Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Email: vrai@westernu.edu
ORCID: https://orcid.org/0000-0001-6286-2341
Explor Endocr Metab Dis. 2025;2:101450 DOI: https://doi.org/10.37349/eemd.2025.101450
Received: August 20, 2025 Accepted: October 12, 2025 Published: November 24, 2025
Academic Editor: Nikolaos Papanas, Democritus University of Thrace, Greece
The article belongs to the special issue Regulators of Glucose Homeostasis, Lipid Metabolism and Energy Balance
Psychiatric medication is vital in the treatment of a wide range of mental and behavioral health conditions, but has moderate metabolic consequences. The common side effects are weight gain, dyslipidemia, increased adiposity, elevated body mass index, increased insulin resistance, and metabolic alterations. Metabolic risk is lower with antidepressants than with antipsychotics. The side effects are linked to the metabolic syndrome, increasing the risk of heart disease, stroke, and type 2 diabetes. Cardiovascular diseases, dysglycemia and diabetes, atherogenic dyslipidemia, and metabolic syndrome are common complications associated with the use of antipsychotics. Therefore, it is essential to comprehend the metabolic alterations and develop strategies for early detection and intervention to mitigate these effects. This review discusses the metabolic alterations associated with common antipsychotic medications, followed by strategies to attenuate the effects.
Psychiatric medication is vital in the treatment of a wide range of mental and behavioral health conditions, but many come with moderate metabolic consequences. Second-generation antipsychotics (SGAs), in particular, clozapine, olanzapine, and quetiapine, are strongly linked to substantial weight gain, dyslipidemia, increased adiposity, elevated body mass index (BMI), metabolic alterations, and increased insulin resistance [1–3]. Selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine and sertraline are also associated with increased appetite, weight gain, and glycemic disturbance, especially with comorbid cardiovascular disease (CVD) [3]. These effects are mediated by well-characterized biological mechanisms, including liver and mitochondrial dysfunction, inflammation, receptor dysfunction, and alterations in the microbiome, which this review will explore. In addition to the effects of the medications themselves, there is a greater risk of developing metabolic illnesses in psychiatric patients [4, 5]. As such, there is a well-established bidirectional relationship between diabetes and depression [6, 7]. While some studies suggest that these effects are mediated by shared risk factors like BMI and lipid abnormalities, others indicate a possible causal relationship due to the underlying biological mechanisms of the diseases that are not fully understood [8].
When considering the metabolic effects of these medications, it is important to recognize the potential pre-existing interplay between metabolism and psychiatric illness. Currently, depression is becoming more recognized as a risk factor for coronary artery disease (CAD), with prospective studies showing a contribution to the development and progression of atherosclerosis [9]. Additionally, dyslipidemia is consistently observed in mood disorders and schizophrenia, regardless of medication use/regimen, which may indicate inherent physiological links between psychiatric disease and metabolic dysfunction [10]. It is also important to note that these patterns are not limited to individuals with severe mental illness (SMI), but it is also predominant in outpatient psychiatric patients with less severe illness [11]. However, the effects may be more pronounced in SMI, with reported figures showing about 70% of deaths in SMI patients come from physical illness, most commonly cardiovascular conditions [12, 13].
Given the complexity of these interactions, there is growing interest in identifying early indicators of medication-induced metabolic dysfunction in psychiatric populations [14–16]. Traditional markers such as BMI and fasting glucose fail to detect subclinical minor metabolic disturbances that would indicate early medication-induced dysfunction [8, 17]. Consequently, the biomarker approach has gained more traction. For example, some studies show no significant elevation of C-reactive protein (CRP) when controlling for medication type and lifestyle factors [15], whereas others show elevated CRP in SSRI- and serotonin-norepinephrine reuptake inhibitor (SNRI)-resistant depression [16]. Mechanistically, adipocytokines, namely adiponectin and leptin, and their role in the Janus kinase/signal transducers and activators of transcription (JAK/STAT), phosphoinositide 3-kinase/protein kinase B (PI3K/Akt), and mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase (ERK) pathways are gaining increased recognition for their involvement in neuropsychiatric states [17]. While the exact mechanisms are still uncertain, leptin seems to have some neuroprotective actions involving the JAK2/STAT3/peroxisome proliferator-activated receptor gamma coactivator (PGC)-1 or PI3K/Akt/mammalian target of rapamycin (mTOR) pathways [18]. These findings add nuance and variability to the landscape of neuropsychiatric biomarkers, indicating potential overlapping pathways between mood regulation and metabolic signaling [14].
This review synthesizes current literature on the metabolic consequences of a broad spectrum of psychiatric medications and the role of biomarkers as a method for diagnosis and monitoring, by connecting drug class effects, comorbidity patterns, and emerging treatments.
