Affiliation:
1Service of Gastroenterology and Hepatology, University Virgen de la Victoria Hospital, 29010 Málaga, Spain
2Instituto de investigación Biomédica de Málaga (IBIMA)-Plataforma Bionand, University of Málaga, 29010 Málaga, Spain
3Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), 28029 Madrid, Spain
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0002-8376-9573
Affiliation:
1Service of Gastroenterology and Hepatology, University Virgen de la Victoria Hospital, 29010 Málaga, Spain
†These authors share the first authorship.
ORCID: https://orcid.org/0000-0002-1384-7193
Affiliation:
1Service of Gastroenterology and Hepatology, University Virgen de la Victoria Hospital, 29010 Málaga, Spain
2Instituto de investigación Biomédica de Málaga (IBIMA)-Plataforma Bionand, University of Málaga, 29010 Málaga, Spain
3Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), 28029 Madrid, Spain
Email: andrade@uma.es
ORCID: https://orcid.org/0000-0002-1565-0757
Affiliation:
1Service of Gastroenterology and Hepatology, University Virgen de la Victoria Hospital, 29010 Málaga, Spain
2Instituto de investigación Biomédica de Málaga (IBIMA)-Plataforma Bionand, University of Málaga, 29010 Málaga, Spain
3Centro de Investigación Biomédica en Red de Enfermedades Hepáticas y Digestivas (CIBERehd), 28029 Madrid, Spain
ORCID: https://orcid.org/0000-0003-0410-8273
Explor Dig Dis. 2023;2:202–222 DOI: https://doi.org/10.37349/edd.2023.00027
Received: June 19, 2023 Accepted: August 26, 2023 Published: September 18, 2023
Academic Editor: Wen-Xing Ding, University of Kansas Medical Center, USA
The article belongs to the special issue CHOLESTASIS
Drug-induced liver injury (DILI) is an adverse reaction to drugs and other xenobiotics that can have serious consequences and jeopardise progress in pharmacological therapy. While DILI is predominantly hepatocellular, a non-negligible percentage of patients who present with cholestatic damage. Mixed damage is typically lumped together with cholestatic damage in the literature. Drug-induced cholestasis is often caused by the use of some non-steroidal anti-inflammatory drugs (NSAIDs), antibiotics (i.e., amoxicillin-clavulanic acid), statins, and anabolic agents, among others. Drug-associated cholestasis tends to have a more chronic course and mostly affects older population. There is also a genetic predisposition to toxic cholestasis caused by some drugs (amoxicillin-clavulanic acid, statins, etc.). Recently, anatomical alterations of the biliary tract induced by drugs (especially immunotherapy drugs) have been described. Bile duct injury is one of the histopathological findings that have prognostic significance in DILI. A correct differential diagnosis with other causes of cholestasis is mandatory to reach an accurate diagnosis. Ursodexycholic acid, corticosteroids, and replacement therapies have been used as a therapeutic arsenal, although more evidence is needed to establish them as a routine therapeutic management in clinical practice. The breakthrough and validation of biomarkers of cholestasis and bile duct injury is an urgent need for drug development and post-marketing phase.
Drug-induced liver injury (DILI) is an adverse reaction not only to a wide range of drugs commonly used in clinical practice, but also to herbal preparations and dietary supplements. DILI has been classically classified as direct (dose-dependent) or idiosyncratic (no dose-related), but indirect liver injury has emerged as a third type of DILI. Indirect DILIs are those liver diseases that emerge as a consequence not directly related to the action of any drugs (for example, reactivation of hepatitis B virus after the use of an immunosuppressive drug). These types of liver injury may warrant a different clinical approach and treatment. This review focuses on idiosyncratic cholestatic damage [1]. Drugs can cause predominantly hepatocellular injury, cholestatic injury, or an intermediate phenotype called mixed injury. Although the definition of cholestasis may vary from a clinical, biochemical, or histological standpoint, in practice, drug-induced cholestasis is defined by an elevation in alkaline phosphatase (ALP) levels greater than two times the upper limit of normal (ULN) and/or an alanine aminotransferase (ALT)/ALP ratio (in times of ULN) less than 2, when other causes of liver injury have been excluded [2]. The percentage of cholestatic liver injury due to xenobiotics varies between the different registries and ranges from 8% to 56% (Table 1) [3–11].
