Affiliation:
1Department of Pharmacology of Chinese Materia Medica, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
2Pharmaceutical Formulation Department, Fuan Pharmaceutical Group Chongqing Lybon Pharm-tech Co., Ltd, Chongqing 401121, China
Affiliation:
1Department of Pharmacology of Chinese Materia Medica, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Affiliation:
1Department of Pharmacology of Chinese Materia Medica, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Affiliation:
1Department of Pharmacology of Chinese Materia Medica, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Affiliation:
3State Key Laboratory of Natural Medicines, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Email: seki-nj@163.com
Affiliation:
1Department of Pharmacology of Chinese Materia Medica, School of Traditional Chinese Pharmacy, China Pharmaceutical University, Nanjing 211198, Jiangsu, China
Email: liuKcpu@163.com
ORCID: http://orcid.org/0009-0007-3456-7258
Explor Endocr Metab Dis. 2025;2:101441 DOI: https://doi.org/10.37349/eemd.2025.101441
Received: July 10, 2025 Accepted: September 22, 2025 Published: October 13, 2025
Academic Editor: Artemis P. Simopoulos, The Center for Genetics, Nutrition and Health, USA
The article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance
Aim: Baicalin and ginsenoside Rb1 show the ability to promote adipocyte browning, but their effects, especially combined treatment, and the related mechanisms under pathological conditions are less known. The study investigated the regulation of browning markers by baicalin and Rb1 under lipid overload and explored the potential implication of a serine/threonine protein kinase G protein-coupled receptor kinase 2 (GRK2).
Methods: The 3T3-L1 cells under palmitic acid (PA) stimulation and male ICR mice on a high-fat diet (HFD) challenge were used to evaluate the effects of drugs.
Results: GRK2 silencing and overexpression inversely regulated the protein abundance of PGC-1α and UCP-1 in 3T3-L1 adipocytes. Baicalin, Rb1, and their combination decreased the PA-induced elevation of GRK2 while increasing the thermogenetic markers at the protein and mRNA levels. In vivo, the tested drugs restored the expression of thermogenetic and mitochondrial biogenetic markers in the inguinal white adipose tissue (WAT) of HFD-fed mice. Consistently, the drug-treated mice displayed an improved metabolic profile. The baicalin-Rb1 combination showed a more potent effect in some examinations, and its effect was comparable to that of GRK2 inhibitor paroxetine or AMP-activated protein kinase activator metformin.
Conclusions: Baicalin and Rb1, alone or in combination, improved the browning of adipocytes during differentiation and prevented the whitening shift of WAT on an HFD, which was associated with the downregulation of GRK2. The study expands the understanding of the anti-obesity effects of baicalin and Rb1 and the potential of Scutellariae Radix-Ginseng Radix et Rhizoma compatibility for treating obesity-associated metabolic diseases.
Obesity is a global public health problem characterized by excess body fat accumulation and is tightly associated with a series of metabolic diseases, notably type 2 diabetes mellitus (T2DM), cardiovascular and cerebrovascular diseases, and certain types of cancer [1–4]. The primary cause of developing obesity is the imbalance between food intake and energy expenditure. Therefore, strict diet control and regular physical exercise are adopted to combat obesity. However, it is hard to achieve the required standards for many obese people [3].
There are three types of adipocytes. The white adipocyte is mainly responsible for energy storage and mobilization, the brown adipocyte is in charge of energy expenditure, thereby decreasing lipid accumulation in peripheral tissues and blood glucose, and the beige or brite adipocyte is located in the depots of white adipose tissue (WAT), especially inguinal WAT, but exhibits a brown-like phenotype [5]. Importantly, there is an interconversion or transdifferentiation between three phenotypes [6–10]. In response to cold or certain pharmacological agents, such as β3-adrenergic receptor agonists, white or beige adipocytes may switch to a brown-like phenotype, which is called britening or browning. In contrast, when exposed to warm or certain over-nutrition circumstances, or with aging, the brown adipose tissue (BAT) or beige adipocytes may shift towards a white-like phenotype, which is described as whitening [11–13]. These findings provide a strategy for promoting browning and preventing whitening to manage obesity and metabolic dysfunctions [8].
Some traditional Chinese medicines show potential to regulate the adipocyte functions. Scutellariae Radix and Ginseng Radix et Rhizoma have been reported to prevent adipogenesis, obesity, and related disorders [14–18]. They also applied in pairs in several famous Chinese herbal formulae, such as XiaoChaiHu Tang from Treatise on Febrile Diseases, Banxia Xiexin decoction from Shang Han Lun, Xiaoxuming decoction from Valuable Prescriptions for Emergency, and Huangqin Renshen Decoction from ShenShiFang in WaiTaiMiYao, to treat gastrointestinal and cardiovascular diseases. Baicalin and ginsenoside Rb1 (hereafter described as Rb1) are the representative active ingredients derived from Scutellariae Radix and Ginseng Radix et Rhizoma, respectively. Both of them can promote the browning of adipocytes during in-vitro differentiation or post-differentiation of normal preadipocytes [19–23]; However, the effect of their combination and their influence on adipocyte browning under pathological conditions, for example, under free fatty acids (FFA) stimulation or high-fat diet (HFD) feeding, is less understood. Accordingly, the underlying mechanism of the browning regulation by the combined treatment needs more in-depth elucidation.