Metabolic side effects of psychiatric medications, especially SGAs, create challenges in the management of psychiatric illnesses, which warrant continued research on these topics. There is clear evidence that SGAs have the most severe metabolic effects, likely due to their action on a variety of different pathways, including mitochondrial dysfunction, inflammatory signaling, receptor pathways, and peripheral tissue dysfunction [2, 18, 19]. Corresponding mechanistic studies have strengthened the evidence of this relationship, showing dose-dependent mitochondrial impairment, oxidative stress, and inflammatory mediator upregulation in liver, adipose, and skeletal muscle [20–22]. SGAs may induce dysbiosis and reduce short-chain fatty acid (SCFA) production, causing increased intestinal permeability, insulin resistance, and systemic inflammation [7, 23]. SSRIs are generally considered to be of lower risk compared to other classes of antidepressants; however, side effects can cause low-grade inflammation, weight gain, and glucose dysregulation, highlighting the need to study the metabolic consequences across different classes of medications [17, 24] (Table 1 and Figure 1).
Potential benefits and metabolic risks associated with selective serotonin reuptake inhibitors (SSRIs) and second-generation antipsychotics (SGAs) [2, 7, 17–24].
| Medication/Class | Potential benefits | Metabolic risk |
|---|---|---|
| SSRIs | Metabolic risk is lower with antidepressants than with antipsychotics. Acute SSRI administration improves glucose-dependent insulin release. LDL levels decrease while HDL levels increase. | Chronic use induces mild to moderate mitochondrial dysfunction and is associated with persistent elevation in CRP, IL-6, and TNF-α.Contribute to insulin resistance, leptin resistance, and weight gain.Increase type 2 diabetes risk. |
| Clozapine, olanzapine (SGAs) | Clozapine: Superior efficacy in treatment-resistant schizophrenia, reduces risk of suicide and tardive dyskinesia, decreases the rate of relapse, and improves the quality of life.Olanzapine: May help with treatment-resistant depression and is less likely to cause tardive dyskinesia. | Greatest antagonism to H1 and 5-HT2C.Greatest risk of weight gain, dyslipidemia, and glucose dysregulation among SGAs.Induce inflammation and ROS generation.Reduce hepatic mitochondrial complex activity.Induce gut dysbiosis. |
| Quetiapine (SGA) | In CNS, they suppress TNF-α and nitric oxide production in microglia. | Intermediate mitochondrial toxicity.Marked increase in serum CRP.Doubles MPO activity.Reduced hepatic mitochondrial activity (less than clozapine and olanzapine). |
| Risperidone (SGA) | Lower H1 and 5-HT2C affinity corresponds to modest weight gain. | Reduces complex I activity and inhibits ATP production.Increases depolarization of mitochondrial membrane and accumulation of ROS (milder than clozapine and olanzapine). |
| Aripiprazole (SGA) | Minimal activity at H1 and 5-HT2C.Most metabolically favorable among SGAs.Safest profile due to minimal impacts on mitochondrial function and appetite. | Increased risk of weight gain, hyperglycemia, and diabetes.Associated with increased levels of cholesterol and triglycerides. |
LDL: low-density lipoprotein; HDL: high-density lipoprotein; CRP: C-reactive protein; IL-6: interleukin-6; TNF-α: tumor necrosis factor alpha; 5-HT2C: 5-hydroxytryptamine 2C; ROS: reactive oxygen species; CNS: central nervous system; MPO: myeloperoxidase.
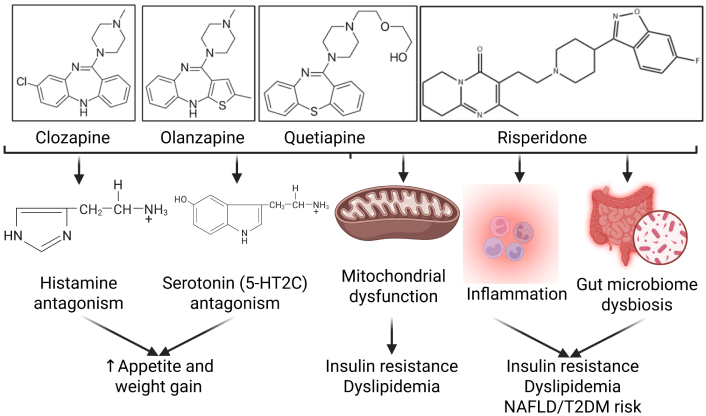
Mechanisms of metabolic dysfunction associated with antipsychotics. Antipsychotics contribute to weight gain, increased appetite, insulin resistance, dyslipidemia, and increased risk for non-alcoholic fatty liver disease (NAFLD) and type 2 diabetes mellitus (T2DM). The chemical structures for clozapine (PubChem Identifier: CID 135398737), olanzapine (PubChem Identifier: CID 135398745), quetiapine (PubChem Identifier: CID 5002), and risperidone (PubChem Identifier: CID 5073) were drawn based on the images from PubChem. The chemical structures for histamine and serotonin were used from BioRender. 5-HT2C: 5-hydroxytryptamine 2C. Created in BioRender. Rai, V. (2025) https://BioRender.com/a02nqtn.
