Affiliation:
1Service de Biologie Clinique, Hôpital Foch, 92150 Suresnes, France
2UMRS-1176, Hôpital du Kremlin-Bicêtre, 94270 Le Kremlin Bicêtre, France
ORCID: https://orcid.org/0000-0003-1935-5402
Affiliation:
1Service de Biologie Clinique, Hôpital Foch, 92150 Suresnes, France
2UMRS-1176, Hôpital du Kremlin-Bicêtre, 94270 Le Kremlin Bicêtre, France
Email: m.vasse@hopital-foch.com
ORCID: https://orcid.org/0000-0002-8784-7209
Explor Immunol. 2023;3:554–564 DOI: https://doi.org/10.37349/ei.2023.00120
Received: January 19, 2023 Accepted: November 06, 2023 Published: December 05, 2023
Academic Editor: Satish Kumar Gupta, Indian Council of Medical Research, India
The article belongs to the special issue Autoantibodies Associated to Thrombosis and Hemostasis
Protein Z (PZ) is a vitamin K-dependent protein that acts as a cofactor for the inhibition of activated factor X by the PZ-dependent protease inhibitor, an anticoagulant protein of the serpin superfamily. The presence of antibodies against PZ (aPZ-Abs) was first described in women with unexplained recurrent embryo loss, pre-eclampsia, or foetal death, independently from habitual antiphospholipid/anti-cofactor antibodies. Other studies suggested that aPZ-Ab could be associated with a small birthweight for the gestational age. The mechanism of action of these antibodies is not yet understood. At this time, even aPZ-Abs are frequently observed in patients with lupus anticoagulant or anticardiolipin antibodies, there is no evidence that aPZ-Abs increase systemic venous or arterial thrombotic risk. The comparison of the various published studies shows that the threshold suggesting an obstetric risk is not clearly defined. At present, it is not known whether one isotype of immunoglobulin (G or M, or both) is particularly involved in certain obstetric manifestations, or these antibodies persist during time, or can be induced by infectious diseases. Consequently, detection of these antibodies is not routinely warranted and should only be performed in randomized clinical trials.
Protein Z (PZ) is a vitamin K-dependent protein identified in human plasma in 1984 and characterized by structural homology with other vitamin K-dependent proteins (factor VII, IX, X, and PC) [1]. It is composed of a γ-carboxyglutamic acid (Gla)-domain containing 13 Gla residues, two epidermal growth factor (EGF)-like modules, and a serine protease-like module (Figure 1A) [2]. However, it was evident that PZ cannot directly down-regulate coagulation, since the aspartate and histidine residues in the serine protease-like module are replaced by threonine and alanine, respectively [2, 3]. It is only in 1998 that a protein that inhibits factor Xa (fXa) in the presence of PZ and calcium was identified in human plasma [4]. This protein, called “PZ-dependent protease inhibitor” (ZPI) belongs to the serpin superfamily [5]. In human plasma, ZPI is in excess relative to PZ, and all PZs are complexed to ZPI [6]. PZ enhances the rate of fXa inhibition by ZPI more than 1,000-fold in the presence of procoagulant lipids and calcium [5]. Both the serine protease-like module and the second EGF-like module of PZ are crucial for the interaction between PZ and ZPI [7], and specific interaction between the Gla domains of PZ and fXa contributes to a 6-fold acceleration of the ZPI inhibition of fXa on phospholipid membranes [8, 9]. ZPI inhibits fXa in a temporary fashion based on the facile regeneration of fXa activity and concomitant formation of inactive, cleaved ZPI following the reaction (Figure 1B) [10]. Besides fXa, ZPI also inhibits fXIa in the absence of PZ [5].
