Affiliation:
Department of Internal Medicine, Ospedale Civile di Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41125 Modena, Italy
Email: a.lonardo@libero.it; lonardoamedeo2@gmail.com
ORCID: https://orcid.org/0000-0001-9886-0698
Explor Dig Dis. 2023;2:188–201 DOI: https://doi.org/10.37349/edd.2023.00026
Received: April 20, 2023 Accepted: June 14, 2023 Published: August 30, 2023
Academic Editor: William Ka Kei Wu, The Chinese University of Hong Kong, China
The article belongs to the special issue Advances in Hepato-gastroenterology: Diagnosis, Prognostication, and Disease Stratification
Nonalcoholic fatty liver disease (NAFLD) is an umbrella definition that describes the ectopic deposition of fat within the liver that occurs in the absence of inciting factors other than the metabolic syndrome and its individual features. NAFLD has a multi-factorial pathogenesis which determines heterogeneous clinical phenotypes and variable natural course spanning from liver-related (steatohepatitis, fibrosis, cirrhosis, hepatocellular carcinoma) to extrahepatic outcomes (cardio-metabolic and cancer). This narrative review article leverages the key aspects of disease natural history as the background information to discuss studies that may inform strategies to risk-stratify NAFLD patients. Evaluation of hepatic fibrosis with non-invasive tools, including blood-based biomarkers and imaging-based elastometry techniques, seemingly retains the core information useful to predict the heterogeneous outcomes listed above. Additionally, genetic testing and metabolomic profiles may also be utilized to this end. In conclusion, a comprehensive understanding of the variable hepatic, cardio-metabolic and cancer outcomes of NAFLD may enable physicians and researchers to risk-stratify and accurately identify the multilayered prognosis of NAFLD individuals while also defining homogeneous patient subsets to enroll in clinical trials.
Primary steatosis describes the ectopic deposition of fat within the liver that occurs in the absence of inciting factors such as alcohol, drugs, genetic and endocrine disorders [1]. Nonalcoholic fatty liver disease (NAFLD) is an umbrella definition embracing primary steatosis, nonalcoholic steatohepatitis (NASH), NASH-cirrhosis and NASH-hepatocellular carcinoma (HCC), namely the entire spectrum of alcohol-like liver lesions in individuals who, while not drinking of excessive amounts of alcohol, do exhibit dysmetabolic features [2, 3]. Of concern, paralleling the global epidemic of diabesity, NAFLD is rapidly emerging as a globally prevailing cause of chronic liver disease eventually culminating in progressive liver fibrosis, cirrhosis, and liver failure in a proportion of individuals [4]. Irrespective of whether primary steatosis will lead to hepatic damage under the form of concurrent inflammatory changes (NASH) and abnormal extracellular matrix deposition (NASH-fibrosis, NASH-cirrhosis, and NASH-HCC). NAFLD and the metabolic syndrome (MetS), with which it has a dynamic bi-directional relationship, are typically associated with severe extra-hepatic manifestations and complications, such as cardio-renal diseases, metabolic decompensation, and certain forms of extra-hepatic cancers [5–7].
Such a remarkable hepatic and extra-hepatic phenotypic changeability seemingly results from disease heterogeneity as affected by variable genetic background, epigenomic mechanisms, metabolic factors, and environmental cofactors [8, 9]. Importantly, this heterogeneity of clinical phenotypes raises the key question of predicting the odds of disease progression in any given individual patient, aimed at developing effective prevention and management strategies [10]. While the best approach to accomplish this goal remains undefined both in primary care and in specialist practice, ruling out advanced fibrosis with validated noninvasive scores [e.g., either NAFLD fibrosis score (NFS) or fibrosis 4 (FIB-4) index] presently holds on as the leading paradigm [11]. However, although other simple methods for risk stratification, such as evaluation of gamma-glutamyl transferase (GGT) and fatty liver index score (which also includes GGT assessment) are available [12, 13], it is anticipated that multi-stratified techniques to categorize NAFLD patients more accurately will be adopted in the near future [14].
Alongside with increasingly advocated personalized and precision medicine approaches, risk stratification has become more and more investigated in the hepatological arena aimed at identifying those primary care patients needing hepatological consultation; as well as those who may benefit most of personalized follow-up schedules and more intensive treatment approaches given that they are prone to the highest risk of severe complications [11, 15]. Additionally, the identification of homogeneous patient populations is of key importance in correctly describing subsets of enrollees in randomized clinical trials (RCTs) of novel drug treatments therefore possibly decreasing the presently disappointing results of RCTs [16].