G protein-coupled receptor (GPCR) kinase 2 (GRK2) is a member of the GRK family. Early studies have shown that GRK2 can bind to a series of agonist-bound GPCRs to desensitize receptor signaling transduction [24]. It is now clear that GRK2 may act as a signaling node with multiple regulatory effects on glucose uptake, insulin sensitivity, inflammation, organ fibrosis, and other pathophysiological processes [25, 26]. Vila-Bedmar R and colleagues [27] found that the expression of BAT characteristic markers was higher in the BAT and epididymal WAT from GRK2+/– mice under cold exposure compared with wild-type littermates, and GRK2+/– mice showed increased energy expenditure and decreased respiratory exchange ratio. Consistently, tamoxifen-induced GRK2 ablation (GRK2–/–) mice displayed increased thermogenetic markers uncoupling protein-1 (UCP-1) and carnitine palmitoyltransferase-1 in BAT compared to the control [28]. These findings revealed the impact of GRK2 on the regulation of browning. Based on the effects of baicalin and Rb1 on obesity-related metabolic dysfunctions and our preliminary investigation, we proposed that the regulation of GRK2 may be associated with the effects of baicalin and Rb1. Here, we selected palmitic acid (PA)-stimulated 3T3-L1 preadipocytes and the HFD-induced mouse obesity model to investigate the impacts of baicalin and Rb1 on GRK2 expression and thermogenetic markers. We observed that baicalin and Rb1, individually or in combination, facilitated the expression of thermogenesis and mitochondrial biogenesis-associated markers under lipid overload, which was partially due to the downregulation of GRK2.
Baicalin, baicalein, ginsenoside Rb1, and ginsenoside compound K (hereafter described as CK) were the products of Herbest (Baoji, China), with a content ≥ 98%. 1,1-Dimethylbiguanide hydrochloride (purity ≥ 99 %, hereafter described as metformin, used in vitro) was purchased from Haiwei Gene (Beijing, China), and Glucophage (hereafter described as metformin, used in vivo) was the product of Sino-US Shanghai Bristol-Myers Squibb (Shanghai, China). Paroxetine hydrochloride was obtained from Yuancheng Gongchuang (Wuhan, China). PA (content > 99 %) was the product of Sinopharm (Shanghai, China). Bovine serum albumin (BSA) was from Tonybio (Shanghai, China). Tribromoethanol (Avertin) was from Aibei Biotech (Nanjing, China). Negative control siRNA, GRK2-targeting siRNA, vector pEX-3 plasmids, or GRK2 overexpression pEX-3 plasmids were provided by GenePharma (Shanghai, China).
The 3T3-L1 preadipocytes with the mycoplasma contamination identification and STR identification, derived from Shanghai Cell Bank, were cultured in a complete Dulbecco’s modified Eagle’s medium (DMEM, Gibco), which contained 10% fetal bovine serum (FBS, Sbjbio, Nanjing, China), 25 mmol/L glucose, and 1% penicillin-streptomycin (Beyotime, Shanghai, China). After growing to confluence, preadipocytes were induced to differentiate with 500 μmol/L methylisobutylxanthine, 1,000 nmol/L dexamethasone, and 10 μg/mL insulin for two days, then cultured with insulin for another two days, and finally incubated in the complete DMEM for four days. The successful differentiation was verified by the oil red staining (Figure S1). During the whole period of differentiation, cells were exposed to PA (100 μmol/L) with or without baicalin, baicalein, Rb1, CK, baicalin combined with Rb1, paroxetine, or metformin. The medium was changed every other day.
Six days after the beginning of differentiation, 3T3-L1 cells were transfected with negative siRNA, GRK2 siRNA, vector pEX-3, or GRK2 pEX-3 in the presence of Lipofectamine-2000 (Thermo Fisher Scientific) for six hours according to the recommendation of GenePharma. These cells were cultured in a complete medium for one day before Western blot analysis. GRK2 siRNA: sense: 5’-GUGAAUGCAGCUGACGCUUTT-3’, antisense: 5’-AAGCGUCAGCUGCAUUCACTT-3’; Negative control: sense: 5’-UUCUCCGAACGUGUCACGUTT-3’, antisense: 5’-ACGUGACACGUUCGGAGAATT-3’.
The study was in accordance with the Guide for the Care and Use of Laboratory Animals. Male SPF-grade ICR mice (6–7 weeks old, weighing 18–24 g) were obtained from Jiangsu Huachuang Sino Pharmatech Co., Ltd. Mice were divided into seven groups: (1) normal control, fed with a low-fat diet (LFD, 10% fat) purchased from Nantong Trophic (Jiangsu, China) for eight weeks; (2) HFD (Nantong Trophic) model control, fed with a HFD (60% fat) for eight weeks; (3) drug treated groups, fed with HFD for eight weeks and orally treated with baicalin (40 mg/kg), Rb1 (20 mg/kg), baicalin (20 mg/kg) and Rb1 (10 mg/kg), paroxetine (5 mg/kg), or metformin (Glucophage, 200 mg/kg), respectively, for the last four weeks.
Mice were treated with HFD for 50 days before measurement. In the OGTT, overnight fasted mice were orally administered 2,000 mg/kg glucose, and the contents of glucose in tail-tip blood samples were measured with glucose test strips (Yuyue, Danyang, Jiangsu, China) at 0, 0.5 h, 1 h, 1.5 h, and 2 h following glucose challenge. In ITT, mice were fasted for eight hours and challenged with 0.75 U/kg insulin via subcutaneous injection.
In the eighth week of HFD feeding, overnight fasted mice were anesthetized with Avertin (i.p. injection, 0.25 g/kg) for collecting blood from the orbital sinus. The contents of glucose and FFA in the serum were detected using commercial kits (Nanjing Jiancheng, China), and blood insulin was determined using an ELISA kit, a product of Fankew, obtained from Shanghai Kexing (Shanghai, China).
After blood collection, mice were euthanized via cervical dislocation. The epididymal WAT (eWAT), inguinal WAT, and interscapular BAT were isolated. The sections from paraffin-embedded adipose tissues were used for routine H&E staining and the immunohistochemistry of UCP-1. Sections were scanned with a slide scanner (Pannoramic MIDI, 3DHISTECH Ltd., Hungary).