Moreover, mitochondrial activity disruption and impaired ATP synthesis can be caused by psychiatric medications, which have been linked to antipsychotic treatment [25, 26]. These effects contribute to fatigue, insulin resistance, and diminished aerobic capacity, reinforcing the mechanistic correlation between antipsychotics and metabolic dysfunction [26, 27] (Figure 1).
Type 2 diabetes is characterized as a metabolic disorder due to relative insulin deficiency that leads to states of hyperglycemia. It was found that with prolonged usage of antipsychotic and antidepressant medications, there was an increase in the risk of developing and worsening type 2 diabetes [3]. Both first-generation antipsychotics and SGAs, such as amisulpride and ziprasidone, induce hyperglycemic states due to impairing glucose regulation and increasing metabolic syndrome risk [28]. Antipsychotic medications have been found to impair glucose metabolism and clearance through antagonistic effects on H1 and 5-hydroxytryptamine 2A (5-HT2A), which normally both play roles in glycogen synthesis and glucose uptake, especially in skeletal muscle cells [29]. Additionally, antipsychotics were found to increase levels of free fatty acids, which, in excess amounts, can lead to insulin receptor degradation and inactivation along with impaired ability for insulin to reduce glucose production [30]. Consequently, through both of these mechanisms, patients with chronic usage of antipsychotic medications are at higher risk of worsening and developing type 2 diabetes.
SSRIs, on the other hand, are one of the most commonly prescribed antidepressant medications, and these medications alter metabolic pathways that also increase the risk for developing diabetes. One study found that with SSRI usage, there is activation of insulin substrate receptor 2 kinases that eventually lead to phosphorylation of selective inhibitory serine sites that lead to insulin inhibition [31]. SSRIs have also been found to disrupt the hypothalamic-pituitary-adrenal axis, cause weight gain, which can factor into exacerbating type 2 diabetes, and contribute to insulin resistance with chronic usage [32]. Additionally, with the antidepressant sertraline, this drug has exhibited promotion of beta cell injury and apoptosis, creating an insufficient insulin response and leading to hyperglycemic conditions [32]. There is also evidence that SSRIs block the activity of the Na/serotonin symporter, which then leads to increased glycogenolysis and a depletion of glycogen storage [33]. Both of these mechanisms demonstrate how chronic usage of SSRI antidepressants can increase a patient’s risk of developing type 2 diabetes and worsen symptoms of diabetes.
With the use of psychiatric medications, it is important to understand the importance of biomarkers that are present and how they may change with prolonged usage of those medications. For example, the severity of some psychiatric disorders can be correlated with RNA biomarkers [34]. Additionally, alterations in levels of excitatory glutamate neurons and inhibitory gamma-aminobutyric acid (GABA) interneurons have been seen in patients with depression [35]. With antidepressant usage, certain biomarker levels, such as interferon (IFN), depended on which drug was being used. With venlafaxine, a significant decrease in IFN was observed, whereas with paroxetine, the levels increased. With sertraline, the levels of IFNγ did not drastically change with treatment [36]. Another biomarker that has been found to change following antidepressant usage is lipid levels. Low-density lipoprotein (LDL) levels decrease while the high-density lipoprotein (HDL) levels increase [37]. Overall, although biomarkers can help provide insight into the severity of diseases and metabolic function after treatment usage, they still require further research to determine a more solidified causative and definitive relationship.
Many studies demonstrate that psychiatric medications contribute to the development and progression of obesity, metabolic alterations, hypertension, CVD, and metabolic syndrome [1, 7, 12]. As discussed previously, these medications have varying severities of metabolic side effects, dependent upon the underlying mechanisms rooted in mitochondrial dysfunction, inflammation, neurotransmitter receptor antagonism, and endocrine disruption [3, 19, 24, 38, 39]. This section will synthesize the clinical evidence of the metabolic risks associated with common psychiatric medications, antipsychotics, and SSRIs.
