysis through glutaminase to sustain the tricarboxylic acid (TCA) cycle and nucleotide synthesis [20]. The effects of Myc suppress tumor immunity by promoting lactate production and upregulating immune checkpoints via programmed death ligand 1 (PD-L1) [19]. This transcriptional regulator also promotes mitochondrial biogenesis and lipid production, creating a hypermetabolic state [19]. The transcription factor Nrf2 serves as a critical defender against oxidative stress in tumors by scavenging reactive oxygen species (ROS). Nrf2 protects cancer cells from apoptosis [21]. NF-ĸB activates via toll-like receptors (TLRs) [22]. The effects of NF-ĸB are the upregulation of HK2 and GLUT1, but it also promotes TAM’s IL-10 and transforming growth factor β (TGF-β) secretion [22, 23]. NF-ĸB also boosts indoleamine 2,3-dioxygenase (IDO), which converts tryptophan into kynurenine. This activates aryl hydrocarbon receptor (AhR) on Tregs, increasing this population, but as a consequence diminishing the immune response against cancer cells [24].
Tumors are not just clones of malignant cells. Tumors are a mixture of different groups of cell types, such as neutrophils, macrophages, lymphocytes, natural killer (NK) cells, natural killer T (NKT) cells, fibroblasts, etc. [25]. Macrophages and dendritic cells (DCs) are some of the main populations of immune cells that infiltrate the tumor tissue [26]. Solid tumors contain tumor-associated myeloid cells (TAMC), which include (I) TAMs, (II) Ang2-expressing monocytes, (III) myeloid-derived suppressor cells (MDSCs), (IV) tumor-associated neutrophils (TANs), and (V) tumor-associated dendritic cells (TADCs) [27]. Gene expression profiles in tumors influence the cellular composition of the tumor microenvironment (TME) [28].
Beyond the tumor’s metabolic adaptability, stromal cells exhibit what is known as “reverse Warburg effect” [29]. Cancer-associated fibroblasts (CAFs) metabolize glucose to lactate, which is important for tumor cells through MCTs for oxidative phosphorylation (OXPHOS) [29]. This metabolic symbiosis promotes metastasis and therapy resistance, while it simultaneously fills the TME with lactate, promoting M2 polarization [16, 17, 30].
Extracellular matrix (ECM) also exhibits tumor-associated changes, which promote the progression of cancer [25]. ECM remodeling is an important feature in tumorigenesis, and it is a vital point of cell extrinsic metabolic regulation [31]. The cellular distribution of tumors is not random; pathologists use the morphology and distribution of cancer cells as criteria to identify them [25]. The remodeling in the ECM is promoted by an alteration in the ECM deposition and degradation homeostasis as a consequence of tumor-secreted factors like TGF-β, vascular endothelial growth factor (VEGF), and matrix metalloproteinases (MMPs) [7, 25]. The interstitium exhibits a change in composition depending on the nutrient consumption of the tumor and its metabolism [32].
TLRs are pattern recognition receptors expressed on a variety of immune cells—including macrophages, DCs, and lymphocytes—as well as on many tumor cell types [22]. While they primarily function as damage-associated molecular patterns/pathogen-associated molecular patterns (DAMPs/PAMPs) recognizers, TLRs also act as metabolic regulators [33]. TLR3 induces a metabolic switch from OXPHOS to glycolysis, leading to lactate accumulation [34]. The metabolic transition promoted by TLR3 involves the transcription factor HIF-1α, which allows tumors and some immune cells (like macrophages and DCs) to adapt to hypoxia [34].
In most solid tumors, hypoxia is a key feature of the TME, which involves oxygen partial pressure (pO2) below 15 mmHg [32]. The ECM is remodeled by the influence of hypoxia, which contributes to the development of altered metabolism [35].