Percentage of cholestastic DILI in different registries reported in the literature
| Registry | Spanish DILI Registry | Latin America | DILIN Registry (USA) | India | China | Korea | Japan | Pakistan | |
|---|---|---|---|---|---|---|---|---|---|
| Type of study | Prospective | Prospective | Prospective | Prospective | Retrospective | Prospective | Prospective | Retrospective | |
| Total DILI cases | 843 | 367* | 899 | 1,288 | 1,985 | 371 | 307 | 462 | |
| Pattern of damage, n (%) | Hep | 482 (57) | 199 (62)* | 484 (53.8) | 362 (29.7) | 1,422 (71.6) | 283 (76.3) | 197 (64) | 116 (25.1) |
| Chol | 173 (21) | 123 (38)# | 210 (23.4) | 521 (42.8) | 254 (12.8) | 33 (8.9) | 48 (16) | 260 (56.2) | |
| Mix | 188 (22) | NA | 205 (22.8) | 334 (27.4) | 309 (15.6) | 55 (14.8) | 62 (20) | 86 (8.7) | |
| Main implicated agents, individually (pharmacological group) | Amoxicillin-clavulanate (Antiinfectives) | Amoxicillin-clavulate (NA) | Amoxicillin-clavulanate (Antiinfectives) | Antituberculous (Antiinfectives) | Azithromycin (Antibiotics) Gu-kang capsule (Chinese traditional patent medicine) | NA (Herbal medications) | NA (Anti-inflammatory drugs) | Antituberculous (Antiinfectives) | |
| HILI cases, n (%) | 32 (4) | 29 (8) | 85 (10) | 179 (13.9) | 563 (28) | 270 (73) | 27 (8.7) | 42 (9) | |
| References | [3] | [4, 5] | [6] | [7] | [8] | [9] | [10] | [11] | |
* 322 patients were cases due to conventional medicines, 29 by herbs and 16 by anabolic androgenic steroids (AAS); # percentage of cholestatic and mixed DILI cases together. Percentage of pattern of damage only includes results of conventional medicines. Chol: cholestatic; DILIN: Drug-Induced Liver Injury Network; Hep: hepatocellular; HILI: herbal and dietary supplements-induced liver injury; Mix: mixed; NA: not available
Drug-induced cholestasis is a phenotype of hepatotoxicity with specificities in causative agents, risk factors, clinical presentation, and prognosis that will be addressed in this review article.
Drug-induced cholestasis diagnosis based on the thorough exclusion of all possible potential causes of cholestasis (Figure 1). This approach requires an accurate medical history, a proper clinical assessment, and a stepwise analysis of the pattern of biochemical alterations and other complementary tests.
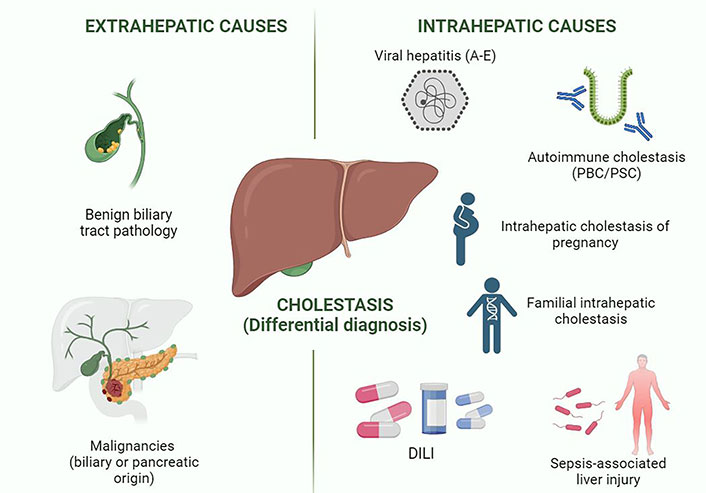
Differential diagnosis in cholestatic syndrome. This figure shows the different entities included in the differential diagnosis of cholestasis in clinical practice. Viral hepatitis A and E are the hepatotropic viruses that typically cause cholestatic alteration. Created in BioRender.com. PBC: primary biliary cholangitis; PSC: primary sclerosing cholangitis
Cholestatic DILI can manifest clinically in a range of presentations, including asymptomatic ALP elevation, mild non-specific symptoms (fatigue, fever, anorexia, weakness, vomiting, chills, right upper quadrant pain, pruritus, skin rash, etc.) to severe protracted jaundice, ascites, coagulopathy, and encephalopathy [1, 12].
Clinical suspicion should be guided by a detailed medical history including exposure to all drugs, herbs, and dietary supplements consumed in the last 6 months. However, clinicians should bear in mind that there are drugs with a longer latency such as minocycline, statins, metrotexate, or nitrofurantoin [13, 14]. A meticulous history should include information about start and stop dates of each suspected agent, eventual dosage changes, and previous exposure. Worsening of liver injury after the re-administration of the drug (rechallenge) which often happens inadvertently, and improvement after cessation of the drug (dechallenge) need to be looked for to support a DILI diagnosis [15]. Other situations that may cause cholestasis such as alcohol consumption, total parenteral nutrition, or causes of hepatic ischaemia (heart failure, sepsis, or hypotension) must be investigated [16].
In most cases of DILI, the diagnostic suspicion is triggered by an alteration in liver biochemical blood test. The initial laboratory tests should include ALT, ALP, total bilirubin, and direct bilirubin levels as well as international normalized ratio (INR) and albumin, which determine the severity [16, 17].
Around 10–20% of the population have minimal alterations in liver biochemical blood test [15], so to define correctly DILI, it is necessary to meet one of the following requirements: a) > 5 ULN elevation in ALT, b) > 2 ULN elevation in ALP in absence of other causes (e.g., bone pathology) and especially when accompanied by elevated gamma-glutamyltransferase (GGT), c) > 3 ULN elevation in ALT and > 2 ULN elevation in total bilirubin concentration. In patients with a baseline abnormal liver test, ULN is replaced by the mean values prior to the initiation of the hepatotoxic agent [2, 15, 17].