The RIPA lysis buffer (Fdbio Science, Hangzhou, China) was applied for protein extraction. After routine sodium dodecyl sulfate-polyacrylamide gel electrophoresis, proteins were transferred to polyvinylidene difluoride membranes (Millipore), reacted with anti-GRK2, anti-peroxisome proliferator-activated receptor-gamma coactivator 1 α (PGC-1α), anti-UCP-1, anti-p-Akt, anti-Akt, or anti-β-actin, and Horseradish peroxidase-conjugated secondary antibodies (Fdbio Science) successively. Following development in the chemiluminescence reagent (Fdbio Science), protein bands were scanned with an imaging system (Tanon, Shanghai, China) and analyzed using Image J 1.8.0 software. The primary antibodies: Phospho-Akt (S473), #4060, and Akt, #4691, cell signaling technology; GRK2, ab137666, Abcam; PGC-1α, 66369-1-Ig, Proteintech (Wuhan, China); UCP-1, DF7220, Affinity; β-actin, AP0060, Bioworld (St. Paul, MN, USA).
The TransZol (TransGen Biotech, Beijing, China) was used for the extraction of total RNA. cDNAs were synthesized using a 5× All-In-One RT Master Mix kit (TransGen Biotech) and mixed with the PerfectStart®Green qPCR Super Mix (TransGen Biotech). The qPCR was operated on a real-time PCR system (CFX96, Bio-Rad) with parameters as 94°C, 30 s, one cycle; 94°C, 5 s, and 60°C, 30 s, 44 cycles; 60°C, 60 s, one cycle; and 95°C, 15 s, one cycle. The mRNA expression was quantified by the 2–ΔΔCt method, and the β-actin acted as a reference. Table 1 lists the primer sequences.
Primer sequences.
| Species | Gene | Primer sequence (5’–3’) | Accession number |
|---|---|---|---|
| Mouse | Actb (β-actin) | F: GCAAGTGCTTCTAGGCGGAC | NM_007393.5 |
| R: AAGAAAGGGTGTAAAACGCAGC | |||
| Mouse | Grk2 (GRK2) | F: GCGCCAGCAAGAAGATCCT | NM_001289480.1 |
| R: GCAGAAGTCCCGGAAAAGCA | |||
| Mouse | Ucp1 (UCP-1) | F: AGGCTTCCAGTACCATTAGGT | NM_009463.3 |
| R: CTGAGTGAGGCAAAGCTGATTT | |||
| Mouse | Nrf1 (NRF1) | F: TCTGCTGTGGCTGATGGAGAGG | NM_001164230.2 |
| R: GATGCTTGCGTCGTCTGGATGG | |||
| Mouse | Cox4i1 (COX-IV) | F: GTACCGCATCCAGTTTAACGA | NM_053094.2 |
| R: CCATACACATAGCTCTTCTCCCA | |||
| Mouse | Ppara (PPARα) | F: AGAGCCCCATCTGTCCTCTC | NM_001113418.2 |
| R: ACTGGTAGTCTGCAAAACCAAA | |||
| Mouse | Cidea (CIDEA) | F: TGCTCTTCTGTATCGCCCAGT | NM_001285731.1 |
| R: GCCGTGTTAAGGAATCTGCTG | |||
| Mouse | Prdm16 (PRDM16) | F: CCACCAGCGAGGACTTCAC | NM_001177332.2 |
| R: GGAGGACTCTCGTAGCTCGAA | |||
| Mouse | Ebf2 (EBF2) | F: GCGGTTCCAGGTCGTGTTGTC | NM_001113197.2 |
| R: GCCTTCTTGCTCTCCTTCCATGC | |||
| Mouse | Tbx1 (TBX1) | F: CACCAAGGCAGGCAGACGAATG | NM_011537.3 |
| R: CATCTACGGGCACAAAGTCCATGAG | |||
| Mouse | Ppargc1a (PGC-1α) | F: TATGGAGTGACATAGAGTGTGCT | NM_008904.2 |
| R: CCACTTCAATCCACCCAGAAAG |
GRK2: G protein-coupled receptor kinase 2; UCP-1: uncoupling protein-1; NRF1: nuclear respiratory factor 1; COX-IV: cytochrome c oxidase IV; PPARα: peroxisome proliferators-activated receptor α; CIDEA: cell death inducing DFFA like effector A; PRDM16: PR domain-containing 16; EBF2: early B cell factor 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α.
Data are expressed as mean ± SD and analyzed with GraphPad Prism 8.01 (GraphPad Software, Boston, MA, U.S.A.). The statistical difference between the two groups was analyzed using an unpaired t-test or an unpaired t-test with Welch’s correction after the normality analysis using the Shapiro-Wilk test and the variance analysis using the F test. For the comparison between multiple groups, the normality and variances were assessed using the Shapiro-Wilk test and Brown-Forsythe test, respectively, and the statistical difference was analyzed using one-way analysis of variance (ANOVA) coupled with Tukey’s post hoc test or the Kruskal-Wallis test coupled with Dunnett’s test. The P-value less than 0.05 is considered statistically significant.
In the preliminary experiment, we have demonstrated that the classic cocktails can induce the differentiation of 3T3-L1 cells successfully, as evidenced by oil red staining (Figure S1). Under this premise, we first explored whether GRK2 suppressed the expression of marker proteins of brown and/or brite adipocytes in differentiated 3T3-L1 cells. The protein abundance analysis showed that GRK2 silencing lowered the protein level of GRK2 and enhanced that of PGC-1α, UCP-1, and Akt phosphorylation at residue S473 under PA treatment (Figure 1A–E), even though PA attenuated the protein levels of PGC-1α and UCP-1 slightly. In contrast, GRK2 overexpression exhibited a contrary effect on the protein expression (Figure 1F–J). This result, coupled with other effects of GRK2 found in obesity, insulin resistance, and adipose tissue browning by other researchers, indicates that GRK2 might serve as a potent regulatory target for adipocyte browning or whitening.