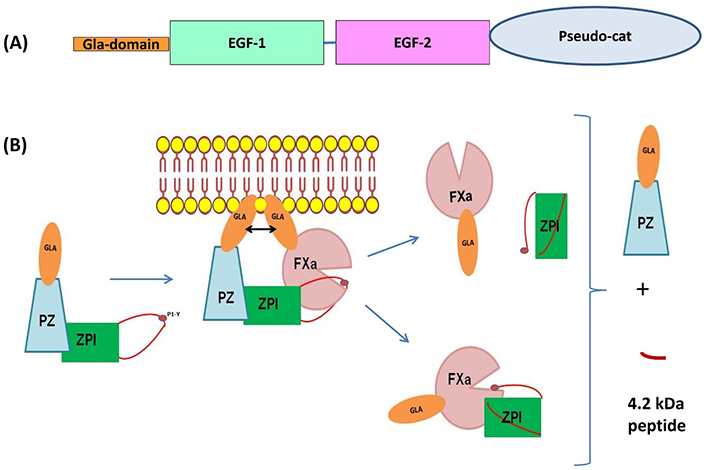
Schematic representation of the structural domains of PZ and the mechanism of fXa inhibition by the complex composed of PZ and the ZPI. (A) Organization of PZ in different domains: in orange, the Gla-domain contains 13 carboxyglutamic acid residues, two EGF domains, and a carboxyl-terminal region which is highly homologous to the catalytic domain of serine proteases (pseudo-cat). The principal sites of interaction with ZPI are located in EGF-2 and in the pseudo-cat region; (B) inhibition of fXa by the complex PZ-ZPI. After interaction of the reactive loop of ZPI with fXa on the phospholipid surface, the ternary complex dissociates, releasing free PZ, a small 4.2 kDa ZPI cleavage peptide, and either the ZPI-fXa complex or fXa and cleaved ZPI. Consequently, ZPI appears to inhibit fXa in a temporary fashion based on the facile regeneration of fXa activity
The consequences of PZ deficiency on the thrombotic risk are unclear [11]. A meta-analysis by Sofi et al. [12] concluded a significant association between low levels of PZ with arterial and venous thrombosis or pregnancy complications. It is precisely in the context of obstetrical complications that the presence of antibodies against PZ (aPZ-Abs) was first described [13].
This review will focus on aPZ-Ab, not only in obstetrical complications, but in other vascular diseases, and will discuss the future trends for understanding the interests of aPZ-Ab detection.
A possible link between obstetrical complications and PZ deficiency was described by Gris et al. [14] who reported a high frequency of PZ deficiency in women with a first primary episode of early foetal death, from the 10th to the end of the 15th week of gestation. Using a new commercially available enzyme-linked immunosorbent assay (ELISA) for the detection of aPZ-Ab [immunoglobulin G (IgG) and IgM], they observed that IgG and IgM levels were higher in 112 women with at least 3 recurrent embryo losses before the 8th week of gestation and in 41 women with one unexplained episode of foetal death from the 10th week than in a series of 191 healthy mothers [13]. IgM levels were also higher in a series of 18 women with severe pre-eclampsia, and in a series of 20 women with recurrent embryo losses associated with PZ deficiency than in the control group. A dose effect was evidenced between aPZ-Ab levels and the risk of pathologic pregnancy. The global risk of pathologic pregnancies increased with increasing levels of aPZ-Ab and became statistically significant when the levels of aPZ-Ab were superior to the 75th percentile (pctl) of the control group (IgG values > 7.1 arbitrary units (AU)/mL and IgM values > 5.3 AU/mL; Table 1).
