Affiliation:
1Independent Consultant, Philadelphia, PA 19147, USA
Email: koljerry@yahoo.com
ORCID: https://orcid.org/0009-0001-5671-5924
Affiliation:
2Allysta Pharmaceuticals Incorporated, Belmont, CA 94002, USA
ORCID: https://orcid.org/0000-0002-2285-7529
Explor Endocr Metab Dis. 2025;2:101449 DOI: https://doi.org/10.37349/eemd.2025.101449
Received: August 08, 2025 Accepted: November 10, 2025 Published: November 14, 2025
Academic Editor: Harold Lebovitz, State University of New York Downstate Medical Center, USA
The article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance
In this Commentary, we highlight the following issues concerning the development of increasingly more powerful incretin-based therapeutics (IBT) that to date have not been addressed with the attention they may deserve: 1. The appropriateness of BMI-based inclusion criteria for a drug capable of producing weight loss approaching that seen after bariatric surgery; 2. significant limitations inherent in communicating the results of an obesity trial involving a potent IBT; 3. the one-size-fits-all dosing strategies in trials may introduce new challenges for sponsors in the race to develop increasingly powerful IBT; 4. the currently imposed limitations on what can be communicated in the approved IBT product label create an advantageously unlevel playing field for opportunists such as compounding pharmacies. Proposals on how to address these issues are made in the text. While it is realized that the presented topics and solutions are not without controversy, they are intended to provoke further discussion.
This Commentary provides a personal perspective on the information that appears to be missing or not explicitly stated in published clinical trials involving increasingly more potent incretin-based therapeutics (IBT) for the treatment of obesity. Here, the term ‘incretin-based therapeutics’ (IBT) is used broadly to include combination or hybrid molecules whose mechanism of action is not limited to one mediated by specific incretin receptors. In the Commentary, a number of recently published clinical trials are referenced. The selection shown in Table 1 intends to illustrate the points made; it should not be construed as an issue confined solely to trials in that list.
The selection of therapeutics and trials discussed in this Commentary.
| Therapeutic | Study description | Ref. |
|---|---|---|
| CagriSema: a combination of cagrilintide (a long-acting, human amylin analogue) and semaglutide [a glucagon-like peptide-1 (GLP-1) receptor agonist] | REDEFINE-1: a 68-week efficacy and safety trial investigating CagriSema (a fixed dose combination of cagrilintide 2.4 mg and semaglutide 2.4 mg) compared to the individual components cagrilintide 2.4 mg, semaglutide 2.4 mg, and placebo, all administered s.c. once-weekly in 3,417 randomized adults with obesity or overweight (BMI of 27 kg/m2), NCT05567796.Primary end point: percentage of weight loss at week 68 with CagriSema versus placebo. | [3] |
| Retatrutide: an agonist of the glucose-dependent insulinotropic poly-peptide, glucagon-like peptide 1, and glucagon receptors | A 48-week phase 2 study in 338 adults with overweight or obesity (BMI 27 kg/m2) randomized to weekly s.c. retatrutide (1 mg, 4 mg, 8 mg, or 12 mg or placebo), NCT04881760.Primary end point: Percentage change in body weight from baseline to 24 weeks. | [4] |
| Semaglutide: a GLP-1 receptor agonist | SELECT: a cardiovascular (CV) outcome trial in 17,604 adults 45 years or older, with an initial BMI of 27 kg/m2 and established CV disease, randomized 1:1 to weekly s.c. semaglutide 2.4 mg or placebo, NCT03574597.Primary end point: a composite of death from cardiovascular causes, nonfatal myocardial infarction, or nonfatal stroke in a time-to-first-event analysis. | [5] |
| High-dose semaglutide | STEP-UP: a 72-week efficacy and safety phase 3 trial investigating subcutaneous semaglutide 7.2 mg compared to semaglutide 2.4 mg and placebo, all administered once weekly in 1,407 randomized adults with obesity (BMI 30 kg/m2), NCT 05646706.Primary end point: percentage of weight loss at week 72 with semaglutide 7.2 mg versus placebo. | [10] |
| Tirzepatide: a glucose-dependent insulinotropic polypeptide (GIP) and GLP-1 receptor agonist | SURMOUNT-1: a phase 3 trial, in 2,539 overweight or obese adults randomized in a 1:1:1:1 ratio to receive once-weekly, subcutaneous tirzepatide (5 mg, 10 mg, or 15 mg) or placebo for 72 weeks, NCT04184622.Coprimary end points: percentage change in weight from baseline and a weight reduction of 5% or more. | [9] |
A few years ago, enticed by promising proof-of-concept clinical trial results with a new generation of highly potent IBT, major pharmaceutical companies began to show optimism about offering convenient medical treatments capable of achieving weight loss comparable to that seen after bariatric surgery [1]. This goal is currently being actively pursued and seems close to realization; however, it also introduces a number of challenges, some of which are discussed below in a Q&A format.