Obesity is one of the most prevalent and significant metabolic side effects of psychiatric medications, especially SGAs [2, 40]. For example, one meta-analysis of 100 randomized controlled trials (RCTs) compared the metabolic side effects of antipsychotics and found that clozapine and olanzapine induced the greatest weight gain, with average increases of more than 4 kg in 12 weeks relative to placebo groups. Meanwhile, quetiapine caused a more modest 2.1 kg of weight gain, with other SGAs showing little or no weight gain [1]. These findings align with epidemiological studies, which have shown high rates of obesity in psychiatric patients, with 40–70% of schizophrenia patients and 20–30% of bipolar patients having obesity [40].
SSRIs have also been shown to contribute to obesity through less direct mechanisms than SGAs [16]. Studies have shown that long-term SSRI treatment causes weight gain through chronic inflammation and hormonal dysregulation [16]. These effects were noted with chronic use, in particular, with some modest weight gain in short-term use. Additional effects can also be attributed to patient diets and lack of physical activity, which contribute to their weight gain. Further research should be conducted to determine the potential mechanisms underlying this phenomenon or whether these results are merely due to common risk factors.
CVD is the leading cause of death in patients with schizophrenia, bipolar disorder, and major depressive disorder, and has been shown to cause 30–50% of the excess mortality and 17–22% of life years in patients with SMI [12, 41]. This strongly emphasizes the importance of selecting the safest possible treatment regimen in these populations. As with other metabolic effects, SGAs have been shown to elevate the risk of hyperlipidemia and CVD more than other psychiatric medications, though SSRIs also pose a risk [1]. As with obesity, SSRIs have the greatest risk of causing cardiovascular events with chronic use [15, 16]. Additionally, as mentioned earlier, the disruption of the gut microbiome is a key piece to be aware of, as it can lead to an increase in pro-inflammatory and pro-atherogenic metabolites such as trimethylamine-N-oxide (TMAO) and lipopolysaccharides (LPSs) [42]. With elevated levels of these metabolites, there is a higher risk of developing cardiovascular inflammation and eventually CVD. Elevated TMAO levels have been consistently seen with higher mortality rates with CVD [42].
The metabolic side effects associated with psychiatric medications are multifaceted, involving mitochondrial dysfunction, chronic inflammation, oxidative stress, neurotransmitter receptor interactions, and changes in gut microbiota [2, 7, 17, 21, 38, 39]. These mechanisms vary across pharmacological classes but appear to contribute to the development of obesity, insulin resistance, dyslipidemia, and CVD [1–3, 12, 39].
Mitochondrial dysfunction has consistently been shown as an essential mechanism in metabolic side effects of psychiatric medications, namely SSRIs and antipsychotics [2, 16, 20, 22, 24, 43]. One study compared mitochondrial function in patient-derived fibroblasts exposed to multiple SGAs, including clozapine, olanzapine, risperidone, quetiapine, and aripiprazole. Their findings showed that clozapine and olanzapine produced the most significant effects on mitochondrial dysfunction. This resulted in decreased oxygen consumption, faster ATP depletion, and an increase in reactive oxygen species (ROS) production. Quetiapine exhibited intermediate mitochondrial toxicity, whereas aripiprazole and risperidone produced the mildest effects [2]. The findings of mitochondrial disruption align well with mechanistic studies, which help contextualize the dysfunction. Madireddy and Madireddy [39] showed that dysfunction at oxidative phosphorylation complexes I and III leads to accumulation of superoxide radicals, which causes downstream lipid peroxidation, mitochondrial DNA (mtDNA) damage, and impaired mitochondrial membrane potential.
The contribution of ROS to mitochondrial dysfunction is especially significant in quetiapine, which is activated by myeloperoxidase (MPO) in inflammatory cells. This occurs via the conversion of quetiapine into quinone-imine and semiquinone radicals, which damage mitochondria, elevate ROS levels, and induce apoptosis [22]. This toxic mitochondrial effect is amplified under conditions of systemic inflammation, which elevates MPO levels, as shown in broader studies linking oxidative stress and mitochondrial dysfunction to metabolic disease [17, 39]. Clinically, patients with quetiapine overdose exhibited markedly elevated serum MPO and CRP levels correlating with more severe presentations, including tachycardia and sedation [22]. This likely contributes to less severe scenarios contributing to cumulative oxidative mitochondrial injury in patients taking medication more longitudinally, especially in patients with elevated baseline inflammation [17].
Risperidone has also been shown to impair mitochondrial function specifically by reducing complex I activity and inhibiting ATP production in peripheral blood monocytes, but these effects are markedly less severe than clozapine and olanzapine [43]. There has also been some evidence that risperidone increases depolarization of the mitochondrial membrane and accumulation of ROS in lymphocytes with glutathione depletion, which implies that oxidative stress is seen even in lower-risk antipsychotics.