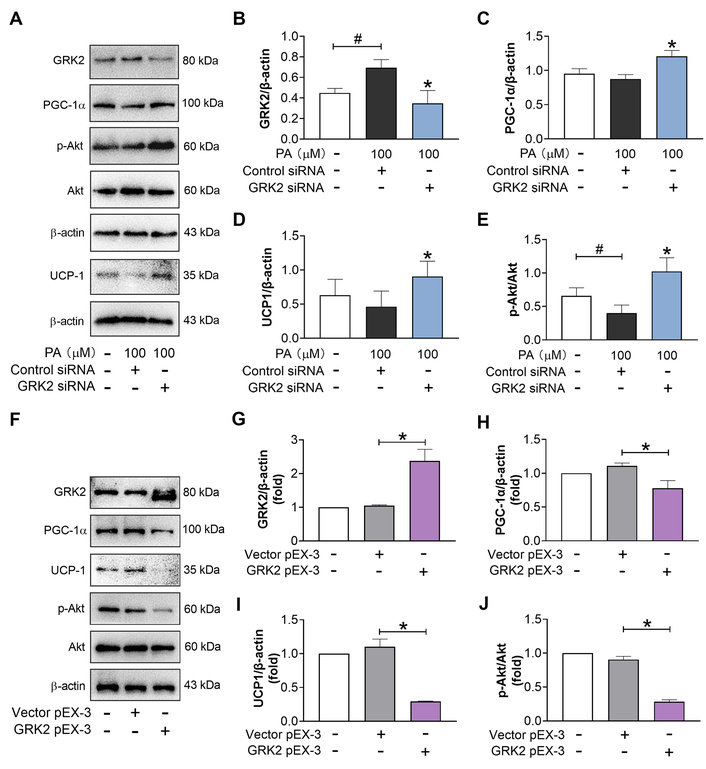
GRK2 prevents the protein expression of browning markers during the late period of differentiation of 3T3-L1 cells. (A–E): Effect of GRK2 silencing (n = 5). (F–J): Effect of GRK2 overexpression (n = 3). #: P < 0.05 compared with blank; *: P < 0.05 compared with model (PA + Control siRNA) or vector pEX-3. GRK2: G protein-coupled receptor kinase 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α; UCP-1: uncoupling protein-1; PA: palmitic acid.
Next, the influence of baicalin and Rb1 on the browning regulation was investigated. Based on previous studies, 0–100 μmol/L Rb1 and 10–50 μmol/L baicalin did not change the viability of 3T3-L1 adipocytes but promoted the browning process under physiological conditions [22, 23, 29]. Because the blood concentrations of both baicalin and Rb1 after oral administration are low, we selected 10 μmol/L for the study. These drugs, individually or in combination, were incubated with 3T3-L1 cells during the whole period of differentiation. Because there is an interconversion between baicalin and baicalein in vivo, and Rb1 can metabolize into a rare ginsenoside CK, both baicalein and CK were also included in the screening experiment. Compared with non-differentiated cells, standard differentiation with insulin, dexamethasone, and methylisobutylxanthine induced increased expression of GRK2, PGC-1α, and UCP-1, although the elevation was not always significant (Figure 2A–H). As described above, PA elevated the abundance of GRK2 while not changing that of PGC-1α and UCP-1. Paroxetine, a GRK2 inhibitor, lowered the GRK2 expression and increased that of PGC-1α and UCP-1 (Figure 2A–H), further indicating the negative role of GRK2 in adipocyte browning. Baicalin, baicalein, Rb1, and CK, at a dose of 10 μmol/L, prevented the upregulation of GRK2 and facilitated the induction of PGC-1α and UCP-1 to varying degrees under PA stimulation (Figure 2A–H), suggesting that they had the potential to regulate the browning program. In addition, the combination of baicalin and Rb1 also regulated the expression of these three proteins in a dose-dependent fashion (Figure 2I–L). The effect of the highest dose (5 μmol/L baicalin + 5 μmol/L Rb1) was comparable to that of metformin (500 μmol/L). Together, these data indicate that baicalin, Rb1, and their combination can boost the browning trend during the white adipocyte differentiation, which is related to GRK2 regulation.
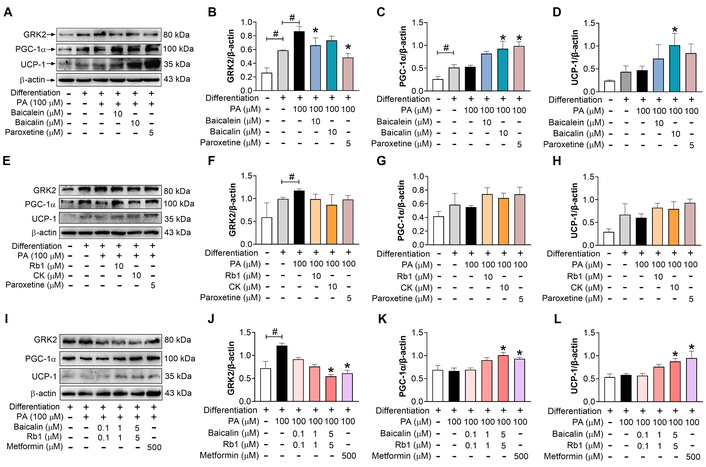
Baicalin, Rb1, and their metabolites downregulate GRK2 expression and improve the browning of 3T3-L1 cells under PA treatment. Cells were exposed to PA and different compounds during the whole period of differentiation. (A–D): Effects of baicalin and baicalein (n = 3). (E–H): Effects of Rb1 and CK (n = 3). (I–L): Effect of baicalin-Rb1 combination (n = 5). #: P < 0.05 compared between two indicated groups; *: P < 0.05 compared with model (differentiation-PA). GRK2: G protein-coupled receptor kinase 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α; UCP-1: uncoupling protein-1; PA: palmitic acid; CK: compound K.