Comparisons of the different studies analysing the signification of aPZ-Ab in women with obstetrical complications
| References | Controls (n) | Cut-off (AU/mL) | Patients | Conclusions |
|---|---|---|---|---|
| [13] | 191 healthy mothers, matched for age, number of pregnancies, and time elapsed since the end of the last pregnancy | IgG: 75th pctl: 7.1 98th pctl: 14 IgM: 75th pctl: 5.3 98th pctl: 11.9 | 112 women with unexplained primary recurrent embryo losses before the 8th week of gestation 41 women with 1 unexplained episode of foetal death from the 10th week 18 patients with 1 episode of severe pre-eclampsia | IgG levels higher in women with recurrent embryo losses and in women with foetal death. IgM levels higher in women with recurrent embryo losses, foetal death, and severe pre-eclampsia. |
| [15] | 283 age-matched multiparous non-pregnant women | IgG: 75th pctl: 9.2 95th pctl: 12.5 99th pctl: 15 IgM: 75th pctl: 4.7 95th pctl: 8.8 99th pctl: 12.3 | 265 women with medically confirmed diagnosis of recurrent spontaneous miscarriage (RSM) | Increase in RSM risk with increasing levels of anti-PZ IgM and IgG titers and increased prevalence of elevated anti-PZ (IgG and IgM) > 99th pctl. |
| [16] | 45 non-pregnant women 70 women with a normal pregnancy | IgG: 75th pctl: 5.3 90th pctl: 7.3 95th pctl: 18.2 IgM: 75th pctl: 21.1 90th pctl: 26.9 95th pctl: 36 | 123 women with pre-eclampsia 51 women with a neonate with small birthweight (SBW) 51 women with foetal death | Non-pregnant women had higher median aPZ-Ab (IgG and IgM) levels than women with a normal pregnancy. aPZ-Ab (IgG) are higher in women with a neonate with SBW than in pregnant controls. aPZ-Ab (IgG) are higher in women with a neonate with SBW than in women with pre-eclampsia or foetal death. aPZ-Ab (IgM) are more often > the 90th pctl in women with SBW and in women with pre-eclampsia when the placenta had villous infarcts or persistent muscularization of basal plate arteries. aPZ-Ab (IgM) > the 75th pctl in case of inflammation of the umbilical vessels in women with foetal death. |
| [17] | 71 normal pregnant women | IgG: 50th pctl: 2.9 IgM: 50th pctl: 12.3 | 67 women in spontaneous labour at term 138 women with an episode of spontaneous preterm labour (49 with term delivery; 35 with preterm delivery and intra-amniotic infection; 54 with preterm delivery without intra-amniotic infection) | No differences between pathological pregnancies and the controls. |
Anti-PZ-Ab (IgG and IgM) levels were not correlated with plasma PZ concentrations both in controls and patients, except in the subgroup of patients with recurrent embryo losses associated with PZ deficiency, where plasma PZ levels were inversely correlated to IgM antibody levels. Additionally, aPZ-Ab presence was not correlated with classical antiphospholipid antibodies.
Similar results were reported in the study of Sater et al. [15], who observed in a series of 265 women with RSM (defined as three or more clinically detectable pregnancy losses before 20 weeks of gestation with the same partner) compared with 283 age-matched multiparous women, a positive dose-effect relationship for IgG and IgM aPZ-Ab with increased RSM risk. Although the same assay kit was used in both studies, the cut-offs associated with an increased risk of RSM were higher than those described by Gris et al. [13] (10.8 AU/mL for IgG and 12.3 AU/mL for IgM).
Whereas in the study of Gris et al. [13], samples were collected at least 6 months after the last obstetric accident, slightly higher levels of IgG and IgM aPZ-Ab were detected in controls with at least 4 pregnancies, which cannot rule out a pregnancy effect. Erez et al. [16] compared the levels of aPZ-Ab in normal pregnant (gestational age between 20 weeks and 41 weeks) and non-pregnant women. They observed that normal pregnant women had a lower median plasma concentration of aPZ-Ab than non-pregnant women (median IgG levels were 3.2 AU/mL for normal pregnancy versus 5.2 AU/mL for non-pregnant women, and median IgM levels were 13.3 AU/mL for normal pregnancy versus 19.2 AU/mL for non-pregnant women), with a negative correlation of IgM with gestational age. These variations should be taken into consideration for the interpretation of the results. In contrast to the study of Gris et al. [13], Erez et al. [16] did not observe any correlation between aPZ-Ab (IgG or IgM) level and parity. Still contrary to the study of Gris et al. [13], they did not observe an increase in levels of aPZ-Ab in either a series of 123 women presenting with pre-eclampsia or 51 women with foetal demise. However, among women with pre-eclampsia, the proportion of patients with villous infarcts or persistent muscularization of basal plate arteries was higher in those with aPZ-Ab IgM concentration superior to the 90th pctl (26.9 AU/mL) than in those with aPZ-Ab (IgM) inferior to the 90th pctl. Similarly, among patients with foetal demise, the proportion of inflammation of the umbilical vessels was higher in patients with IgM aPZ-Ab levels above the 75th pctl (21.1 AU/mL). Lastly, they studied the presence of aPZ-Ab in a series of 51 women with small for gestational age (SGA) neonates, defined as a birthweight below the 10th pctl. The median level of aPZ-Ab from the IgG subtype was higher in this group of women than in pregnant controls, and the proportion of patients in the SGA group who had aPZ-Ab (IgG) levels superior to the 75th pctl (5.3 AU/mL) was higher than that observed in normal pregnancy.