Question 1. Suppose the implicit objective of the trials is to achieve treatment results comparable to those achieved with bariatric surgery. Why are these trials not conducted in people in whom bariatric surgery is typically a serious alternative treatment option?
Despite discussions to lower the BMI criteria, bariatric surgery continues to be primarily performed in individuals with severe (BMI > 40 kg/m2, class 3) or class 2 obesity (BMI 35–39.9 kg/m2) if associated with obesity-associated severe comorbidity [2]. In contrast, in a typical clinical trial with a highly potent IBT, the lower limit of the qualifying BMI range is 30 kg/m2 or 27 kg/m2, if obesity-related comorbidities are present [3–6]. Such broad BMI selection criteria are in line with regulatory requirements, e.g., recently reiterated by the FDA in the updated draft guidance [7]. With such BMI inclusion criteria, however, the emerging problem to address (yet missing in regulatory guidelines) is the risk of overtreatment by reaching a BMI falling into the underweight category and the resulting signs and symptoms of sarcopenia. Interestingly, to date, the published data offer insufficient insight into how widespread this has already been, let alone presenting such data as adverse events of special interest, subject to mandatory reporting, akin to hypoglycemia in glucose-lowering therapy trials. Notably, in the REDEFINE-1 trial, investigators were permitted to adjust dosing when patients reached the lower limit of the normal BMI range (18.5 kg/m2) or exhibited related health concerns (in reference [3] and its supplementary materials). Consequently, only ~74.7% of participants in the CagriSema arm escalated to the full dose, and only ~57.4% reported completing the trial at that dose. Nevertheless, by the end of 68 weeks of the trial, 2.9% of participants in the CagriSema arm who were overweight at baseline ended up in the underweight category (BMI < 18.5 kg/m2), as referenced from [3] supplementary materials. Anonymous testimonials [8] from participants in ongoing trials of Eli Lilly’s next-generation obesity drug indicated that, in response to a feeling of losing too much weight, patients started making various self-made adjustments to the dosing regimen or switching to calorie-dense foods. With the provided rationale and the emerging signals from trials with IBT, it appears reasonable to reopen the discussion on the regulatory path for developing this class of therapeutics that limits the risk of overtreatment. A starting point for such a discussion could be the pros and cons of limiting study enrollment to participants who would otherwise have qualified for and benefited from bariatric surgery. Moreover, in the scope of the discussions should be patient-centric issues heard first-hand from participants of trials involving a powerful IBT who had experienced an extreme weight loss with the resulting coping strategies and psychological burden, i.e., topics that are unrepresented in the standard quality-of-life questionnaires.
Question 2. Is the competition to show a larger average weight loss a reasonable battle?
The existing regulatory guidelines recommend that the weight-reduction drug’s efficacy be assessed using the mean percentage change in body weight as the primary endpoint [7]. Waterfall graphs, like those shown in Figures 1 and 3, showing the percentage of individual total weight loss, are now commonly published for key trials involving an IBT (as part of the core paper [9] or the supplementary materials [3, 4, 6]). They clearly show that average weight loss is hardly representative of the broad spectrum of responses observed. In other words, using the average percentage of weight loss to publicly communicate the extent of clinically meaningful benefit for any given patient to whom the therapy is prescribed may be misleading. This brings us to caution about the fallacy of averages. What follows is the need for a more meaningful public disclosure of the study results. One alternative is discussed below.
Question 3. Can the analogy from Continuous Glucose Monitoring (CGM) reporting be drawn to show BMI ranges achieved on treatment?