Within the rapidly evolving scenario described above, the present narrative review article will address the heterogenous pathogenesis of NAFLD and its variable clinical course, as well as the background information for analyzing those available tools useful for predicting the risks of progressive fibrosis, cirrhosis, HCC, metabolic decompensation, cardio-nephro-vascular outcomes, and extra-hepatic cancer. With this backset, my review specifically emphasizes surrogate indices of fibrosis given that the role of liver biopsy out of clinical trials has become increasingly limited [17].
Although this is not a systematic review, it is important to declare the strategy of bibliographic research followed to ensure reproducibility of results. My bibliographic research was based on PubMed Central (PMC) database using the following keywords [risk stratification (Title/Abstract)] and [NAFLD (Title/Abstract)]. This research was updated as of the 5th of March 2023 and identified 207 titles. Additional titles were retrieved based on cross-references, consultation of the author’s personal archives, and by specific research necessary to address reviewers’ concerns.
The natural history of NAFLD follows a remarkably variable and largely unpredictable course in the individual patient. For example, the details of why some individuals follow a benign, indolent course while others progress from simple steatosis to progressive liver fibrosis, cirrhosis, and HCC remain incompletely understood [18]. Conversely, we do know that it is hepatic fibrosis, rather than NASH, that determines the prognosis and the clinical course of NAFLD, although NASH is an acknowledged critical step towards the progression to the most severe NAFLD forms in a subset of NAFLD patients [18, 19].
Multiple factors are involved in dictating poor clinical outcomes in a proportion of NASH patients [20]. A robust line of research indeed indicates that, in NASH, pathogenic heterogeneity mirrors differential patterns of response to metabolic stress, susceptibility to intrahepatic lipotoxicity, as well as variable efficacy in the hepatic repair-response to metabolic injury [21]. Recent analysis has found that most individuals exhibit metabolic dysfunction as the primary disease driver (hence the quintessential pathogenic “factor” of NAFLD) [9]. However, a much more sophisticated interpretation of NAFLD pathogenesis has identified active cross-talks of myriads of different disease modifiers. These have collectively been named “cofactors” and comprise sex, genetic polymorphisms, comorbid conditions, gut dysbiosis, infections and lifestyle habits. Cofactors affect NAFLD clinical phenotypes, while also determining distinct prognostic trajectories and, probably, variable responses to therapy [8, 9].
An improved understanding of NAFLD factors and cofactors that affect disease expressivity and outcomes, in its turn, may translate into the benefits of designing more personalized and precision medicine approaches to offer maximally effective management strategies to those NAFLD individuals with the highest risk of poor sequels [16, 22, 23].
Schematically, the natural course of NAFLD comprises hepatic and extra-hepatic events and liver histology changes seemingly play a major role in the determination of both.
Several seminal studies have depicted the natural course of NAFLD [24]. Here I report on two of among the most recently published. In 2016, Vilar-Gomez et al. [25] followed for an average of 5.5 years 458 patients with biopsy-proven NAFLD with either fibrosis stage 3 (F3) bridging fibrosis (n = 159), or compensated cirrhosis (299 patients with Child-Turcotte-Pugh class A cirrhosis) recruited in a multi-national study involving Spain, Australia, Hong Kong, and Cuba. Data have shown that the risk of either hepatic decompensation or HCC were higher among individuals with cirrhosis than among patients with F3 fibrosis; conversely, those with F3 fibrosis compared to patients with cirrhosis, had a higher risk of incident vascular events and extra-hepatic cancers. Finally, the cumulative incidence of extra-hepatic malignancies was higher in patients with F3 fibrosis than among those with cirrhosis. Death or liver transplantation, hepatic decompensation, and HCC were strongly associated with baseline cirrhosis and mild steatosis. Details on fibrosis staging adopted in NAFLD/NASH arena may be retrieved elsewhere [26, 27]. In short, in both principal classification systems, i.e., the NAFLD activity score (NAS, NASH Clinical Research Network) and steatosis, activity, and fibrosis (SAF)/fatty liver inhibition of progression (FLIP) algorithm, F3 identifies bridging fibrosis, namely an advanced form of pre-cirrhotic disease [28]. This seminal study was first in demonstrating that NAFLD-cirrhosis is associated with liver-related clinical complication, whereas those with bridging fibrosis are at risk principally of extra-hepatic cancers and vascular events [25].