In order to categorize DILI according to its biochemical pattern, the calculation of the R value is used. This is defined as the result of dividing ALT/ULN-ALT by ALP/ULN-ALP, using the first serum values available during the event. If the R value is ≤ 2, the pattern is defined as cholestatic while values ≥ 5 classify it as hepatocellular liver injury and intermediate values as mixed liver injury. Cholestatic DILI can also be defined as an increase in ALP above 2 times the ULN [18]. Approximately, 20–40% of all DILI cases have a cholestatic pattern. Mixed liver injury typically behaves like cholestatic liver injury so some analysis in the literature combines both [19]. Moreover, studies on hepatic histological findings show similar features in biopsies from cases of mixed and cholestatic injury [20]. Consistent biochemical pattern of liver injury observed for many drugs is considered a useful clue in the diagnosis of DILI, but clinicians should bear in mind that this “signature” is not so straightforward as some drugs have been associated with various biochemical profiles [16]. In addition, the analytical parameters (likewise R value) may be dynamic during the evolution of hepatocellular cases. Therefore, the same drug may initially produce hepatocellular damage, which may later turn into mixed damage or even cholestatic damage. This is because the dynamics of ALT resolution is much faster than that of ALP [17]. This could be a confounding issue to define the pattern damage in some cases.
Furthermore, laboratory workup to exclude alternative causes of liver damage is needed. It is recommended to rule out infectious hepatitis in suspected DILI including routine test for anti-hepatitis E virus (HEV), immunoglobulin M (IgM; or HEV-RNA), and hepatitis C virus (HCV)-RNA [16, 17]. While most infectious hepatitis manifests with a hepatocellular injury pattern, some microorganisms such as Epstein-Barr virus, hepatitis A virus, Salmonella typhi, and Coxiella burnetii can cause cholestatic liver injury [19].
Other laboratory tests recommended in the aetiological assesment of liver damage are ceruloplasmin levels (Wilson’s disease), autoantibodies, and immunoglobulin G (IgG; autoimmune hepatitis) and, especially in cholestatic cases, antimitochondrial antibodies levels to rule out PBC [15].
Imaging tests beyond abdominal ultrasound (which is typically normal) are usually not necessary in a suspected case of DILI. This allows an initial assessment of biliary obstruction, liver cirrhosis, or focal parenchymal lesions. However, when a cholestatic pattern is present and/or jaundice or abdominal pain predominates, it is recommended to perform computerized tomography (CT) or magnetic resonance cholangiography to exclude other aetiologies.
In the presence of biliary tract strictures in a patient with suspected DILI, a diagnosis of drug-induced secondary sclerosing cholangitis, characterised by jaundice and slower resolution, should be considered. This disorder has been described with exposure to amiodarone, atorvastatin, amoxicillin-clavulanate, gabapentin, infliximab, 6-mercaptopurine, nivolumab, sevoflurane, ketamine, and venlafaxine [16, 17, 21, 22].
Liver biopsy should be considered in patients with suspected DILI who worsen or non-resolve the biochemical abnormalities despite drug discontinuation. However, it should be emphasised that the time to resolution of cholestatic DILI is slower than for the hepatocellular pattern [19]. Histological findings may be helpful in the differential diagnosis by identifying other cholestatic diseases like antimitochondrial antibody-negative PBC, small duct PSC, or hepatic overlap syndromes [12].
Moreover, biopsy can be a useful tool to establish prognosis of DILI cases, because some histological findings (ductular reaction, microvesicular steatosis, higher degrees of necrosis, and hepatic fibrosis) have been associated with an increased risk of liver failure and death [20].
In order to confidently attribute manifestations of liver injury to a particular drug, a structured assessment is necessary. For this purpose, various causality scales have been developed. The DILIN structured expert opinion process scores DILI cases from 1 (definite) to 5 (unlikely) according to the likelihood of being caused by a given drug. However, this scale has a complex applicability due to the lack of DILI experts and low external validity. In the pharmacovigilance arena and clinical practice, the Rousell Uclaf causality assessment method (RUCAM) [18], also known as Council for International Organizations of Medical Sciences (CIOMS)/RUCAM scale, has been used since more than 30 years, although this tool is critiqued due to its complexity and poor reproducibility among raters. Recently, in a collaborative work, a revised electronic causality assessment method (RECAM) has been developed, which has some advantages over RUCAM by eliminating risk factors, simplifying the latency and dechallenge fields and the ability to include data on histological findings, other diagnostic tests or rechallenge if any; nevertheless, the reliability of scale needs to be validated in future studies [15, 23].
The most common form of drug-induced liver damage is separately the hepatocellular pattern, which also tends to carry out higher risk of liver related-death and liver transplantation [1, 3]. However, a multitude of agents widely prescribed in daily clinical practice are also associated with cholestatic liver damage. We describe herein the main medications and herbals leading to cholestatic liver damage (Table 2).