Regarding SSRIs, chronic use has been shown to induce mild to moderate mitochondrial dysfunction characterized by ROS generation, membrane potential disturbance, and decreased electron transport chain (ETC) activity [16, 24]. This is likely caused by serotonin’s modulation of mitochondrial calcium flux and oxidative phosphorylation in peripheral tissues [16]. While these effects are less severe than those of SGAs, these results indicate they may pose relevant metabolic risks, particularly in long-term users.
Low-grade inflammation is both a consequence of mitochondrial dysfunction and an active driver of metabolic disturbances associated with psychiatric medications [17, 39]. Mitochondrial dysfunction increases ROS production, which functions as damage-associated molecular patterns (DAMPs) that activate inflammatory signaling pathways [21]. This causes elevated pro-inflammatory cytokines, including interleukin (IL)-6, tumor necrosis factor (TNF)-α, and CRP, further impairing mitochondrial function. This, therefore, creates a pathological feedback loop that exacerbates oxidative stress and metabolic dysfunction [39].
Unsurprisingly, clozapine and olanzapine consistently induce the highest level of inflammation among antipsychotics, in tandem with their mitochondrial toxicity [2, 21]. In addition to metabolic dysfunction, these mechanisms contribute to serious adverse effects, including myocarditis [21]. Mitochondrial functional data further show that clozapine and olanzapine are associated with the highest levels of ROS generation relative to other medications, highlighting their role in metabolic risk caused by inflammation [2].
Quetiapine may reduce or increase inflammation depending on the immediate biological environment [38]. In the central nervous system (CNS), quetiapine suppresses TNF-α and nitric oxide production in microglia, which implies localized anti-inflammatory effects. However, in adipose stem cells and macrophages, quetiapine promotes pro-inflammatory cytokine production [38], indicating a potential converse effect on inflammation. Clinical research has shown that quetiapine is associated with a marked increase in serum CRP and doubles MPO activity, correlating with systemic inflammation [22]. Inflammation-related effects are not unique to antipsychotics. Long-term SSRI use has also been associated with persistent elevations in CRP, IL-6, and TNF-α, which contribute to metabolic dysregulation and antidepressant resistance [15, 16, 20].
Antipsychotic-induced weight gain is a well-documented phenomenon and is mediated by histamine (H1) and serotonin 5-HT2C receptors [1, 19]. This provided a comprehensive review of histamine’s role in metabolic syndrome, which showed that H1 receptor antagonism disrupts hypothalamic regulation of satiety, increases food intake, and reduces energy expenditure [19]. These findings directly correlate with the metabolic side effect profiles seen across SGAs [1] (Table 1). Clozapine and olanzapine exhibit the greatest antagonism to H1 and 5-HT2C receptors, which corresponds to the greatest rates of weight gain, dyslipidemia, and glucose dysregulation among SGAs [1]. Quetiapine has been shown to have intermediate receptor affinity, which may explain its moderate metabolic risk relative to clozapine and olanzapine [1]. Risperidone has lower H1 and 5-HT2C affinity, which causes a modest weight gain profile by comparison. Aripiprazole has minimal activity at these receptors and thus is most metabolically favorable among SGAs [1]. SSRIs contribute to metabolic dysregulation through distinct mechanisms that do not involve H1 or 5-HT2C antagonism [16, 44]. Acute SSRI administration has been shown to improve glucose-dependent insulin release in pancreatic beta cells, allowing for increased overall ATP production [44]. While this initially improves insulin signaling, chronic exposure to SSRIs has been shown to contribute to insulin resistance, leptin resistance, dysregulated adipokine signaling, and weight gain [16, 44] (Table 1).
Hepatic mitochondrial dysfunction is a key component of the metabolic effects of antipsychotic medications [2, 39, 44]. One study reported that clozapine and olanzapine reduce hepatic mitochondrial complex activity, leading to decreased fatty acid oxidation, which progresses toward non-alcoholic fatty liver disease. The same study also showed that quetiapine reduces mitochondrial activity in the liver, albeit to a lesser extent than clozapine and olanzapine [2]. Moreover, skeletal mitochondrial impairment from olanzapine has been shown to further decrease insulin resistance via glucose oxidation [2, 39]. Adipose inflammation is a consistent finding of antipsychotic-induced metabolic dysfunction due to increased production of pro-inflammatory cytokines, specifically in olanzapine, clozapine, and quetiapine [17, 21, 38].