Cancer cells can adapt to their changing microenvironment. Specifically, cancer stem cells (CSCs) present a different transitioning phenotype between a quiescent mesenchymal-like (M) and a replicative epithelial-like (E), also called epithelial-mesenchymal transition (EMT) [18]. CSCs rely on glutamine and lactate metabolism imported from the stroma [9]. Moreover, CSCs produce TGF-β, which biases the macrophage polarization toward an M2 phenotype [36]. ROS play a pivotal role in regulating EMT in breast cancer stem cells (BCSCs), acting both as signaling molecules and inducers of oxidative stress [37]. Moderate ROS levels, often generated via mitochondrial activity or enzymes like NADPH oxidase (NOX) and MMP-3, can activate EMT-related pathways and transcription factors such as snail and NF-κB, promoting mesenchymal phenotypes and BCSC-like properties [37]. BCSCs typically maintain lower ROS levels than non-stem cancer cells through upregulated antioxidant defenses, preserving their quiescence, therapy resistance, and plasticity [37]. This redox balance allows BCSCs to transition between epithelial and mesenchymal states during metastasis [37]. EMT is fundamental in the process of cellular invasion and metastasis. The interplay between EMT and metabolic plasticity suggests the capacity of tumor cells to adapt to nutrient-restricted environments due to metabolic reprogramming [38]. Cancer cells’ plasticity provides the cell with the capability of switching between distinct stages of differentiation in order to survive in the tumor’s harsh microenvironment [9]. The metabolic reprogramming of tumors and immune cells is driven by oncogenic pathways and immunosuppressive metabolites, as summarized in Table 1. These molecules establish a protumoral environment by polarizing macrophages and disrupting effector immune cells.
Oncogene and metabolite effects in the tumor microenvironment
| Category | Molecule | Effect | Reference |
|---|---|---|---|
| Oncogenes | HIF-1α | Upregulates glycolysisPromotes M2 polarization via lactateSuppresses CD8+ T cells | [12–14] |
| Myc | Enhances glutaminolysis and glycolysisDrives immune evasion by lactate and PD-L1 upregulation | [19, 20] | |
| NF-ĸB | Induces IL-10 and TGF-β secretion, inducing M2 polarization | [22, 34] | |
| Nrf2 | Antioxidant response suppresses ROSSustains immunosuppression | [12, 13] | |
| Metabolite | Lactate | Activates GPR132 inducing M2 polarizationInhibits NK and CD8+ T cells | [43, 53] |
| L-arginine | Impairs T cell function (anergy) | [46, 48] | |
| Tryptophan (kynurenine) | Expands T reg population through AhR activation | [42, 55] | |
| Glutamine | Promotes T cell exhaustion | [13, 42] |
HIF-1α: hypoxia-inducible factor 1 alpha; PD-L1: programmed death ligand 1; NF-ĸB: nuclear factor kappa-light-chain-enhancer of activated B cells; IL-10: interleukin-10; TGF-β: transforming growth factor β; Nrf2: nuclear factor erythroid 2-related factor 2; ROS: reactive oxygen species; GPR132: G protein-coupled receptor 132; NK: natural killer; AhR: aryl hydrocarbon receptor
Macrophages are part of the mononuclear phagocytic system and are heterogeneous cells that show very different functions and phenotypes depending on distinct microenvironment cytokines [39]. Under different stimuli, undifferentiated macrophages can be polarized into two forms, and they exhibit different functions (Table 2) [40]. Classically activated macrophages (M1) are involved in host defense and are activated by interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), and have antitumoral activity. Alternatively activated macrophages (M2) are activated by IL-10, IL-4, and