Although the published waterfall graphs allow for discerning the magnitude of individual weight loss, they do not indicate the individual BMIs achieved at the end of treatment, i.e., whether it is still in the obese range, has reached a normal/healthy BMI, or has fallen into an underweight category. This can be presented as a ‘temperature’ graph akin to one commonly used to report time ranges for CGM values. A nice attempt to present BMI data this way was shown in one of the publications from the SELECT trial [9] and, more recently, in the supplementary materials accompanying the published results from the REDEFINE-1 trial [3]. In SELECT, the average weight loss achieved in the semaglutide 2.4 mg arm was 10.2%. Even with such moderate average weight loss, as shown in Figure 1, there was a profound shift in BMI categories, with more patients ending up in the overweight category and some emerging with BMI categorized as healthy. The graph would have been complete if the percentage of patients in the underweight BMI range had been added (even if it is zero) as a separate category and color-coded accordingly. This is important because 12% reaching a BMI of less than 25 kg/m2 out of over seven thousand is a large number [9]. In REDEFINE-1, six participants were reported to have reached the underweight category [3] during the study, despite active precautions to avoid crossing the lower boundary of the normal BMI range. The limitation of showing shifts in BMI categories is that they do not adequately inform about the magnitude of the actual BMI decline. For example, a shift from the overweight (defined at baseline as BMI 27–29.9 kg/m2) to the normal BMI category (BMI 18.5–24.9 kg/m2) could indicate a drop anywhere from 2.1 to 11.4 kg/m2.
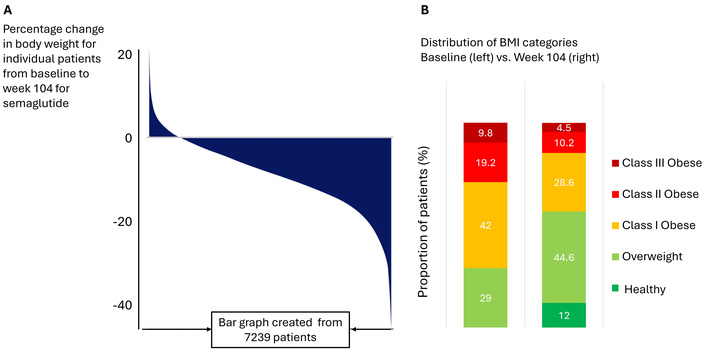
Individual and by BMI category weight loss in the SELECT trial. A replica of a waterfall (panel A) showing individual percentage change in body weight from the baseline and stacked (‘temperature’; panel B) bar graphs showing changes in weight categories in the study population between baseline and week 104 of treatment with semaglutide; here, the category ‘healthy’ was defined as BMI < 25 kg/m2. Adapted from [9]. © The Author(s) 2024. Licensed under a CC-BY 4.0.
Given the great informative value of waterfall and ‘temperature’ graphs, a combination approach could be considered, whereby waterfall graphs are split by BMI category at baseline and superimposed on the temperature graph, as proposed in Figure 2. The Figure illustrates how data on individual weight changes from baseline to the end of treatment are mapped to starting and ending BMI values and BMI category, which, in the view of these authors, could be of great value for understanding the actual individual outcomes. It would also make sense to create such data by sponsors for the monitoring purposes of a trial with a highly potent IBT to ascertain patients’ safety in real time. In this case, individual BMI changes could be monitored at each study visit and scrutinized against pre-defined safety margins.
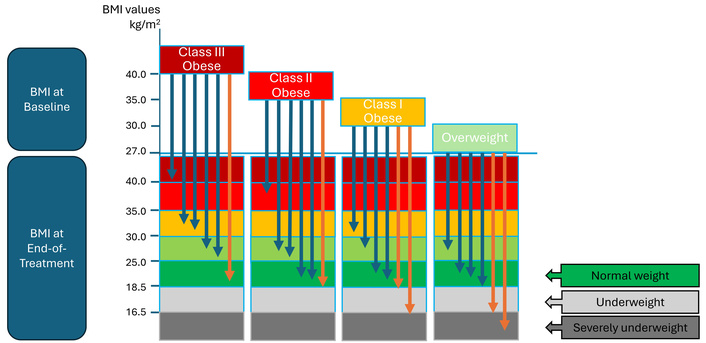
A hypothetical graph showing individual (arrows) magnitudes of BMI changes superimposed on the observed shifts in BMI categories in a trial with a highly potent incretin-based therapeutic. In this case, the individual changes shown are between baseline and the end of treatment. Blue arrows show individuals in whom the observed magnitude of weight loss is considered safe; orange arrows indicate individuals in whom the observed weight loss is considered excessive. Such data can also be generated for each study visit, which could help assess both the absolute magnitude and the rate of the observed weight loss. The definitions of BMI categories can be adjusted for ethnicity.