It is reasonable to confirm the browning-facilitating impact of baicalin and Rb1, individually or in combination, and to compare their effects under an equal concentration of 10 μmol/L. The Western blot measurement showed that, under PA stimulation, 10 μmol/L of baicalin, Rb1, and baicalin-Rb1 combination had a comparable effect on the protein expression of GRK2 and p-Akt (Figure 3A, B, E). For the protein abundance of PGC-1α and UCP-1, the influence of combined treatment, as well as baicalin, seems more potent (Figure 3A, C, D). At the level of relative mRNA expression, PA decreased the mRNA expression for the thermogenesis-related markers, including PGC-1α, UCP-1, the cell death inducing DFFA like effector A (CIDEA), and cytochrome c oxidase IV (COX-IV) (Figure 3G–J), which was reversed to some extent by the tested drugs, paroxetine, and metformin. All these drugs blocked the increase in the gene expression of GRK2 (Figure 3F). These results demonstrated that baicalin, Rb1, and their combination might facilitate or restore the browning possibility during adipocyte differentiation in the presence of PA.
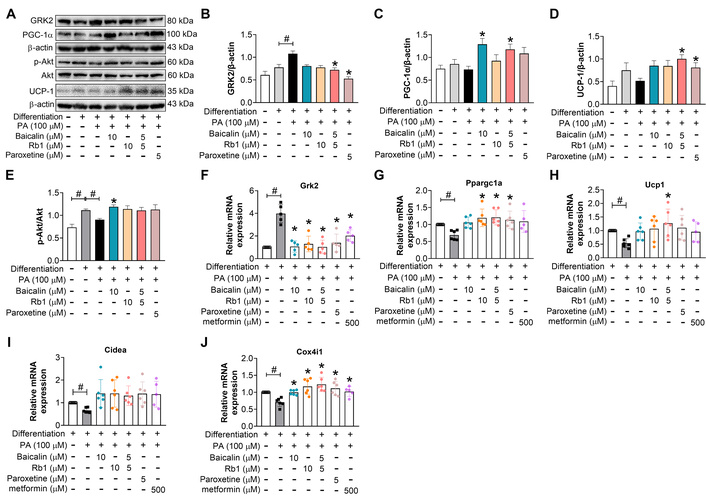
Baicalin, Rb1, and their combination facilitate the browning trend during 3T3-L1 cell differentiation under PA-loading conditions. (A–E): The protein expressions (n = 5). (F–J): The gene expressions (n = 5). #: P < 0.05 compared with differentiation-blank; *: P < 0.05 compared with model (differentiation-PA). GRK2: G protein-coupled receptor kinase 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α; UCP-1: uncoupling protein-1; PA: palmitic acid.
Then, the effects of these drugs were explored in vivo. Baicalin, Rb1, and combined treatment ameliorated the HFD-induced metabolic disorders, as evidenced by decreased body weight, blood glucose, insulin, homeostatic model assessment of insulin resistance (HOMA-IR), FFA, and improved blood glucose in OGTT and ITT (Figure 4A–G). In addition, compared with the subcutaneous WAT (sWAT) and BAT from model mice, which turned white in comparison to the normal group, those from the mice receiving treatments showed a more brown color (Figure 4H). These compounds also decreased the adiposity indexes of epididymal WAT (eWAT) and sWAT (Figure 4I). The combined treatment showed a more significant effect, which was similar to that of metformin and paroxetine (Figure 4I). We did not evaluate the BAT index, because the loss and whitening of BAT make it difficult to work out the exact weight of BAT. These data indicate that baicalin and Rb1 can prevent HFD-induced whitening of inguinal WAT and interscapular BAT.
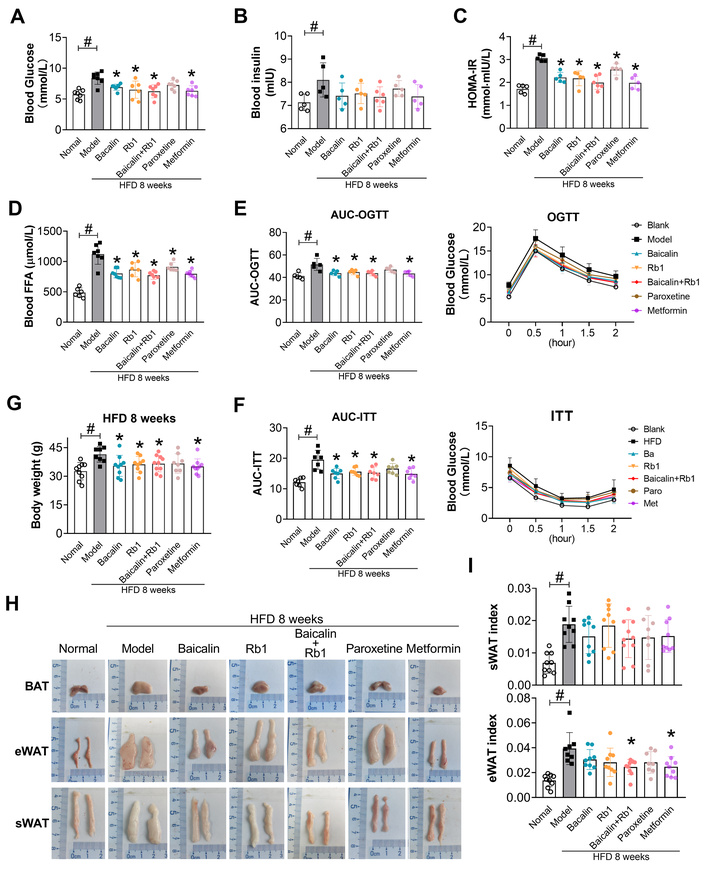
Baicalin and Rb1 improve the metabolic profile in HFD-fed mice. (A): Fasted blood glucose (n = 7). (B): Fasted blood insulin (n = 5–6). (C): HOMA-IR (n = 5–6). (D): Fasted blood FFA (n = 6–7). (E): OGTT (n = 5). (F): ITT (n = 7). (G): Body weight gain after eight-week HFD (n = 8–10). (H): Gross appearance of interscapular BAT, eWAT, and inguinal sWAT. (I): sWAT, and eWAT index (n = 8–9). #: P < 0.05 compared with normal (LFD feeding); *: P < 0.05 compared with model. HFD: high-fat diet; HOMA-IR: homeostatic model assessment of insulin resistance; OGTT: oral glucose tolerance test; ITT: insulin tolerance test; BAT: brown adipose tissue; eWAT: epididymal white adipose tissue; sWAT: subcutaneous white adipose tissue; FFA: free fatty acids; LFD: low-fat diet.