In recent literature, gut microbiota is being increasingly recognized as a key factor in psychiatric medication-induced side effects. Studies have shown that olanzapine and clozapine induce gut dysbiosis, characterized by a depletion of SCFA production and an elevated Firmicutes/Bacteroidetes ratio, which implicates subsequent metabolic disruption [7]. Clinically, in patients with schizophrenia, clinical data show patients exhibit reductions in SCFA-producing bacteria, which led to elevated LPS, insulin resistance, and hepatic steatosis [23, 45]. Definitive clinical data are lacking on gut microbiome effects for other antipsychotics, quetiapine, risperidone, and aripiprazole, but some have shown that these medications may shift microbiota composition and increase inflammation [7]. With regard to SSRIs, preliminary data suggest that they can influence microbial composition in addition to their effects on gastrointestinal (GI) motility [16].
Combining these mechanisms, oxidative stress, mitochondrial dysfunction, chronic inflammation, tissue dysfunction, and microbiome alterations form a complex network of metabolic side effects in psychiatric medications that must be considered [2, 7, 17, 21, 22, 38, 39]. SGAs, clonazepam, and olanzapine exhibit both the most severe metabolic side effects and have the most mechanistic disturbances [1, 38]. Quetiapine, risperidone, and SSRIs demonstrate some unfavorable metabolic effects primarily related to mitochondrial dysfunction and metabolic dysregulation [16, 38, 43]. The antipsychotic aripiprazole has the safest profile due to minimal impacts on mitochondrial function and appetite [1, 2].
Clozapine and olanzapine exhibit the highest cardiometabolic burden, which is consistent with the metabolic, pro-inflammatory characteristics [1, 7, 21]. Among SGAs, quetiapine and risperidone carry intermediate risks, while aripiprazole has the safest profile [22, 38]. SSRIs do not carry the same level of risk; however, long-term use can lead to chronic inflammation and hormonal dysregulation [15, 16, 20]. Importantly, there is a bidirectional relationship between psychiatric illnesses and metabolic dysfunction, further deepening the need to study this topic [6, 7]. Confounding factors such as lifestyle, diet, and socioeconomic status significantly interact with antipsychotic medications to influence metabolic changes, making it difficult to isolate the medication’s effect alone. These factors can independently cause metabolic abnormalities and, when combined with antipsychotic use, can worsen a patient’s overall cardiometabolic risk. Sedentary lifestyles are more common among patients with conditions like schizophrenia due to negative symptoms and social withdrawal. A lack of physical exercise directly contributes to weight gain and metabolic syndrome. Tobacco use is more prevalent in patients with psychosis and is independently linked to insulin resistance, dyslipidemia, and a higher risk of type 2 diabetes. It also increases sympathetic-adrenal activity, which can contribute to metabolic issues. Sleep disturbances, which are often comorbid with SMIs, can negatively impact metabolic health [46]. The appetite-stimulating effects of some antipsychotics, particularly SGAs, can lead patients to consume diets high in calories and sugar. Factors like cognitive deficits and low motivation can make it difficult for patients to plan and prepare nutritious meals, leading them to consume more fast food and sugary drinks. Lower socioeconomic status is often associated with limited access to healthcare and specialized medical services, which can delay proper metabolic monitoring and intervention. “Food deserts” and financial constraints can limit access to nutritious, affordable food options, contributing to a poor diet. Studies have shown that lower educational attainment and income are associated with poor health outcomes and are predictors of obesity. The environment in which a person lives, influenced by socioeconomic factors, can affect their cardiometabolic health [47].
There has been some development of adjunctive therapies, including metformin administration with SGAs, to reduce weight gain and insulin resistance, but they remain underutilized in clinical practice [48]. Clinically, future care should focus on educating providers on the benefits of precision and personalized approaches to manage side effects in at-risk patients. Precision and personalized approaches for managing antipsychotic side effects in at-risk patients integrate genetic information, careful monitoring, and tailored lifestyle and medication strategies. This shifts treatment from a trial-and-error approach to a proactive, patient-specific one, especially for vulnerable individuals. For patients at risk for adverse reactions, such as movement disorders like extrapyramidal symptoms, pharmacogenomic testing can help clinicians identify safer and more tolerable medication options. Genetic variations can affect how quickly a patient metabolizes a drug. For instance, testing can help identify patients who are “poor metabolizers” of certain antipsychotics and may require lower doses to minimize side effects. Certain gene variants have been associated with a higher likelihood of developing specific side effects like weight gain or tardive dyskinesia. Regular systematic monitoring is crucial for detecting side effects early, especially in at-risk patients. Further, considering the timing of medication, ruling out other causes of metabolic side effects, sleep pattern, and hygiene, and switching medication may also be helpful depending on the patient [49–51].