IL-13; they promote wound healing and regulate immune responses [40]. During diseases, macrophages polarize to a specific phenotype depending on epigenetic and genetic factors [40]. Epigenetic factors such as DNA methylation, mediated by enzymes like DNA methyltransferase 1 (DNMT1) and DNMT3b, affect the balance between M1 and M2 macrophages, with DNMT1 promoting M1 polarization by silencing suppressor of cytokine signaling 1 (SOCS1) and DNMT3b modulating M1/M2 differentiation [40]. Conversely, ten-eleven translocation 2 enzyme (TET2) facilitates inflammatory responses through demethylation [41]. Histone modifications further refine macrophage polarization, where enzymes such as protein arginine methyltransferase 1 (PRMT1), SET domain-containing lysine methyltransferase 7 (SET7), and Jumonji domain-containing protein 3 (JMJD3) enhance M1 activation, while SET domain bifurcated 2 (SETDB2), enhancer of zeste homolog 2 (EZH2), and lysine-specific demethylase 1 (LSD1) promote M2 polarization [21]. In the TME, epigenetic changes in TAMs contribute to immunosuppression, with factors like BRD4, ERK-1/2 phosphorylation, and histone deacetylation reinforcing their tumor-supportive roles [21, 41]. However, inhibitors such as suberoylanilide hydroxamic acid (SAHA) have shown potential in reducing macrophage migration and immune suppression, highlighting the therapeutic potential of targeting these epigenetic mechanisms in cancer and chronic inflammatory diseases [21]. M2 macrophages can be divided into 4 subtypes: M2a, M2b, M2c, and M2d [40, 42]. M2 macrophages display pro-malignancy and anti-inflammatory activities (Figure 3) [16, 27, 36]. They promote the tumor progression by causing angiogenesis and a metastasis of the tumor [36]. Most TAMs are thought to resemble M2 macrophages [43, 44]. These cells play an important role in connecting inflammation with cancer.
Differences between M1 and M2 macrophages
| Macrophages | M1 | M2 |
|---|---|---|
| Activation | TLR, TNF-α, IFN-γ, CSF2 | IL-4, IL-10, IL-13, TGF-β, PGE2 |
| Secretion | IL-6, IL-8, IL-1β, IFN-γ, TNF-α | IL-10, IL-4, EGF, TGF-β, IL-19 |
| Markers | HLA-DR, CD11c, CD86, iNOS, pSTAT1 | CD136, CD204, CD206, VEGF, cMAF |
| Function | Pro-inflammatory, microbicidal, anti-tumoral | Anti-inflammatory, wound healing, pro-tumoral |
TLR: toll-like receptor; TNF-α: tumor necrosis factor alpha; IFN-γ: interferon-gamma; CSF2: granulocyte-macrophage colony-stimulating-factor 2; IL: interleukin; TGF-β: transforming growth factor β; PGE2: prostaglandin E2; EGF: epithelial growth factor; HLA-DR: human leukocyte antigen-DR isotype; CD: cluster of differentiation; iNOS: inducible nitric oxide synthase; pSTAT1: phosphorylated signal transducer and activator of transcription; VEGF: vascular endothelial growth factor; cMAF: cellular microenvironment-associated factor. References: [24, 58]
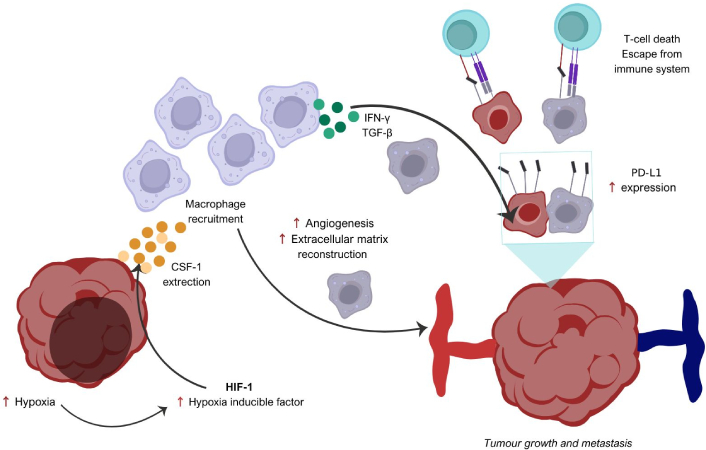
Role of tumor-associated macrophages. M2 macrophages contribute to tumor development by interacting with the tumor microenvironment (TME). Tumor-associated macrophages (TAMs), recruited by CSF-1 secretion, promote cell proliferation by secreting growth factors such as EGF and FGF. VEGF, PDGF, TGF-β, and MMPs produced by macrophages induce neoangiogenesis, lymphangiogenesis, and facilitate tumor metastasis and extracellular matrix (ECM) remodeling. TAMs also impair the function of immune cells, including dendritic cells, CD8+ T cells, and NK cells, thereby creating an immunosuppressive effect. CSF-1: colony-stimulating factor-1; EGF: epithelial growth factor; FGF: fibroblast growth factor; VEGF: vascular endothelial growth factor; PDGF: platelet-derived growth factor; TGF-β: transforming growth factor β; MMPs: matrix metalloproteinases; NK: natural killer; PD-L1: programmed death ligand 1. Adapted from Padzińska-Pruszyńska et al. [56]. © 2024 The Authors. CC BY 4.0