In 2021, expanding these findings further, Sanyal et al. [29] by following for a median of 4 years 1,773 adults with biopsy-proven NAFLD found that, after adjusting for confounding factors, the stage of fibrosis predicted the odds of any incident event of hepatic decompensation and increased all-cause mortality. In agreement with other reports, this study also found that, compared to those with absent-to-early fibrosis stages (F0 to F2) individuals with fibrosis stage F4 exhibited a higher risk of incident type 2 diabetes (T2D) and chronic kidney disease (CKD) [29]. However, in this study, incident cardiac events and extra-hepatic cancers did not vary across fibrosis stages [29].
As to the risk of primary liver cancer, data suggest that a dysmetabolic milieu, such as seen in NAFLD, is a definite risk factor/co-factor for HCC and an increasingly acknowledged risk factor for cholangiocarcinoma as well [30]. In agreement, a recent meta-analysis by Yi et al. [31] found that cholangiocarcinoma was strongly associated with NAFLD [odds ratio (OR) 2.05 1.53–2.75].
In NAFLD, differently from chronic liver disease owing to other etiologies, HCC risk is not restricted to those exhibiting NAFLD-cirrhosis. Indeed, also non-cirrhotic NAFLD, namely uncomplicated steatosis and NASH, may be precursor lesions of HCC although, among these patients, the incidence of HCC fails to reach those thresholds which justify screening campaigns [32].
The pathomechanisms underlying the association of metabolic fatty liver syndromes with primary liver cancer have recently been reviewed [30, 33].
Extra-hepatic outcomes may be classified as cardiovascular, metabolic and (extra-hepatic) cancer [34, 35].
Probably the best evidence that NAFLD per se is a strong cardiovascular risk factor comes from a meta-analytic review published by Mantovani et al. [36]. These authors analyzed 36 published longitudinal studies collectively totaling information regarding 5,802,226 adults. During a median 6.5-year follow-up, 99,668 incident cases of both fatal and non-fatal cardiovascular events were observed and NAFLD was shown to be linked with a moderately increased risk of fatal or non-fatal cardiovascular disease (CVD) events [pooled random-effects hazard ratio (HR) 1.45, 95% confidence interval (CI) 1.31–1.61; I2 = 86.18%]. However, the magnitude of this risk markedly increased in parallel with increasing NAFLD severity, particularly the stage of fibrosis (pooled random-effects HR 2.50, 95% CI 1 68–3 72; I2 = 73.84%). All risks occurred irrespective of common cardiometabolic confounding factors (e.g., age, sex, adiposity, and diabetes). Finally, these findings were not modified by sensitivity analyses supporting the notion that NAFLD undoubtedly is a strong determinant of both morbidity and death owing to CVD [36].
While it is often repeated that “NAFLD is the hepatic manifestation of the MetS”, it is rather true that the NAFLD-MetS relationship is mutual and bi-directional. Several studies document this notion [3]. For example, Ballestri et al. [37] were first in evaluating, in a meta-analytic review, 20 published studies totaling 117,020 individuals. Analysis of data has shown that NAFLD, irrespective of whether diagnosed by either liver enzymes or ultrasonography, significantly increased the risk of incident T2D and MetS over a median 5-year follow-up. Along the same line, Mantovani et al. [38] by analyzing 501,022 individuals enrolled in 33 studies and followed for a median 5-year time found that, compared to non-NAFLD controls, those with NAFLD were prone to an increased risk of incident diabetes and that more “severe” NAFLD and fibrosing NAFLD were both associated with an even higher risk of incident diabetes irrespective of common confounding factors (age, sex, adiposity), of sensitivity analyses, and without any evidence of significant publication bias.
Since 2008, innumerable studies have addressed with various disease and outcome definitions the association of NAFLD with CKD [39]. Such a robust and prolific line of research has also prompted some meta-analytic reviews. Among the most updated of such studies, Mantovani et al. [40], based on their selection criteria, worked on 13 published papers totaling 1,222,032 individuals (28.1% with NAFLD) who, over a median follow-up of 9.7 years, featured 33,840 cases of incident CKD stage ≥ 3 (defined as estimated glomerular filtration rate < 60 mL/min per 1.73 m2, with/without proteinuria). Data have shown that NAFLD was linked with a 1.45-fold increased long-term risk of incident CKD stage (HR 1.43, 95% CI 1.33 to 1.54; I2 = 60.7%) independent of conventional confounding factors (age, sex, adiposity, arterial hypertension, diabetes); findings were not altered by sensitivity analyses and no significant publication bias was revealed by funnel plot [40].
These findings may have a relevance in risk-stratification of NAFLD, although some methodological issues have been identified regarding the original studies that were included in this review [39].