Given the understanding and findings regarding the mechanistic metabolic disruptions of these drugs, future research should prioritize biomarker strategies for early detection and precision management of metabolic risk. Incorporation of biomarkers like CRP and IL-6 has shown some benefit in the early identification of metabolic disruption in high-risk patients [26, 27]. Biomarkers provide a quantitative link between the mechanisms discussed in Metabolic alterations with antipsychotics and the clinical outcomes we have explored here. Chronic low-grade inflammation (elevated CRP, IL-6, and TNF-α) is a hallmark of psychiatric illness and psychotropic medication use, regardless of treatment status, and compared to healthy controls [52]. Recent studies have corroborated this evidence, showing that SSRIs and SGAs exacerbate inflammatory biomarkers linked to insulin resistance and increased cardiovascular risk [15, 16]. These observed effects are likely due to oxidative stress, as MPO levels in quetiapine overdose patients had a strong correlation to CRP levels and tachycardia, which indicated that these oxidative stress biomarkers are indicative of the cardiometabolic burden in psychiatric populations [22].
Further, regular monitoring of patients taking psychotropic medications to monitor their weight, blood pressure, blood sugar, and lipid levels should be done in clinical practice because of the different sensitivity of metabolic effects (Table 2). Emphasizing the importance of maintaining a healthy diet, getting regular exercise, and smoking cessation can help mitigate metabolic risks. This demonstrates the importance of a holistic treatment perspective in clinical settings where physicians may be able to utilize various methods to prevent metabolic dysfunctions. In some cases, adjustments to medication dosages or switching to alternative medications with fewer metabolic side effects may be necessary. In some cases, combination therapies with other medications, like metformin, may be considered to manage weight gain or other metabolic issues associated with psychotropic drugs. Finally, addressing metabolic issues early on is crucial to preventing the development of more serious conditions like type 2 diabetes and CVD.
Summary of the severity of metabolic effects of various drugs along with the underlying mechanism [15, 16, 20, 22, 26, 27, 38, 46].
| Drug/Class | ||||||
|---|---|---|---|---|---|---|
| Clozapine, olanzapine | Quetiapine | Risperidone | Aripiprazole | SSRIs | ||
| Metabolic effect | Weight gain | High | Moderate | Moderate | Low | Minimal |
| Mitochondrial dysfunction | High | Moderate | Moderate | Low | Low–moderate | |
| Inflammation | High | Moderate | Moderate | Low | Moderate | |
| Dysbiosis | High | Moderate | Moderate | Low | Low–moderate | |
| Overall metabolic risk | Very high | Moderate | Moderate | Low | Low | |
SSRIs: selective serotonin reuptake inhibitors.
Long-term longitudinal data reveal that metabolic alterations from antipsychotic drugs, particularly SGAs, often begin with rapid weight gain and progress over years to significant disturbances in glucose and lipid metabolism. Management strategies focus on early and regular screening, lifestyle interventions, switching to lower-risk medications, and adding adjunctive medications [53]. A study by Abo Alrob et al. [54] studying a patient population of 91 patients found that six months of treatment with SGAs may result in elevated systolic pressure, elevated triglyceride, and impaired glucose levels in 44%, 54.9%, and 31.9% of patients, respectively. Evidence-based strategies have been developed to monitor, prevent, and manage the metabolic side effects associated with antipsychotic medication. Consensus guidelines recommend screening for metabolic issues at baseline and regularly thereafter, including weight or BMI, fasting plasma glucose, and fasting lipid profiles. Clinicians should consider an individual’s specific risk factors, such as family history of diabetes, ethnicity, and age, when prescribing antipsychotics, as some populations are more vulnerable. Switching to an antipsychotic with a lower metabolic risk, such as aripiprazole or ziprasidone, can help manage weight and metabolic abnormalities. However, this carries a risk of psychiatric relapses and should only be done in consultation with a psychiatrist. For patients who cannot switch their antipsychotics, adjunctive medications may be added. Metformin is the best-studied option for weight and glucose management, though its effects tend to be modest [55, 56].
Altering the gut microbiome to mitigate antipsychotic-induced metabolic effects is a promising, emerging strategy, with research showing probiotics, prebiotics, and dietary fiber can counteract side effects like weight gain and insulin resistance, particularly by targeting beneficial bacteria like Akkermansia muciniphila. While promising in animal models and limited human trials, further research is needed to understand the complex interactions and establish personalized approaches for psychiatric patients. Supplementation with probiotics, especially Akkermansia muciniphila, shows potential in animal and human studies to improve weight gain, insulin resistance, and liver function associated with antipsychotic use. Prebiotics and dietary fibers can modulate the gut microbiome and have shown promise in reducing weight gain and improving metabolic parameters in conjunction with probiotics. Fecal microbiota transplantation (FMT) is also being considered as a strategy to restore a healthy gut microbiome and counteract metabolic disturbances [57–60]. The notion of altering microbiomes to mitigate the effects on other CVDs is supported by the fact that therapeutic modulation of gut microbiota has beneficial effects [61].