In certain circumstances, TAMs produce IL-10 and TGF-β. This fact, in turn, induces monocytes to express the molecule PD-L1, which leads to the suppression of cytotoxic T cell responses against the tumor [24, 45]. TGF-β secreted by M2-like macrophages prevents cytolytic activities of NK, and it inhibits the maturation and functioning of DCs [46]. IL-10 suppresses the T-cell differentiation and inhibits the function of cytotoxic T-cells and NK [47]. M2-like macrophages are rich in arginase-1 (Arg1), which promotes dysregulation in the T-cell receptor (TCR), causing CD8+ T-cell anergy [48]. As a consequence of tumor metabolism, additional effects arise from the mechanisms involved in HIF-1α expression, including the inhibition of CD8⁺ T cells—mediated by mTOR-driven accelerated glycolysis—and the recruitment of Tregs through the chemokine (C-C motif) ligand 20 (CCL20)/C-C chemokine receptor type 6 (CCR6) axis [14, 43].
The role of TAMs in the TME is hard to define due to their heterogeneity in the TME; however, TAMs can display remarkable plasticity and switch from one phenotype to another depending on the TME signals [49, 50]. Macrophage anti-tumoral or pro-tumoral activity of macrophages is TME-influenced [49]. Macrophages are highly sensitive to variations in concentrations of metabolites, oxygen tension, acidification, and other molecular components associated with alterations in the TME [42, 51].
In homeostatic conditions, macrophages exhibit base line metabolism (OXPHOS) [51]. In contrast, there is a metabolic adjustment featured in macrophages in TAMs (aerobic glycolysis and amino acid metabolism) [42, 52]. Macrophages are highly adaptable, with M1 types showing increased glycolysis, glutathione, ferritin, cyclooxygenase 2 (COX-2), and inducible nitric oxide synthase (iNOS) activity, but low COX-1 and Arg-1 [42]. In contrast, M2 macrophages rely on fatty acid oxidation, with lower glutathione, ferritin, and COX-2, but higher COX-1 and Arg-1 activity, and reduced iNOS function [46].
M2 macrophages deplete L-arginine through Arg-1 activity, starving T cells of this nutrient, impairing TCR signaling [48].
TAMs are the largest population of stromal cells that suppress antitumoral activity and stimulate tumor progression [16]. In humans, macrophage polarization is a continuum that comprises two extremes, from the classically M1 macrophages to the alternatively activated macrophages [39]. Due to the insufficient blood perfusion, consequent hypoxia, and glycolytic cell metabolism, there is an excessive amount of lactic acid in the TME [14, 18]. Altered cancer cell metabolism enhances the production of lactate, this metabolite is considered the canonical tumor waste product, and it is also considered one of the regulators of intracellular communication within the TME [32, 43]. In lactate-producing tumors, TAMS originate through the action of G protein-coupled receptor 132 (GPR132) [11, 16]. GPR132 is highly expressed in macrophages, and it functions as an acidic signal-sensing receptor [16, 30]. Tumor-produced lactate makes the extracellular tumor pH around 6.5–7 in comparison to normal extracellular pH values being 7.4 [16, 32]. It has also been reported that the bias in macrophage polarization is due to IL-4, IL-10, and IL-13 synthesized by LTCD4+ cells and growth factors secreted by tumor cells, such as colony-stimulating factor-1 (CSF-1) and GM-CSF [16, 36].