Likelihood score of different cholestatic DILI cases defined by LiverTox (https://pubmed.ncbi.nlm.nih.gov/31643176)
| Pharmacological group | Agents | Likelihood score |
|---|---|---|
| NSAIDs | Celecoxib | B |
| Ibuprofen | A | |
| Metafenamic acid | D | |
| Meloxicam | C | |
| Naproxen | B | |
| Nimesulide | A | |
| Oxaprozin | C | |
| Piroxicam | B | |
| Rofecoxib | C | |
| Sulindac | A | |
| Statins | Atorvastatin | A |
| Simvastatin | A | |
| Fluvastatin | B | |
| Antiinfectives | Amoxicillin-clavulanate | A |
| Penicillins (2nd generation) | B-C | |
| Cephalosporines | B | |
| Macrolides | A-B | |
| TMP-SMX | NA | |
| Nitrofurantoin | A | |
| Ciprofloxacin | B | |
| Levofloxacin | A | |
| Doxycycline | B | |
| Meropenem | D | |
| Terbinafine | B | |
| Amphotericin B | C | |
| Micafungin | D | |
| Itraconazole | B | |
| Psychotropic agents | Chlorpromazine | A |
| Imipramine | B | |
| Amitriptyline | B | |
| Fluoxetine/citalopram | C | |
| Duloxetine | C | |
| Immunomodulator and antineoplastic drugs | Azathioprine | A |
| Eculizumab | D | |
| Cisplatin | C | |
| Durvalumab | B | |
| Pembrolizumab | A | |
| Pexidartinib | B | |
| Nivolumab | A | |
| Atezolizumab | B | |
| Avelumab | B | |
| Herbs | Kratom | B |
| Ashwagandha | C | |
| Tribulus | E | |
| COVID-19 vaccines | BNT162b2, Pfizer | C |
| mRNA-1273 | C | |
| ChAdOx1, AstraZeneca | C | |
| Ad26.COV2.2, Janssen | D | |
| NVX-CoV2373, Novavax | E | |
| Gam-COVID-Vac, Spuntnik V | E | |
| Sinopharm COVID-19 vaccine | E |
Likelihood score: A (well known cause of clinically apparent liver injury), B (highly likely cause of clinically apparent liver injury), C (probable rare cause clinically apparent liver injury), D (possible rare cause of clinically apparent liver injury), E (unproven but suspected cause of clinically apparent liver injury or unlikely cause of clinically apparent liver injury, depend on the involved substance). COVID-19: coronavirus disease 2019; mRNA: messenger RNA; NSAIDs: non-steroidal anti-inflammatory drugs; TMP-SMX: trimethoprim/sulfamethoxazole
The prevalence of NSAID hepatotoxicity in several large prospective DILI registries worldwide oscillates between 3% and 36%, being a strikingly frequent causative agent of DILI in Italy [24–26]. DILI cases attributed to NSAIDs collected in DILIN were analyzed. There were significant rates of autoimmunity traits (38%). The most frequent agent implicated was diclofenac, all cases exhibited a hepatocellular pattern, but subtle increases of ALP were identified. Diclofenac-induced liver injury was often severe [27]. Ibuprofen has been also reported in the literature as causative of DILI, with a predominance of hepatocellular pattern [24]. Nonetheless, an analysis of cases of ibuprofen-related DILI in Spanish and Latin-American revealed an important representation of cholestatic/mixed form of liver damage (42%) [26]. Besides, vanishing bile duct syndrome, which is considered a serious variant of liver damage characterised by bile duct injury and ductopenia has been linked to ibuprofen use [24]. Case reports of celecoxib-and sulindac-induced cholestasis have been published, notably, presenting as fulminant liver failure [28, 29].
On the other hand, there are described cases of long-standing cholestasis due to NSAIDs as flurbiprofen or celecoxib reported in the literature [30, 31]. Liver damage associated with piroxicam and rofecoxib is typically cholestatic or mixed [32–34]. A recent multicentre international study carried out in Latin America and Spain analyzing DILI cases due to nimesulide showed that the percentage of cholestatic/mixed damage was 33%, with a significantly higher proportion of pruritus (37% vs. 5.3%) and lower severity (percentage of acute liver failure, 5.3% vs. 29%) compared to hepatocellular cases [35].
Amoxicillin-clavulanate is currently the main causative agent in most of DILI registries, mainly due to its widespread prescription worldwide [3–11]. While amoxicillin-clavulanate-induced liver injury has been associated with certain genetic susceptibilities [human leukocyte antigen (HLA)-A*02:01, HLA-DRB1*15:01], in earlier genetic studies, other novel genetic markers such as reduced endoplasmic reticulum aminopeptidase 2 (ERAP2) expression or HLA-B*15:18 have recently been identified [36–38]. The latency of amoxicillin-clavulante hepatotoxicity is usually short, but some cases may develop at the end of prolonged treatment and even after discontinuation of therapy [39]. Liver injury is primarily associated with the clavulanic acid component, as the incidence of DILI with amoxicillin-clavulanate is much higher than with amoxicillin alone [40]. Amoxicillin-clavulanate hepatotoxicity affects more frequently elderly patients with a cholestatic phenotype, both biochemically and histologically with instances of vanishing bile duct syndrome [41–43]. At the contrary, amoxicillin-clavulanate-hepatotoxicity typically present with hepatocellular pattern in younger patients with short latency [6].
Flucloxicillin-induced liver damage is well-documented in the literature, and it is a classic example of cholestatic DILI ranging from mild liver injury to aggressive instances leading to vanishing bile duct syndrome [44]. It is well-known that DILI related to flucoxacillin is an immune-mediated reaction as it is strongly associated with HLA-B*57:01 carriages being more common in females [45]. Other second-generation penicillins, such as cloxacillin, dicloxacillins, and oxacillins, have been also reported to cause cholestatic DILI [46].