Another study compared aPZ-Ab levels in 71 normal pregnant women, 49 women with preterm labour but term delivery, and 89 women with spontaneous preterm labour before 34 weeks of gestation, 35 women in this last group having an intraamniotic/inflammation [17]. They did not observe any significant variations of aPZ-Ab levels in the different groups. In normal pregnancy, median values and range of aPZ-Ab were 2.9 AU/mL (range: 0–500.5) for IgG and 12.3 AU/mL (range: 0–44) for IgM, showing a large distribution.
Using a homemade ELISA, Pardos-Gea et al. [18] studied the presence of aPZ-Ab in a series of 50 patients with arterial thrombosis and 64 patients with a venous thrombotic event and compared them with a control group of 82 healthy donors from the blood bank. The mean absorbance value in healthy individuals plus 2 standard deviations was indicated to correspond, by definition, to 1 AU of anti-PZ IgG or IgM antibodies, which was the cut-off level of the study. They observed a significant decrease of plasma PZ levels in the group of patients with arterial thrombosis, as well as higher levels of aPZ-Ab of IgG subtype when compared to the controls or to patients with venous thrombotic events. For aPZ-Ab of IgM subtypes, no significant differences were observed between the different groups. They also observed a moderate inverse correlation between aPZ-Ab (IgG) levels and PZ concentration in the global analysis of controls and patients, whereas no correlation was found for IgM. As the vast majority of the patients with arterial or venous thrombosis had aPZ-Ab levels below the cut-off (1 AU/mL), the authors concluded that there was no relationship between aPZ-Ab and venous or arterial thrombosis (Table 2).
Comparisons of the different studies analysing the signification of aPZ-Ab on the thrombotic risk
| References | Controls (n) | Cut-off (AU/mL) | Patients | Conclusions |
|---|---|---|---|---|
| [18] | 82 donors from the blood bank | 1 (for IgG or IgM)* | 50 with arterial thrombosis, 64 with venous thrombosis | aPZ-Ab (IgG) higher in patients with arterial thrombosis, but the vast majority < 1 AU/mL |
| [19] | 33 healthy volunteers | IgG: 75th pctl: 4.4 IgM: 75th pctl: 5.3 | 102 patients with lupus anticoagulant (LAC; 62 with thrombosis, 33 asymptomatic) | IgM > 75th pctl more frequent in the patient group. Similar frequency of elevated aPZ-Ab (IgG or IgM) in patients with and without thrombosis |
| [20] | 59 healthy volunteers | IgG: 95th pctl: 10 IgM: 95th pctl: 10 | 86 consecutive patients with anticardiolipin (aCL) antibodies | Frequency aPZ-Ab in the patient group: 40.7% The frequency of aPZ-Ab increases in patients with a double or triple positivity of antiphospholipid antibodies Higher frequency of aPZ-Ab in patients with LAC (57.7%) than in patients without LAC (25.6%) Similar frequency of elevated aPZ-Ab (IgG or IgM) in patients with and without thrombosis High frequency of aPZ-Ab in patients with foetal loss |
| [21] | 23 healthy volunteers | NR | 21 with central retinal vein occlusion | No significant difference in the frequency of aPZ-Ab between patients and cases |
*: whereas all the studies used the same commercial assay, this one used a homemade assay. NR: not-reported
Another study evaluated the frequency of aPZ-Ab and the consequences on the thrombotic risk in a series of 102 patients with persistent LAC [19]. A group of 33 healthy volunteers without any history of thrombosis served as the control group. Among the patients, LAC was identified in 69 cases in the context of the search for an aetiology to a thrombotic event, whereas it was discovered in 33 cases mainly because of the prolongation of the activated partial thromboplastin time, without thrombosis. The positivity of aPZ-Ab levels, defined as levels exceeding the 75th pctl of aPZ-Ab levels in the healthy controls, were 4.4 AU/mL for the IgG and 5.3 AU/mL for the IgM subtype. The prevalence of positive aPZ-Ab was significantly higher for IgM subtype in patients than in the control group, but not for IgG. A similar prevalence of positive aPZ-Ab was observed in the group of patients with thrombotic events or in the group of asymptomatic patients, therefore the authors concluded that these antibodies did not significantly increase the risk of thrombosis of patients with LAC. Lastly, it was underlined that elevated aPZ-Ab levels were more prevalent in women with LAC and foetal loss (52% were positive for IgG and 65% for IgM) than in those with LA and normal pregnancy (33% were positive for IgG and 53% for IgM). The difference was not statistically significant, probably because of the small number of women with pregnancy (39 cases) in their series.