Lactate has also been found to repolarize M1-like macrophages towards the M2-like phenotype [43]. TAM densities have been found to be higher in areas of the tumor that are hypoxic, avascular, and necrotic [53]. TAMs tend to exhibit a M2-like phenotype bias; this macrophage phenotype secretes cytokines such as VEGF, TGF-β, and IL-8 [40]. Alternatively activated macrophages’ cytokines are proangiogenic factors and matrix remodeling promotors; they also have an effect on adaptative immunity and immune escape, contributing to cancer cell proliferation, survival, and metastasis [11, 16, 23]. Proangiogenic factors such as VEGF promote tumor vascularization, and they stimulate the capacity of the tumor to metastasize [23]. However, M2 macrophages are also believed to foster cancer metastasis through cytokines including CCL17, CCL18, CCL22, IL-10, VEGF, and TGF-β [16]. The major pathogenic activity of TAMs is their suppressive effect on anticancer immune response [23]. TGF-β and IL-2 secreted by M2 macrophages weaken the anticancer ability of NK cells and cytotoxic lymphocytes [43]. A positive feedback loop to promote metastasis has been described, lactate produced by cancer cells and GPR132 on macrophages form a ligand-receptor pair, which induces metastasis and paracrine invasion [16].
Ji et al. [54] recently explored the role of the YTH N6-methyladenosine RNA binding protein 2 (YTHDF2) gene in macrophage polarization. In their study, knockdown of YTHDF2 in gastric cancer cells resulted in a reduced proportion of CD206+ (M2) macrophages, while the proportion of CD80+ (M1) macrophages increased [54]. Nonetheless, additional studies are needed, as YTHDF2 displays a complex dual role in cancer, functioning both as an oncogene in some contexts and as a tumor suppressor in others [54]. This intricate behavior could be key to understanding its potential as a therapeutic target. Another current study established that extracellular cell-free mitochondrial DNA induced by kinase inhibitors in hepatocellular carcinoma (HCC) polarized macrophages to an M2 phenotype through the TLR9 pathway [55]. Overall, macrophage polarization is not induced by a single mechanism; it is a TME-influenced phenomenon [43, 49, 54, 55].
On the other hand, TLR agonists push the TAMs to a M1-like phenotype bias [50]. TLR7 and TLR3 promote a switch to M1-like phenotype macrophages [50]. However, not all TLRs are associated with tumor inhibition, and their effect/pathway changes according to the type of cancer [22].
Cancer is a highly heterogeneous disease driven by genetic instability and dynamic interactions within the TME [17, 32]. A hallmark of this disease is metabolic reprogramming—particularly the Warburg effect—which enables cancer cells to sustain high proliferation rates and survive in nutrient- and oxygen-deprived environments. This metabolic shift results in the excessive production of lactate, a metabolite increasingly recognized as more than just a waste product [14, 17, 18]. Emerging evidence highlights lactate’s role as a signaling molecule, particularly in the modulation of immune cells such as TAMs [32, 40, 43, 53]. Malignant metabolism orchestrates cancer cell properties through the production of oncometabolites such as lactate, the canonical tumor waste product [5, 12]. Tumor-produced lactate induces the polarization of TAMs towards an M2 phenotype through the GPR132 acidic-signal sensing [11, 16]. M2 macrophages play an important role in tumor progression, angiogenesis, immune escape, and metastasis [23, 43, 56].