Cephalosporin-induced liver damage with a cholestatic presentation was reported by DILIN group in 2015. Cefazolin was the most predominant drug within this class of antibiotics (19 cases) but other 14 patients receiving different types of cephalosporins suffered cholestatic damage, experiencing a more severe course and leading to death in 2 cases [47].
Erytrhomycin is a frequent causative agent of DILI in some countries as Sweden [48]. Its type of damage is predominantly cholestatic and its course is favourable in the majority of cases [49]. Erytrhomycin is one of the instances of drugs which comply with Hy’s law (ALT ≥ 5× ULN + total bilirubin ≥ 2 mg/dL) but nevertheless has a normally benevolent course. Azitrhomycin is another macrolide with a well-known potential to cause cholestatic damage, often prolonged, with instances of vanishing bile duct syndrome [50–52].
TMP-SMX hepatotoxicity often causes cholestatic damage, this type of phenotype accounting for approximately 60% in some series [53]. In comparison to other drugs, TMP-SMX has a not negligible proportion of severe events in terms of deaths or liver transplantation [48]. The hepatotoxicity is attributed to the sulfonamide component [53]. In a recent analysis of vanishing bile duct syndrome cases reported in the literature, TMP-SMX represented 19% of total cases [54].
A recent analysis of two prospective cohorts of patients with nitrofurantoin-induced liver injury showed a predominance of female sex (96%), positive autoantibodies (65%), and hepatocellular damage. Nonetheless, near to 9% developed a mixed/cholestatic reaction. Notably, in these series, 22% of patients were characterized by a persistent elevation of transaminases and required immunosuppressive therapy to get a complete normalisation of liver profile [13]. A linkage of nitrofurantoin-hepatoxicity with HLA-DR6, DR-2, and HLA-DRB1*11:04 has been published [55]. Liver biopsy specifically showed chronic hepatitis with autoimmune-like features and instances of cirrhosis associated with nitrofurantoin have also been previously reported [55]. Likewise, nitrofurantoin has been associated with fulminant hepatitis [56].
Quinolones have been associated with cases of cholestasis and of vanishing bile duct syndrome in the literature attributed to levofloxacin and ciprofloxacin [57–60]. Rarely, other antibiotics such as doxycycline have been tagged as culprit of a cholestatic DILI [61], and meropenem linked to several reports of induced vanishing bile duct syndrome to date [62, 63].
While most antifungals involved in DILI tend to manifest a hepatocellular pattern, some other cause predominantly cholestatic damage with marked jaundice and pruritus. Probably the most representative of this last group is terbinafine [64]. In a genome-wide association study (GWAS) of European population with DILI, a strong linkage between HLA-A*33:01 and cholestatis DILI due to terbinafine was identified. Indeed, this allele was associated with cholestatic/mixed injury regardless the causative drug [65]. Cases of cholestasis-induced by amphotericin B, micafungin, and itraconazole have also been reported [66–68].
Statins are a common cause of DILI worldwide [69, 70]. Statin-associated DILI has a genetic background as a specific polymorphism on chromosome 18 (rs116561224) has been identified [65]. In the Spanish DILI Registry, atorvastatin was one of the drugs linked to cholestatic damage in elderly patients [71]. Atorvastatin-induced liver injury is a well-defined entity whose pattern of damage is largely cholestatic although it may be presented as autoimmune-like liver disease [72–75]. Simvastatin-induced cholestasis has been reported but it patterns of damage is mostly hepatocellular [76, 77].
Chlorpromazine is a prototypical, long-standing example of a hepatotoxic drug. This agent causes cholestatis and it is responsible for chronic cholestasis and even ductopenia, leading to cirrhosis [78]. Other psychotropic agents which classically have been related to cholestatic damage are tricyclic antidepressants (imipramine or amitriptyline) or selective serotonin reuptake inhibitors (fluoxetine, duloxetine, or citalopram) [79–82].
Azathioprine is an immunomodulator widely used in autoimmune diseases. In some series, percentage of DILI due to azathioprine ranges from 3% to 10%, and typically presents during the first 3 months of therapy [83–85]. Although azathioprine-induced liver damage is predominantly hepatocellular, azathioprine-induced cholestasis is a well-established entity with many cases reported in the literature. In some instances, in which a liver biopsy was performed, nodular regenerative hyperplasia was a prominent histological feature [86–89].
Eculizumab is a monoclonal antibody to complement factor 5, which blocks complement activation and is used to treat paroxysmal nocturnal hemoglobinuria and also hemolytic uremic syndrome. This agent has been linked to cholestatic injury [90]. Cases of cholestasis-induced chemotherapeutics, as cisplatin, have been previously published [91]. The vast majority of immunotherapy-related DILI is biochemically hepatocellular, but in some cases a cholestatic or mixed damage arises [92]. It is worth to highlight a particular type of immunecheckpoint inhibitors-induced liver damage known as secondary sclerosing cholangitis. This entity is characterized by cholestatic pattern, with structural change (diffuse biliary duct dilatation and thickening of the bile ducts) [93]. It has been reported cases of secondary sclerosing cholangitis due to nivolumab, avelumab, pembrolizumab, durvalumab, and atezolizumab [94–102]. Cases of cholestasis due to checkpoint inhibitors have a poorer response to standard treatment compared to hepatocellular pattern with greater rates of steroid-resistance in some published cases [94, 95, 98].