Similar conclusions could be drawn from the study of Andriamandimbisoa et al. [20], who analysed the frequency of aPZ-Ab in a series of 86 consecutive patients with aCL antibodies and studied the clinical signification of these aPZ-Ab in terms of thrombosis or foetal loss. Using the only commercially available kits and a series of 59 healthy controls, they defined positive samples as being above the 95th pctl. The values of cut-offs were 10 AU/mL, for both IgG and IgM, thresholds proposed by the manufacturer. The frequency of aPZ-Ab was 40.7% in the patient group versus 6.8% in a group of volunteers (P < 0.0001). The frequency of aPZ antibodies is higher in patients with LAC (57.7%) than in patients without LAC (25.6%, P = 0.02) and significantly increased in patients with a double or triple positivity of antiphospholipids antibodies. Similar to the study by Sailer et al. [19], there were no significant differences in aPZ-Ab frequency between patients with and without thrombotic events. Interestingly, among the 8 women with recurrent foetal losses, aPZ-Abs were observed in 7 cases, strengthening the hypothesis that aPZ-Ab may be associated with obstetrical complications.
Lastly, aPZ-Abs were studied in a prospective series of 21 patients under 60 years of age with central retinal vein occlusion [21]. One patient had a positive aPZ-Ab (it is not specified the subtype IgG or IgM) versus none in the 23 controls. Cut-offs for positivity were not reported.
It is now well-established that viral, bacterial, and parasitic infections can induce antiphospholipid antibodies [22]. Therefore, some studies evaluated the presence of aPZ-Ab in patients with bacterial or viral diseases. Nien et al. [23] compared the presence of aPZ-Ab in a series of 42 pregnant women with pyelonephritis, compared to aPZ-Ab in 71 pregnant women without infection. They did not classify the patients as positive or negative for aPZ-Ab detection but only evaluated the levels of aPZ-Ab. They did not observe any significant differences between both groups.
Research on aPZ-Ab was performed in 2 children with an ischemic stroke following varicella [24]. IgM aPZ-Abs were in the grey zone (between 10 AU/mL and 20 AU/mL) for the two children, whereas IgG aPZ-Abs were below 10 AU/mL.
Over the past 3 years, it has become apparent that inappropriate immune responses contribute to the pathogenesis of severe coronavirus disease 2019 (COVID-19) [25, 26]. We evaluated the presence of aPZ-Ab at diagnosis in a series of 243 patients with proven COVID-19. IgM aPZ-Abs were more frequently present than IgG in COVID-19 patients, and the frequency significantly increased with the severity of the disease. Whereas the frequency of positive aPZ-Ab (> 10 AU/mL) in patients who were immediately discharged from the emergency department was 26.6% and 3.3%, for IgM and IgG, respectively, the frequency of these antibodies was 55.5% for IgM and 25.4% for IgG for the patient requiring intensive care unit admission (unpublished data). As for the various autoantibodies detected in COVID-19, there is not yet evidence of the contribution of these autoantibodies to disease complications or worsening.
Several hypotheses were proposed, but none of them were actually validated. Enhanced immune-complex formation associated with cellular or complement activation was suggested by Gris et al. [13], as observed with anti-β2-glycoprotein (anti-β2GP) and other antiphospholipids [27, 28]. However, to our knowledge, no study evaluating complement activation has been performed so far in women with aPZ-Ab and recurrent foetal loss. Scambi et al. [29] suggested that the activation of complement could explain, in part, the inefficiency of the classical heparin/aspirin treatment in women with obstetrical complications. In agreement with a possible activation of complement by aPZ-Ab, Gris et al. [30] described that women with PZ deficiency and/or aPZ-Ab had poorer outcomes than women with other coagulation abnormalities.