Despite monitoring the side effects with available strategies, managing antipsychotic-induced metabolic issues remains challenging. This may be due to multiple reasons. Many patients with serious mental illness have poor adherence to both their psychiatric and physical health treatments, which can undermine management efforts. Even the most successful interventions often achieve only modest reductions in metabolic risk, and some weight gain may still occur. Some antipsychotics have direct effects on metabolism that are independent of weight gain and contribute to the overall metabolic risk. Data on the long-term metabolic side effects of the newest antipsychotics, such as brexpiprazole, cariprazine, and lumateperone, are still limited [55, 62, 63].
Psychotropic medications, especially SGAs, are integral to psychiatry, but existing literature suggests that there is a consistent correlation to metabolic changes. Clozapine and olanzapine (SGAs) show the greatest metabolic burden through mechanisms of mitochondrial dysfunction, oxidative stress, receptor-mediated appetite changes, inflammation, and gut dysbiosis. Among other SGAs, quetiapine and risperidone are associated with moderate acute metabolic changes, and aripiprazole has the safest profile due to its limited receptor antagonism [1, 2, 7, 19, 21, 23, 38, 39, 44, 45]. SSRIs have a safer profile, but still may be associated with chronic inflammation, insulin resistance, and appetite/weight gain [16, 24, 32, 44]. These findings help explain the elevated cardiometabolic morbidity and mortality in psychiatric patients [4, 6, 7, 12, 41]. Our findings also highlight the importance and role of metabolic biomarkers moving forward. Markers such as CRP, IL-6, leptin, and adiponectin may be integrated into routine care to enable earlier detection of subclinical disturbances and to improve precision management [15–18, 20]. For example, in a clinical setting, precision care for a diabetic psychiatric patient may include extra testing for CRP, IL-6, or lipid changes, potentially at the subclinical level, which could be mitigated by structured lifestyle counseling, adjunctive metformin, or alteration of psychiatric medication regimen. Moreover, the findings from our analysis suggest potential benefits of an interdisciplinary approach between endocrinology, molecular biology, and psychiatry to enhance the safety of treatments and translate mechanistic processes into reductions of the metabolic burden of psychotropic medication use.
Although cross-sectional data and medium-term RCT and clinical data consistently implicate SGAs and other psychotropic medications in metabolic risk, high-quality longitudinal studies confirming the trajectory of metabolic adverse effects (weight gain, lipid changes, hyperglycemia, etc.) would be hugely beneficial for developing standardized data across drug classes and enhancing the precision treatment of patients [1, 25, 26]. However, one current limitation is that mechanistic and biomarker studies related to this topic have been performed largely with animal models; while these help map mechanistic processes, confirmatory studies on human patients are needed. For example, a patient with elevated levels of CRP or IL-6 may be showing signs of pharmacologically induced metabolic disturbance and thus may benefit from an altered medication regimen or treatment plan. Clinical studies show this may come in the form of proactive drug selection, lifestyle modification, and adjunctive therapies [12, 26, 48].
5-HT2A: 5-hydroxytryptamine 2A
Akt: protein kinase B
BMI: body mass index
CRP: C-reactive protein
CVD: cardiovascular disease
IFN: interferon
IL: interleukin
JAK: Janus kinase
LPSs: lipopolysaccharides
MPO: myeloperoxidase
PI3K: phosphoinositide 3-kinase
RCTs: randomized controlled trials
ROS: reactive oxygen species
SCFA: short-chain fatty acid
SGAs: second-generation antipsychotics
SMI: severe mental illness
SSRIs: selective serotonin reuptake inhibitors
STAT: signal transducers and activators of transcription
TMAO: trimethylamine-N-oxide
TNF: tumor necrosis factor
MN: Writing—original draft. SN: Writing—original draft. VR: Conceptualization, Resources, Supervision, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
As the corresponding author, I declare that this manuscript is original; that the article does not infringe upon any copyright or other proprietary rights of any third party; and that neither the text nor the figures have been reported or published previously. All the authors have no conflict of interest and have read the journal’s authorship statement.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 5961
Download: 113
Times Cited: 0
Bingwu Xu ... Yong Zhang
Pirangi Srikanth ... Sukhendu Nandi
Gabrielle St-Arnaud ... Vincenzo Di Marzo
Zhuqi Wang ... Kang Liu
Jerzy W Kolaczynski, Eva Surmacz