Pexidartinib is a potent kinase inhibitor drug with a highly lithified clinical setting, used in the treatment of adults with symptomatic tenosynovial giant cell tumour. Patients treated with this molecule frequently experienced increases of ALP (near to 20%). Furthermore, biopsy revealed bile duct damage and duct loss. Cases of acute liver failure and liver transplantation have been reported [103, 104].
The use of banned ASS is a growing concern worldwide. The fraction of AAS cases in the DILIN Registry is 5% of the total cases [105]. In the Spanish and the Latin American DILI cohorts, the proportion of AAS-induced liver injury is 2.3% and 5%, respectively [5, 106]. AAS hepatotoxicity has been reported extensively in the literature. Liver damage related to anabolic steroids is typically cholestatic, with deep and prolonged jaundice (normally than 3 months) as well as itching, which is sometimes refractory to pharmacological therapy. Hy’s law is commonly fulfilled in hepatocellular cases, but fulminant liver failure has not been reported. Acute kidney injury accompanies AAS-hepatotoxicity in 14–24% of cases, and it is related to the extremely high bilirubin levels [107, 108]. Besides, the long-term use of anabolic steroids has been associated with peliosis hepatis and liver tumours [109].
Herbal products and dietary supplements use are a growing habit nowadays. Although liver damage secondary to herbs usually presents with a hepatocellular pattern, the liver injury related to several herbal products is characterised by a cholestatic/mixed phenotype [5]. Kratom, which is botanical extract from the Mitragyna speciosa consumed in many Southest Asiatic countries is one of the herbs that characteristically presents as long-lasting cholestatic damage, behaving similarly to anabolic steroids [110].
Ashwagandha (also called “Indian ginseng”), derived from Withania somnifera, is taken for its anti-inflammatory and neuroprotective condition in Southest Asia and India. Ashwagandha-related liver impairment is mainly cholestatic or mixed [111]. Neither herb (Kratom and Ashwagandha) has been linked to acute liver failure.
Tribulus is an herbal product (resulting from fruits or roots of Tribulus terrestris) used globally for aphrodisiac purposes and the bodybuilding setting. Liver damage due to tribulus is characterized by profound jaundice accompanied by renal impairment. Histologically, it usually presents as bland cholestasis [112].
Following the establishment of the global vaccination campaign against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus, acute de novo hepatitis cases began to be identified, with typical features of autoimmune hepatitis but temporally coinciding with the SARS-CoV-2 vaccine. Subsequently, case reports and case series of probable hepatitis secondary to the COVID-19 vaccine were published [113]. Recently, an international multicentre study has reported 59 cases of post-vaccination hepatitis, finding significant rates of positive autoantibodies, elevated IgG levels, and biopsies consistent with autoimmune hepatitis. Although 95% of the cases corresponded to a hepatocellular pattern, 5% of them exhibited a mixed pattern. Centralized analysis of biopsies identified 2 cases with bland cholestasis in the histology [114]. In a previous international study of 87 patients, the proportion of SARS-CoV-2 vaccine-related cholestatic and mixed hepatotoxicity was 6% and 10% respectively [115]. A summary of the most representative drugs for the different cholestatic phenotypes of liver damage can be observed in Table 3.
Most representative drugs in different cholestatic phenotypes of liver damage
| Acute cholestasis | Chronic cholestasis | |||||
|---|---|---|---|---|---|---|
| Bland cholestasis | Cholestatic hepatitis | Autoimmune features | Vanishing bile duct syndrome | Secondary sclerosing cholestasis | Autoimmune features | Fibrosis/cirrhosis |
AAS Herbs (tribulus) Warfarin Thiabendazole Oral contraceptives SARS-CoV-2 vaccines | NSAIDs Antiinfectives (amoxicillin-clavulanate, penicillins, macrolides, TMP-SMX, etc.) Statins Tricyclic antidepressants Selective serotonin reuptake inhibitors Azathioprine Eculizumab Cisplatin Herbs (Kratom, Ashwagandha) | Statins (atorvastatin) Nitrofurantoin SARS-CoV-2 vaccines | Antibiotics (amoxicillin/clavulanate, azithromycin, flucloxacillin, quinolones, etc.) NSAIDs (ibuprofen, diclofenac) Psychotropes (chlorpromazine, imipramine, carbamazepine, etc.) | Immunotherapy (nivolumab, pembrolizumab, durvalumab, etc.) Docetaxel Ketamine Atorvastatin | Nitrofurantoin | Chlorpromazine Nitrofurantoin |
Various risk factors associated with the risk of developing toxic cholestasis have been described in last years. An early genetic study using hybridization in 140 well phenotyped DILI patients showed that the frequencies of alleles DRB1*15 and DQB1*06 were significantly increased in patients with the cholestatic/mixed type of liver damage (but not in the hepatocellular injury) in comparison to healthy subjects, suggesting the key role of the adaptive immune system in this type of injury [116].
In the GWAS era, a multinational collaborative study in 862 persons with DILI (excluding those with DILI related to amoxicillin-clavulanate and flucoxacillin) and 10,588 population-matched controls showed a significant genome wide association between DILI and A*33:01 for cholestatic and mixed DILI, but not for hepatocellular DILI. This association was mediated by large effects for terbinafine-, fenofibrate-, and ticlopidine-related DILI (Table 4) [65].