It has been described that the binding of anti-β2GP1 to β2GP1 on the trophoblast surface, leads to placenta activation and production of proinflammatory cytokines and chemokines that recruit neutrophils [31]. A similar mechanism can be considered for aPZ-Ab since PZ is largely expressed in the placenta [32]. This hypothesis is in agreement with the observation of Erez et al. [16], who described that in women with pre-eclampsia, elevated IgM aPZ-Ab antibodies were associated with vascular placental lesions (e.g., failure of transformation of basal plate arteries and villous infarcts). Additionally, in women with foetal demise, elevated aPZ-Abs were associated with inflammatory lesions of the umbilical cord (umbilical phlebitis/chronic vasculitis or umbilical arteritis). Interestingly, PZ biosynthesis was described in human umbilical veins of endothelial cells (HUVECs) [33], strengthening the hypothesis of an interaction between PZ and aPZ-Ab at the cell surface leading to cell activation. However, PZ expression was detected not only in umbilical endothelial cells but also in arteries [33] and veins [34], whereas the association between aPZ-Ab and venous or arterial thrombotic events is not evident from the few studies so far performed [18–20]. However, mechanisms leading to the activation of peripheral vessels can be different from those involved in HUVEC or placenta cells.
Furthermore, it could be of major interest to study the epitopes recognized by the aPZ-Ab. Indeed, PS is another vitamin K-dependent factor which, similar to PZ, is expressed largely on the surface of trophoblastic cells, and it was suggested that PS could protect or restore damaged villi [35]. It has been shown that in some women with recurrent foetal loss, anti-PS antibodies were directed against the EGF domains of the PS. It has been hypothesized that these anti-EGF antibodies could be involved in placental insufficiency [36] since EGF family proteins exert an anti-apoptotic effect on trophoblast cells [37]. As the PZ also presents EGF domains, it could be interesting to analyse whether the aPZ-Abs detected in women with recurrent foetal losses are also directed against the EGF domains of the PZ.
Inhibition of the activating role of PZ on ZPI can also be suggested. Indeed, PZ has no enzymatic activity but enhances the rate of fXa inhibition by ZPI more than 1,000-fold. Therefore, it can be speculated that the coating of PZ by antibodies impairs this functional complex, leading to a hypercoagulable state [13, 38]. To our knowledge, the consequences of aPZ-Ab on a biological hypercoagulability (for example, analysis of F1+2, thrombin-antithrombin complex, or d-dimers elevation) has not been yet performed, which could give clues about the effects of aPZ-Ab on the coagulation. Another problem is that there is no plasma assay to measure the activity of the PZ-ZPI complex, consequently, it is not possible to analyse whether aPZ-Ab can inhibit the fXa inhibitory activity of ZPI. Conversely, one can even imagine that the consequences of aPZ-Ab on the activity of ZPI can be different depending on the epitope of PZ involved. At this time, nothing is none concerning the epitopes recognized by aPZ-Ab. It was shown that the second EGF domain and the serine protease-like domain of PZ interact with ZPI. We previously discussed that antibodies directed against the EGF domain of PZ could inhibit a protective effect of PZ on trophoblastic cells. It can also be hypothesized that antibodies directed against these functional domains of PZ inhibit the interaction with ZPI, leading to a prothrombotic state due to a decreased inhibition of fXa. However, free ZPI is an inhibitor of fXIa, an interesting antithrombotic strategy [39]. Therefore, the loss of inhibition of fXa by the complex PZ-ZPI can be counterbalanced by the inhibition of fXIa by ZPI alone and does not necessarily lead to a prothrombotic state.
Finally, a last unresolved enigma concerns the relationship between PZ deficiency and antiphospholipid antibodies (LAC or aCL). Three independent studies reported that patients with antiphospholipids had a deficiency in PZ [40–42]. None of these studies analysed whether patients had also aPZ-Ab, which could lead to accelerated clearance of the PZ/aPZ-Ab complexes. However, the recent studies that analysed simultaneously the levels of plasma PZ and the presence of aPZ-Ab did not show any effect of aPZ-Ab on plasma PZ levels, except for a subgroup of women with recurrent foetal losses and IgM aPZ-Ab, who had a lower level of plasma PZ [11].