M2 TAMs are associated with more aggressive features of the tumor, augmenting tumor invasiveness, progression, and further dissemination [44, 57]. The most common markers for M2 TAMs are CD206, CD204, and CD163 [58]. Remarkably, CD136+ is a highly specific marker for M2 macrophages [59]. In HCCs study, CD206+ macrophages are significantly associated with more aggressive tumor properties such as multiple tumor numbers and advanced tumor, node, metastasis (TNM) stage, as CD206+ macrophages are linked to poor overall survival [44]. Comparably, a study on non-functional pituitary adenomas (NFPAs) cells cultured with M2 TAMs exhibited significant invasion and proliferation compared to NFPAs cultures with M1 TAMs [57]. M2 TAM polarization was significantly associated with a bigger tumor size and an advanced TNM stage in breast cancer [60]. Similarly, triple-negative breast cancer with high Ki67 was associated with M2 polarization [56].
These findings allow us to propose M2 polarization in the TME as a prognostic factor in different types of cancer [56, 57, 60]. Understanding the dynamics of the tumor and its microenvironment can provide a new perspective in cancer diagnosis, prognosis, and treatment.
For future research, this pivotal aspect in tumor physiology can be targeted to improve treatment outcomes. Despite these advances, key challenges remain. TME is incredibly complex and variable, making it difficult to generalize TAM behavior across tumor types. Moreover, the ability of TAMs to shift between phenotypes complicates efforts to selectively target the M2 subset. Nonetheless, understanding how tumor metabolism shapes immune cell function presents an exciting frontier in cancer therapy.
The interplay between cancer metabolism and the immune landscape of the TME is central to tumor progression. M2 macrophages play a crucial role in tumor progression by promoting immune suppression, angiogenesis, and metastasis. Their polarization is driven by metabolic changes like the Warburg effect, which enhances lactate production and an immunosuppressive TME. Due to their association with poor clinical outcomes in various cancers, M2 macrophages serve as potential prognostic biomarkers and therapeutic targets. Further research should aim to elucidate the molecular mechanisms underpinning M2 polarization and identify specific inhibitors that can selectively modulate this process.
Arg-1: arginase-1
BCSCs: breast cancer stem cells
CCL22: chemokine (C-C motif) ligand 20
CD8+: cluster of differentiation 8+
COX-2: cyclooxygenase-2
CSCs: cancer stem cells
CSF-1: colony-stimulating factor-1
DCs: dendritic cells
DNMT1: DNA methyltransferase 1
ECM: extracellular matrix
EGF: epithelial growth factor
EMT: epithelial-mesenchymal transition
EZH2: enhancer of zeste homolog 2
GLUTs: glucose transporters
GPR132: G protein-coupled receptor 132
HCC: hepatocellular carcinoma
HIF-1α: hypoxia-inducible factor 1 alpha
HK2: hexokinase 2
iNOS: inducible nitric oxide synthase
LDHA: lactate dehydrogenase A
MCTs: monocarboxylate transporters
MMPs: matrix metalloproteinases
NF-ĸB: nuclear factor kappa-light-chain-enhancer of activated B cells
NFPAs: non-functional pituitary adenomas
NK: natural killer
Nrf2: nuclear factor erythroid 2-related factor 2
OXPHOS: oxidative phosphorylation
PD-L1: programmed death-ligand 1
PKM2: pyruvate kinase M2
ROS: reactive oxygen species
TAMs: tumor-associated macrophages
TANs: tumor-associated neutrophils
TCR: T-cell receptor
TGF-β: transforming growth factor beta
TLRs: toll-like receptors
TME: tumor microenvironment
TNM: tumor, node, metastasis
Tregs: T regulatory cells
VEGF: vascular endothelial growth factor
YTHDF2: YTH N6-methyladenosine RNA binding protein 2
JDMO: Conceptualization, Investigation, Writing—original draft, Writing—review & editing, Visualization. MÁPV: Writing—review & editing, Visualization. NEHG: Conceptualization, Supervision, Validation, Writing—review & editing, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