Genetic factors related to cholestasis-induced by xenobiotics
| Association | HLA type | References |
|---|---|---|
General association with cholestatic/mixed DILI (High positive predictive value) | DRB1*15 DQB1*06 | [115] |
| A*33:01¥ | [65] | |
Association with specific agents (High positive predictive value) | ||
Terbinafine Fenofibrate Ticlopidine | A*33:01 | [65, 118] |
| Amoxicillin-clavulanate | A*0201 B1*1501 B*1801 DQB1*02 DQB1*0602 DQR1*06 DRB1*07 DRB1*15 DRB5*0101 | [119] |
| Flucoxacillin | B*5101 B*57-03 | [12, 115] |
| Oral contraceptives | ABCB11 1331T>C polymorphism | [22] |
¥ Excluding cases of amoxicillin-clavulanate and flucoxacillin
In recent decades, progress has been made in discovering the genetic factors that increase susceptibility to DILI, in particular by identifying allelic variations of HLA class I and II [65], as described in Table 4. Some HLA alleles have a high negative predictive value and can therefore be a usable tool in clinical practice to exclude the diagnosis of DILI or to rule out a particular drug, herbal product, or dietary supplement, especially when several potential agents are involved [117, 118]. An increased risk of cholestatic and mixed DILI (especially from terbinafine, fenofibrate, and ticlopidine) has been associated with the HLA-A*33:01 alleles [65, 119].
On the other hand, some HLA alleles associated with DILI due to amoxicillin-clavulanate, one of the drugs that most frequently causes cholestatic DILI, have been identified. The HLA-B1*1501-DRB5*0101-DQB1*0602 haplotypes have been identified in 57–67% of patients with DILI due to this drug while it is only present in 15–20% of the general population [16, 17, 120].
Other alleles identified for amoxicillin-clavulanate hepatotoxicity are HLA-DQR1*06, HLA-B*1801, HLA-A*0201 and, in particular to cholestatic/mixed pattern HLA-DRB1*15, HLA-DQB1*06, HLA-DRB1*07, and HLA-DQB1*02. In addition, Genetic variants have been described in relation to other agents that cause cholestatic injury such as flucloxacillin (HLA-B*5701 and B*57-03) and oral contraceptives (ABCB11; 1331T>C polymorphism) [12, 22, 116].
Age has also been shown to be an important contributor to the phenotypic expression of DILI [70]. In an analysis of the Spanish DILI Registry involving 882 patients, the pattern of liver injury shifted towards cholestatic with increasing age for the top culprit drugs amoxicillin-clavulanate, atorvastatin, levofloxacin, ibuprofen, and ticlopidine, with the best cut-off point for increased odds of cholestatic DILI being 65 years [70, 120, 121]. The mechanisms underlying the increased risk of cholestatic injury in older patients are unknown but, speculatively, a diminished renal clearance and biliary function, would favour a more cholestatic liver reaction to drugs [116]. For instance, prolonged canalicular excretion and exposure of the bile duct cells to amoxicillin-clavulanate, might favour an immune attack against these cells [121]. Indeed, the frequency of DILI events attributed to drugs with potential to inhibit the bile salt pump export protein and that undergo biliary excretion also increases in the elderly [122].
The relative prevalence of cholestatic DILI vary across populations. In the United States, the percentages for cholestatic disease in DILI are higher than in the Spanish and Asian (Korean, Chinese, and Japanese) populations [3, 6, 8–10]. The reasons behind these differences are unclear but differences in causative drugs and in the rate of comorbidities could contribute. Actually, in the American population, there was evident a high prevalence of obesity and underlying metabolic syndrome. In addition, there are already published studies showing that higher comorbidity is associated with higher 6-month mortality in patients with DILI [123].
On the other hand, ethnicity also seems to influence the higher frequency of DILI by some specific agents, as well as differences in the phenotype. The DILIN group carried out an analysis to compare 144 African-Americans and 841 Caucasian patients with DILI. This study showed a higher prevalence of cases of DILI due to TMP-SMX (7.6% vs. 3.6%), methyldopa (4% vs. < 1%) or phenytoin (5% vs. < 1%) and a lower number of cases of amoxicillin-clavulanic acid (4.1% vs. 13.4%) in the African-American population compared to Caucasian patients. Besides, African-Americans are more prone to severe cutaneous reactions, lower average levels of ALT and more severe liver injury leading to worse outcomes, including death and liver transplant [124].
The first and most important therapeutic measure when DILI is suspected is the immediate withdrawal of all possible causative agents and avoidance of rechallenge. This requires a thorough assessment of causality. In addition, close laboratory monitoring is required in the first days and weeks after DILI is detected. Cholestatic injury resolves more slowly than hepatocellular injury and requires longer follow-up. Indeed, ALP levels have at DILI onset may predict both chronicity and prolonged recovery [125, 126].
In clinical trials, the stopping rules used to mitigate hepatotoxicity are helpful but only apply to hepatocellular damage since they use predefined thresholds for ALT and AST. The rationale behind this strategy is that small increases in ALT (< 3× ULN) would not require discontinuation of treatment, as an “adaptation” often occurs. However, there are no defined stopping rules for ALP elevations [17].