Only a limited number of studies evaluated aPZ-Ab and its clinical consequences, and therefore conclusions should be drawn with caution. As for studies on PZ deficiency [14, 43, 44], it seems that anti-PZ antibodies constitute a risk factor for obstetric complications, either on recurrent miscarriages, pre-eclampsia, or intrauterine growth retardation. However, the cut-off indicating an obstetrical risk is not clearly defined: the presence of aPZ-Ab was detected in all pregnant and non-pregnant women in all studies, aPZ-Ab levels were described to be higher (both IgG and IgM) in women without obstetrical complications but with at least 4 pregnancies [13]. This raises two questions: First, are aPZ-Ab really autoimmune antibodies or do they result from allo-immunisation? And second, when does the pathologic transformation of the antibodies occur? Concerning the first point, it should be interesting to analyse whether aPZ-Abs are detected in newborns. For the second question, according to Gris et al. [13], the global risk of pathologic pregnancies increased with increasing levels of aPZ-Ab, the mean variation of risk for an increase of 1 AU for IgG has an odds ratio of 1.2 (95% confidence interval 1.13–1.27) and for IgM, an odds ratio of 1.42 (95% confidence interval 1.32–1.52). Whereas all studies (except the study of Pardos-Gea et al. [18]) used the only commercially available kit to detect aPZ-Ab, the cut-offs to consider the positivity of aPZ-Ab were different, particularly for IgM and clearly deserve further investigation. In addition, it is not clear which Ig subtype is pathological: According to the study of Gris et al. [13], both isotypes are associated with obstetrical complications, whereas for Erez et al. [16] elevated aPZ-Ab of both isotypes are observed in mothers of neonates with SBW, whereas pre-eclampsia and foetal death are associated with elevated IgM aPZ-Ab. All the studies performed so far indicate that aPZ-Abs are independent of classical antiphospholipid antibodies (LAC, aCL, and anti-β2GP1 antibodies) even if they are observed in a high frequency in association with LAC or aCL antibodies and do not seem to be a major risk factor for venous or arterial thrombosis. However, the number of patients included in the studies evaluating the thrombotic risk is low and probably does not have the necessary statistical power to draw reliable conclusions and should be reconsidered in larger studies. Furthermore, as done by Tea et al. [21], research of aPZ-Ab could be interesting in patients with thrombosis in unusual locations (splanchnic, cerebral, retinal, upper-extremity, and renal), and in those whose etiologic biological diagnosis is often not yet identified. The physiopathological role of aPZ-Ab in obstetrical complications is still poorly understood and the identification of epitopes recognized by these antibodies could be of great interest. Although their persistence at a stable level over time has been observed in women with obstetric complications [13], it is not known whether the antibodies observed during infectious pathologies are persistent. Therefore, due to all the uncertainties regarding levels that may be considered pathological, detection of these antibodies is not routinely warranted and should only be performed in randomized clinical trials.
aCL: anticardiolipin
anti-β2GP: anti-β2-glycoprotein
aPZ-Abs: antibodies against protein Z
AU: arbitrary units
COVID-19: severe coronavirus disease 2019
EGF: epidermal growth factor
fXa: factor Xa
Gla: γ-carboxyglutamic acid
IgG: immunoglobulin G
LAC: lupus anticoagulant
pctl: percentile
PZ: protein Z
RSM: recurrent spontaneous miscarriage
SBW: small birthweight
ZPI: protein Z-dependent protease inhibitor
The authors thank Polly Gobin for her editorial assistance and Dr. Jean Amiral for helpful discussion.
TP and SZC: Resources, Visualization, Writing—review & editing. THA: Resources, Writing—review & editing. MV: Conceptualization, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3095
Download: 23
Times Cited: 0
Masahiro Ieko ... Akitada Ichinose
Gary W. Moore
Osamu Kumano ... Jean Amiral
Fariza A. Cheldieva ... Tatiana M. Reshetnyak