Now, let us examine similar data from a trial in which the weight loss achieved was, to the best of the authors’ knowledge, the highest ever reported in a large randomized, placebo-controlled trial with a highly effective antiobesity incretin receptor agonist (in reference [4] and its supplementary materials). In this trial, at 48 weeks, the mean percentage change in the retatrutide groups exposed to the highest dose of 12 mg s.c. weekly approached 25%, and the corresponding waterfall graph (Figure 3) is shown below. The dashed line on the graph shows the approximate position of the average 25% weight loss achieved in this trial. It shows that, to achieve such an average in the highest-dose cohort (62; 54 completers), nearly half had to achieve weight loss > 25%. Since the trial enrolled patients with a BMI of 27 kg/m2 or higher, the question remains which weight category the participants landed in at the end of treatment. No such data has been made available to the public.
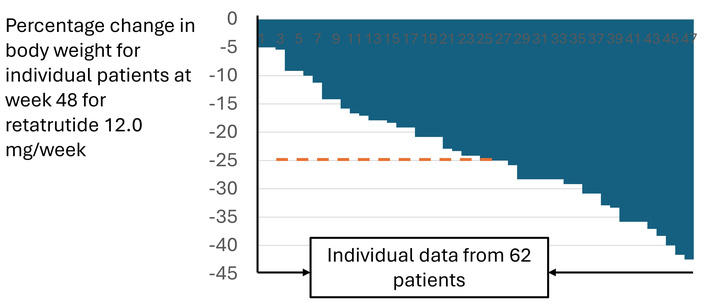
A waterfall graph from the phase 2 trial with retatrutide showing the individual percentage of the weight loss at week 48 of treatment at the target dose of 12 mg per week. Adapted with permission from [4] supplementary materials. © 2023 Massachusetts Medical Society.
What follows is that when dealing with powerful IBT such as tirzepatide [6], retatrutide [3], CagriSema [3], and high-dose semaglutide [10], the emerging question to address is the proportion of patients who ended up being overtreated, i.e., those who have lost too much weight as assessed by BMI (actual or at risk of) falling into the underweight category. In the view of these authors, such information should be recorded as adverse events of special interest subject to mandatory reporting akin to hypoglycemia (or time below range using the CGM metric) in glucose-lowering therapy trials. In addition, significant and/or rapid falls in BMI below defined safety thresholds should prompt a pre-specified (i.e., described in the study protocol) assessment for nutritional deficiencies, lean body mass, and functional status, and, if needed, pre-specified interventions. Without such measures described in the study protocols and replicated in the ensuing publications, the pharma study sponsors risk falling victim to a race-to-the-bottom.
Question 4. What is race-to-the-bottom?
It is essential to recognize that randomized, placebo-controlled, double-blind clinical trials are not typically designed as treat-to-target studies. Instead, they follow a one-size-fits-all approach to dose selection due to regulatory requirements. With the use of powerful IBT, such a trial design becomes increasingly risky as the variability in individual responses persists. As a result, achieving the average target—such as 25% weight loss—may come at the cost of an increased proportion of participants being overtreated. Sponsors of such trials may begin to see this as a new challenge, with the emerging realization that competing in this space increasingly resembles a ‘race-to-the-bottom’ and its consequences. The term has been used in different contexts, but here it is defined as a causal chain in which pharmaceutical companies attempt to outcompete each other by leveraging top-line study results and the assumption that a greater average clinical effect is always better. This often involves selecting drug doses that, given the current regulatory requirement to include participants with broad BMI ranges, for some individuals, may exceed the desired effect by a significant margin. Such a race may lead to unintended consequences, including erosion of the company’s public image and valuation. Eventually, the companies may realize they would rather avoid this type of competition, but they often decide they have no choice but to stay in it.