We further investigated the impacts of baicalin and Rb1 on the thermogenesis-related proteins and genes in inguinal WAT. The histological analysis showed more large adipocytes with weaker UCP-1 staining in the BAT and sWAT from model mice compared to LFD-fed mice (normal) (Figure 5A), which corresponded to upregulation of GRK2 and downregulation of PGC-1α, UCP-1, and p-Akt measured by Western blot (Figure 5B–E). Consistently, the sWAT from model mice exhibited elevated GRK2 mRNA and decreased mRNA expression for brite adipocyte-specific marker TBX1 and the key thermogenetic and mitochondrial biogenetic proteins, including early B cell factor 2 (EBF2), PR domain-containing 16 (PRDM16), CIDEA, PGC-1α, nuclear respiratory factor 1 (NRF1), COX-IV, UCP-1, and peroxisome proliferators-activated receptor α (PPARα) (Figure 6), demonstrating that HFD feeding induced obvious whitening of inguinal sWAT. Paroxetine lowered the expression of GRK2 and abrogated the negative impact of HFD on p-Akt, PGC-1α, and UCP-1 at the protein level (Figure 5), and restored the mRNA expressions of thermogenesis-related genes, especially Tbx1, Ebf2, Prdm16, Ppargc1a, Ucp1, and Ppara (Figure 6), further demonstrating the negative influence of GRK2 on the shift to browning. Baicalin, Rb1, and their combination counteracted all these HFD-induced alterations in the expressions of protein and mRNA to a certain degree. For the measured markers, the combination of baicalin-Rb1 showed a more significant effect, followed by baicalin, and lastly Rb1.
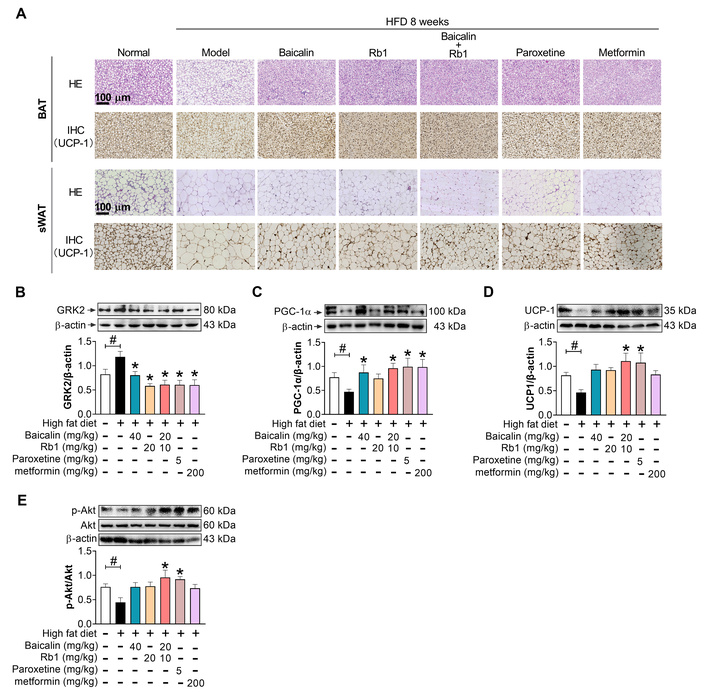
Baicalin and Rb1 differently regulate the protein expression of GRK2 and browning regulators in adipose tissues. (A): H&E staining and IHC of UCP-1 in BAT and sWAT. Scale bar: 100 μm. (B–E): Protein level of GRK2, PGC-1α, UCP-1, and p-Akt in inguinal sWAT (n = 5). #: P < 0.05 compared with normal (LFD feeding); *: P < 0.05 compared with model. HFD: high-fat diet; BAT: brown adipose tissue; UCP-1: uncoupling protein-1; sWAT: subcutaneous white adipose tissue; GRK2: G protein-coupled receptor kinase 2; PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α; LFD: low-fat diet.
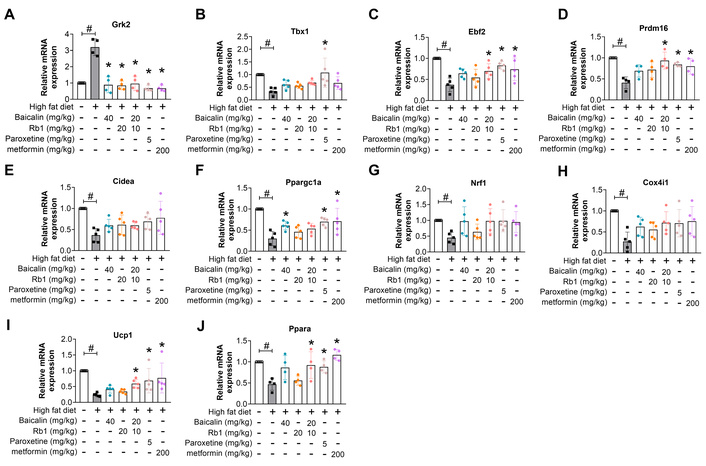
Baicalin and Rb1 regulate GRK2 and thermogenesis-related genes in inguinal sWAT. (A): Grk2. (B): Tbx1. (C): Ebf2. (D): Prdm16. (E): Cidea. (F): Ppargc1a. (G): Nrf1. (H): Cox4i1. (I): Ucp1. (J): Ppara. #: P < 0.05 compared with normal (LFD feeding); *: P < 0.05 compared with model, n = 4–5. GRK2: G protein-coupled receptor kinase 2; sWAT: subcutaneous white adipose tissue.