In addition, initial management includes symptomatic treatment and general supportive care involving the use of antiemetic, analgesics, and parenteral hydration. It is also important to control pruritus, especially in cholestatic DILI, for which cholestyramine, antihistamines, phenobarbital, rifampicin, opioid analogues, ultraviolet B phototherapy, and plasmapheresis have been tried (Figure 2) [12, 16, 127].
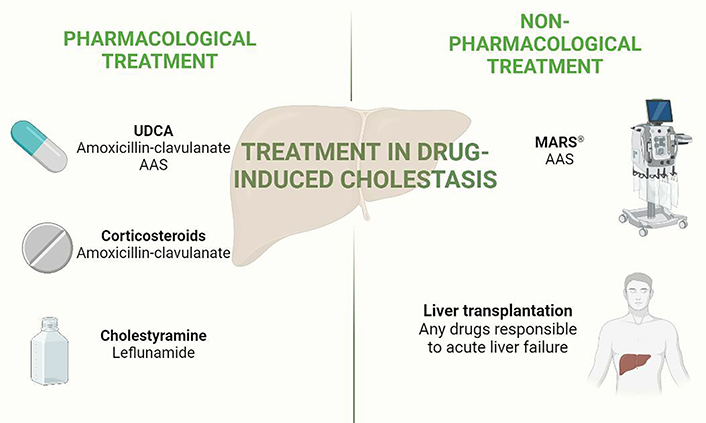
Medical tools available in the management of drug-induced liver cholestasis. This figure shows pharmacological and non-pharmacological tools to treat cases of cholestatic DILI. Bold words: main therapies, normal words: most representative drugs which have been analysed in the main studies commented in the text. Created in BioRender.com. MARS®: Molecular Adsorbent Recirculating System®; UDCA: ursodeoxycholic acid
UDCA has been used for decades in cholestatic DILI to reduce the time to resolution. In cholestatic liver disease, UDCA provides protection against bile salt toxicity, stimulates hepatobiliary secretion, has antioxidant activity, enhances glutathione levels, and inhibits liver cell apoptosis [116, 128]. A recent systematic review suggests that UDCA may be effective and safe in both prevention and treatment of DILI, although the lack of robust trials means that a strong recommendation cannot be made [129].
Corticosteroids have been prescribed empirically, especially in DILI patients with immnoallergic features or when there is no response to other therapies. Their use is controversial as some studies have shown no benefit, or even worsening, in cases of acute liver failure [130, 131]. On the other hand, a recent propensity score-matched analysis showed benefits and lack of serious risk of corticosteroids in DILI. In these cohorts 12% and 20% of the group treated with corticosteroids were cholestatic and mixed respectively, compared to 20% cholestatic and 20% mixed pattern in the untreated arm [132]. Specifically, in cholestatic DILI, there is limited evidence on the use of corticosteroids. Their use could be beneficial in combination with UDCA in terms of rapid reduction of bilirubin and transaminases [133].
Specific therapies have been described for particular forms of DILI. One example is the use of cholestyramine for terbinafine-induced liver injury, which presents with a cholestatic pattern in most cases. Cholestyramine also seems to be beneficial in DILI caused by leflunomide, although this agent mostly induces a hepatocellular pattern. The use of cholestyramine appears to short the course of hepatotoxicity, although these data come from case report and case series [17, 134, 135].
In patients who do not respond to standard medical therapy, the use of extracorporeal liver support systems may be considered, although these methods have not been shown to decrease mortality in patients with liver failure [17, 134]. However, an improvement of cholestasis parameters in patients with anabolic steroid-induced liver injury and DILI related with a few drugs has been reported with the use of extracorporeal liver support system, called albumin dialysis with MARS® [136–139].
Drug-induced cholestasis is a presentation of hepatotoxicity, which varies in causative drugs, severity, histological features, and outcome, often leading to prolonged or chronic damage. Emerging varieties of drug-induced cholestasis that physicians should be aware of include sclerosing cholangitis. Older people are particularly at risk of suffering toxic cholestasis regardless the causative agent. The discovery and validation of biomarkers of cholestasis and bile duct injury is an urgent need for drug development and daily practice.
AAS: anabolic androgenic steroids
ALP: alkaline phosphatase
ALT: alanine aminotransferase
COVID-19: coronavirus disease 2019
DILI: drug-induced liver injury
DILIN: Drug-Induced Liver Injury Network
HLA: human leukocyte antigen
MARS®: Molecular Adsorbent Recirculating System®
NSAIDs: non-steroidal anti-inflammatory drugs
PBC: primary biliary cholangitis
RUCAM: Rousell Uclaf causality assessment method
SARS-CoV-2: severe acute respiratory syndrome coronavirus 2
TMP-SMX: trimethoprim/sulfamethoxazole
UDCA: ursodeoxycholic acid
ULN: upper limit of normal
JMPB and JPTO equally contributed to: Conceptualization and Writing—original draft. RJA: Conceptualization, Writing—original draft, and Writing—review & editing. MGC: Writing—review & editing. All authors have read and agreed to the published version of the manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Grants from
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Maitane Asensio ... Jose J. G. Marin
Ricardo Espinosa-Escudero ... Maria J. Monte
Grégory Merlen ... Thierry Tordjmann
Beatriz Sanchez de Blas ... Marta R. Romero
Carola Dröge ... Verena Keitel
Vasiliy Ivanovich Reshetnyak, Igor Veniaminovich Maev
Elias Kouroumalis ... Argyro Voumvouraki