On the other hand, if compromises are made to the one-size-fits-all strategy, they may happen at a cost. For example, in the REDEFINE-1 trial (Table 1), despite a one-size-fits-all design, dose flexibility was allowed [3]. Consequently, the trial started to resemble a treat-to-target trial with the emergence of the known phenomenon of titration paradox whereby those who ended up the trial on CagriSema at lower than the target dose (average 1.1 mg/week) achieved better average weight loss at 68 weeks compared to participants who had stayed on the target dose of 2.4 mg/week [3].
Question 5. Is the race-to-the bottom unknowingly facilitated by regulatory agencies?
Clinical trial experience—summarized by the FDA mantra “what you study is what you get”—directly dictates which doses, their timing, and delivery devices receive approval, often limiting the ability to individualize treatment.
The gap between FDA-approved prescribing information and the clinical need for individualized therapy has created a space that compounding pharmacies have eagerly filled. Paradoxically, compounding pharmacies do not need to worry about liability for off-label use because the individualized formulations and dosing of approved drug substances (e.g., semaglutide) are de facto off-label by default rather than exception. In this regard, the semaglutide [11] and terzipatide [12] brands indicated for the treatment of obesity are not equally vulnerable, as the latter offers a broader range of approved doses and provides flexibility in delivery options—including single-dose pens and vials. Taken together, there are grounds to allege regulatory agencies such as FDA for unknowingly facilitating the off-label use of the approved drug ingredients such as semaglutide and thus creating conditions an unlevel playing ground between compounding pharmacies and drug substances patent holders (big pharma) thus leaving the game not refereed adequately with potential negative consequences on the quality of the pharmacovigilance, and ultimately the public safety. The steps recently undertaken and communicated by the FDA are acknowledged [13], as are the existing legal boundaries that restrict the agency from taking more decisive action. The simple solution, however, that the agency could have taken instead, would have been revised labeling allowing unrestricted maintenance dosing for the approved brands of IBT.
In this Commentary, we highlight the following issues concerning the development of increasingly powerful IBT: 1. The appropriateness of BMI-based inclusion criteria for a drug capable of producing weight loss approaching one comparable to that seen after bariatric surgery; it is proposed that the existing regulatory guidelines may need to be reopen to allow additional discussion on regulatory path for developing this class of therapeutics that limits the risk of overtreatment. 2. Significant deficiencies in communicating the results of an obesity trial involving a potent IBT; it is proposed that, in communicating the trial results, more emphasis needs to be placed on showing individualized responses that include both individual shifts in BMI categories and the actual magnitude of BMI reduction. 3. The race to develop even more potent IBT with one-size-fits-all dosing strategies in trials may introduce new challenges for sponsors, but if compromises are made to this strategy, they may happen at a cost, indicating that designing a trial for a potent IBT that satisfies regulatory requirements and addresses real-world clinical situations is inherently challenging. 4. The limitations on what can be communicated based on the approved IBT product label, and consequently, applied in the clinic, create an advantageously unlevel playing field for opportunists such as compounding pharmacies; it is proposed that the most straightforward countermeasure could be a revised labeling allowing for unrestricted maintenance dosing for the approved brands of IBT. While the topics presented here are not without controversy, they are intended to provoke further discussion.
CGM: Continuous Glucose Monitoring
IBT: incretin-based therapeutics
The views and opinions presented in the paper represent solely the authors’ own, and do not constitute or knowingly make a reference to, any of the proprietary information or trade secrets.
JWK: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing. ES: Conceptualization, Validation, Writing—original draft. Both authors read and approved the submitted version.
Jerzy W Kolaczynski is a former employee of Novo Nordisk (retired November 2023) and the current holder of the company’s restricted stock (NVO). Eva Surmacz is affiliated with Allysta Pharmaceuticals Incorporated. The data and publication in this article are not subject to any restrictions. There are no other conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The datasets that support the findings of this study are available from the corresponding author upon reasonable request.
The authors received no financial support for the research, authorship, and publication of this article.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2012
Download: 107
Times Cited: 0
Bingwu Xu ... Yong Zhang
Pirangi Srikanth ... Sukhendu Nandi
Gabrielle St-Arnaud ... Vincenzo Di Marzo
Zhuqi Wang ... Kang Liu
Michael Natalizio ... Vikrant Rai