Promoting the browning of WAT is considered a potential strategy to combat obesity and metabolic dysfunctions. We showed that baicalin and Rb1 upregulated thermogenesis-associated markers during adipocyte differentiation under PA stimulation and blocked the whitening shift of inguinal WAT upon HFD challenge, which was partially attributed to suppressing the expression of GRK2. In addition, the baicalin-Rb1 combination exhibited a more potent impact, indicating the beneficial compatibility of bioactive ingredients from Scutellariae Radix and Ginseng Radix et Rhizoma.
The beige adipocyte attracts growing interest from researchers. Multiple lines of evidence reveal that there are three sources of beige adipocytes: de novo adipogenesis of brite adipocytes, transdifferentiation of mature white or brown adipocytes, and proliferation of mature brite adipocytes [2, 30]. In the in vitro study, we used a cellular model of 3T3-L1 preadipocytes. These cells are induced to differentiate into mature white adipocytes with classic cocktails containing methylisobutylxanthine, dexamethasone, and insulin. Previous studies showed that the cocktails exhibit basal browning potential, evidenced by increased expression of the β3-adrenergic receptor and UCP-1, and elevated citrate synthase activity during differentiation [31, 32]. In this context, we applied this method to 3T3-L1 cells together with PA stimulation during the period of differentiation to establish a lipid overload environment, as elevated blood FFA is a characteristic of HFD-induced obesity.
We found that PA showed little effect on the protein abundance of PGC-1α and UCP-1. However, it markedly inhibited the mRNA expression of the tested thermogenetic genes, including Ppargc1a and Ucp1. Both baicalin and Rb1, particularly their combination, increased the protein level of PGC-1α, UCP-1, and p-Akt. PGC-1α is a master regulator for browning, UCP-1 is responsible for dissipating heat, and p-Akt transfers insulin signaling to upregulate PPARγ, which is a transcription factor that can bind to a series of enhancers of BAT-specific genes to facilitate the transcription of corresponding genes [9, 13, 32, 33]. This finding indicates the potential of tested drugs to induce adipocyte browning in the presence of PA.
In the in vivo study, we establish the HFD-induced obesity model in the ICR strain of mice. Although the ICR strain is genetically variable, which might be more similar to humans, several studies revealed that it can also respond to HFD. For example, Jinglei Li et al. [34] have compared the 10-week HFD (53% fat)-induced alterations in four strains of mouse, including C57BL/6 and ICR strains. The results showed that ICR mice developed obesity and insulin resistance in response to HFD feeding, as evidenced by increased body weight, adipose tissue and liver indexes, and serum lipid profile. HFD-fed ICR mice displayed significant glucose and insulin intolerance (reflected by OGTT and ITT) and abnormal histological features of liver and adipose tissue. In our lab, we have successfully reproduced the HFD (60% fat)-induced obesity and insulin resistance model in different batches of the ICR strain. These mice show inflammatory and fibrotic changes in visceral adipose tissues [35]. After 14-week HFD feeding, the liver sections of ICR mice showed obvious lipid droplets inside the hepatocytes (Figure S2). Elevated serum lipid profiles and inflammatory cytokines (data not shown) were observed in these obese mice. Thus, the above studies suggest that HFD can successfully induce obesity and insulin resistance in the ICR strain.
Different from the PA, HFD feeding remarkably lowered the protein abundance of PGC-1α and UCP-1 and mRNA expression pattern for multiple thermogenetic and mitochondrial biogenetic proteins, among which TBX1 is an accepted beige-specific marker; EBF2 and PRDM16 are the master transcription factors regulating the brown adipocyte differentiation; CIDEA serves as a marker of brown adipocytes; NRF1, PGC-1α, and COX-IV are closely related to mitochondrial biogenesis, and PPARα is fatty acid oxidation-related and it also participates the upregulation of UCP-1 [7, 9, 36]. We thought that the more significant influence of HFD should be due to the overall and complex alterations occurring in vivo.
In several traditional Chinese herbal formulae, the ratio of Scutellariae Radix and Ginseng Radix et Rhizoma is 1:1. Theoretically, the doses of baicalin and Rb1 should be designed according to this proportion. However, the average content of baicalin in Scutellariae Radix is nearly 9% while that of Rb1 in Ginseng Radix et Rhizoma is about 0.2% according to the Chinese Pharmacopoeia (Version 2020). Due to the low content and bioavailability of Rb1, we did not use the ratio-based doses. Our results showed that 40 mg/kg baicalin, 20 mg/kg Rb1, and 20 mg/kg baicalin-10 mg/kg Rb1 combination exhibited beneficial effects on PGC-1α, UCP-1, p-Akt, and a series of thermogenesis-related genes, which was in line with their impact on the metabolic profile including body weight gain, adipose tissue indexes, HOMA-IR, and glucose and insulin tolerance. Importantly, we found that the combined treatment still exhibited a more potent effect than individual treatment in vivo, indicating the significance of Chinese medicine compatibility, especially for the treatment of obesity and associated metabolic dysfunctions.
A few studies have reported the mechanisms involved in the regulation of the browning process by baicalin or Rb1, including the activation of AMP-activated protein kinase (AMPK) and PPARγ, as well as the inhibition of Wnt/β-Catenin signaling [20, 21, 23, 29]. We focused on GRK2 because it can regulate mitochondrial function and adipocyte browning, and is implicated in the development of obesity, insulin resistance, T2DM, and other metabolic disorders [24–28, 37–41]. We observed that GRK2 overexpression decreased while its silencing increased PGC-1α and UCP-1 in 3T3-L1 adipocytes, and GRK2 inhibitor paroxetine positively regulated thermogenesis-related markers. This finding is consistent with the reports showing increased BAT activation under physiological conditions or HFD challenge in GRK2+/– and inducible GRK2–/– mice [27, 28]. These findings, together with the reported impact of GRK2 on insulin resistance and other dysfunctions, especially the new role of GRK-biased adrenergic agonists in combating obesity and T2DM [42], strengthen the potential of GRK2 serving as a target for treating metabolic diseases.
The Western blot and qPCR analysis demonstrated the ability of baicalin, Rb1, and their combination to inhibit GRK2 expression under lipid overload, indicating the involvement of GRK2 in the browning regulation by these drugs. Although we did not evaluate the AMPK status in the experiment, the GRK2-AMPK interaction might be involved. In our study, metformin suppressed the GRK2 expression in vitro and in vivo. In turn, the BAT from GRK2+/– mice had an elevated level of p-AMPK and p-acetyl-CoA carboxylase (ACC) upon cold exposure [27]. In a follicle-stimulating hormone-stimulated gluconeogenesis study, Qi and coworkers [43] also showed that GRK2 induced the AMPK hyperphosphorylation at Ser485 and dephosphorylation at Thr172 residue, thus decreasing the AMPK activity. In addition, GRK2 regulated PPARγ and glycogen synthase kinase-3 (GSK3) via Akt because GRK2 can interact with Akt and suppress insulin-induced Akt phosphorylation [44, 45]. GSK3 can attenuate the mRNA expression of several thermogenetic genes and UCP-1 protein abundance in response to isoproterenol [46], and this effect was abolished by GSK3 phosphorylation by Akt. These reports further confirm the regulatory role of GRK2 in adipocyte browning, and it might be a target hub implicated in the effects of baicalin and Rb1 on browning or whitening.
In conclusion, baicalin, Rb1, and their combination can decrease GRK2 while increasing or restoring the protein levels of PGC-1α, UCP-1, and p-Akt. They also regulate the expression of genes associated with thermogenesis and mitochondrial function in both PA-stimulated adipocytes and HFD-fed mice. This effect matches their ability to improve systemic metabolic dysfunction in vivo. These results demonstrate the potential of the drugs to regulate adipocyte browning and whitening in response to lipid overload. The impacts of GRK2 silencing, overexpression, and the GRK2 inhibitor paroxetine revealed that these drugs may regulate browning and whitening via GRK2 downregulation. The combination treatment was more effective than solo treatments, which may partly explain the importance of the Scutellariae Radix-Ginseng Radix et Rhizoma pair.
However, this study still has some limitations. We did not study the browning program under the induction by classic agents, such as thyroid hormone T3, rosiglitazone, or β3-receptor agonist. The measurements of thermogenetic effects or mitochondrial functions are still insufficient. These issues require further research, along with ingredient dose optimization, safety of combined treatment, and the influence of baicalin and Rb1 on crosstalk between GRK2 and some key browning regulators. Moreover, it remains unclear if the ingredient combination can regulate browning under hypermetabolic conditions such as cachexia, which is also worth exploring.
ACC: acetyl-CoA carboxylase
AMPK: AMP-activated protein kinase
BAT: brown adipose tissue
CIDEA: cell death inducing DFFA like effector A
CK: compound K
COX-IV: cytochrome c oxidase IV
DMEM: Dulbecco’s modified Eagle’s medium
EBF2: early B cell factor 2
eWAT: epididymal white adipose tissue
FFA: free fatty acids
GPCR: G protein-coupled receptor
GRK2: G protein-coupled receptor kinase 2
GSK3: glycogen synthase kinase-3
HFD: high-fat diet
HOMA-IR: homeostatic model assessment of insulin resistance
ITT: insulin tolerance test
LFD: low-fat diet
NRF1: nuclear respiratory factor 1
OGTT: oral glucose tolerance test
PA: palmitic acid
PGC-1α: peroxisome proliferator-activated receptor-gamma coactivator 1 α
PPARα: peroxisome proliferators-activated receptor α
PPARγ: peroxisome proliferators-activated receptor γ
PRDM16: PR domain-containing 16
sWAT: subcutaneous white adipose tissue
T2DM: type 2 diabetes mellitus
UCP-1: uncoupling protein-1
WAT: white adipose tissue
The supplementary figures for this article are available at: https://www.explorationpub.com/uploads/Article/file/101441_sup_1.pdf.
Zhuqi W: Investigation, Formal analysis, Visualization, Writing—original draft. JL: Investigation, Validation. Ziqing W and HZ: Investigation. XS: Conceptualization, Supervision, Writing—review & editing. KL: Conceptualization, Supervision, Visualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The animal protocol was approved by the Ethics Committee of China Pharmaceutical University (registration code: 2022-08-006).
Not applicable.
Not applicable.
Datasets are available on request.
The work was supported by the National Natural Science Foundation of China (Grant No. [82174009]). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1674
Download: 103
Times Cited: 0
Bingwu Xu ... Yong Zhang
Pirangi Srikanth ... Sukhendu Nandi
Gabrielle St-Arnaud ... Vincenzo Di Marzo
Jerzy W Kolaczynski, Eva Surmacz
Michael Natalizio ... Vikrant Rai