Two meta-analytic reviews have recently addressed the research question of whether NAFLD is associated with the risk of extra-hepatic cancers. The two studies agreed on some findings and differed for others.
Mantovani et al. [41] retrieved and analyzed 10 published cohort studies, globally totaling 182,202 adults. NAFLD, captured with either imaging techniques or International Classification of Diseases codes, was found in approximately one quarter of cases. During a median 5.8-year follow-up, 8,485 incident cases of extrahepatic cancers were observed and, compared to non-NAFLD controls, NAFLD was significantly associated with an approximately 1.5-fold to 2-fold increased risk of cancers of esophagus, stomach, pancreas or colorectal cancers, and with an approximately 1.2-fold to 1.5-fold increased risk of lung, breast, gynecological or urinary system cancers. All risks occurred irrespective of confounding factors such as age, sex, smoking, obesity, diabetes. Interestingly, the primary pooled analyses exhibited an overall relatively low heterogeneity in most of the studies; moreover, findings were not altered by sensitivity analyses and no significant publication was revealed by funnel plots cases. However, no studies with biopsy-proven NAFLD were available for the analysis [41].
A few months later, also Yi et al. [31] conducted an umbrella meta-analytic review to comprehensively summarize the associations of NAFLD with extrahepatic outcomes. These authors reported that individuals with NAFLD had an increased risk of the following extra-hepatic cancers/tumors: thyroid cancer, pancreatic cancer, esophageal cancer, gastric cancer, colorectal cancer/adenoma, urinary tract cancer, breast cancer, and lung cancer. However, the entity of this risk varied across different cancer types (from HR, 1.25 for lung cancer to HR, 2.63 for thyroid cancer). Conversely, no significant association was found between NAFLD and blood cancer, cancer of female genital tract and prostate cancer outcomes [31].
Based on the notions discussed under paragraphs 2 and 3 above, different methodological approaches may be followed to implement an effective risk-stratification in NAFLD. Although such approaches will be discussed separately below, it is probable that composite scores and complex classifications systems will be adopted soon. One of such approaches takes in consideration the liver status, disease determinants, and extrahepatic feature, hence it is named “LDE” and has been described previously in detail [1, 9, 14].
The principal modifiers of risks in NAFLD and, therefore, the best candidate to implement a stratification of risks include genetics; hepatic status; and cardiovascular risk assessment.
An outstanding panel of experts from USA, Europe, Australia, and Asia, have issued the following criteria to follow to identify those NAFLD patients who should be referred from a primary care pathway to hepatological practice [11]. According to these authorities, sequential steps should be taken. It is important to identify those who have multiple metabolic risk factors; T2D; or imaging-proven steatosis or raised transaminases. Such people should undergo further laboratory testing including FIB-4 evaluation, and those with indetermined risk, be submitted to liver stiffness measurement (LSM). Those patients deemed to be at a high risk, defined by LSM > 12 kPa, should undergo hepatological evaluation [11]. Liver stiffness may be measured with different imaging techniques which are based either on magnetic resonance (magnetic resonance elastography) [42] or on ultrasonography. The latter include transient elastography [43], shear wave elastography [44], acoustic radiation force impulse techniques, and strain elastography technique [45].
Several studies have addressed risk stratification with reference to variable liver outcomes (Table 1).
Risk stratification of liver outcomes in NAFLD
| Author, year [Ref.] | Series | Findings | Conclusion |
|---|---|---|---|
| Dongiovanni et al., 2018 [46] | 9,414 individuals from three study populations were recruited: the liver biopsy cohort, the Swedish Obese Subjects Study and the population-based Dallas Heart Study | Intra-hepatic fat accumulation was associated with liver disease and dysmetabolic traits Genetic variants affect liver damage proportionally to their steatogenic capacity | Long-term accumulation of fat in the liver causes CLD |
| Labenz et al., 2018 [47] | 261 non‐cirrhotic biopsy-proven NAFLD German patients were enrolled | LSM identified advanced fibrosis with an AUC of 0.81 (95% CI 0.72–0.91) while NFS, FIB‐4, and APRI exhibited a lower performance (AUCs of 0.74, 0.71, and 0.67, respectively) | LSM outperformed wet tests in ruling out advanced fibrosis |
| Ioannou et al., 2019 [48] | 7,068 individuals with NAFLD-cirrhosis identified in 2012 were evaluated for the development of incident HCC retrospectively till January 2018 | 7 variables, namely age, sex, BMI, diabetes, platelet count, serum albumin and serum AST/√ALT ratio, selected out of 25 considered potential predictors, were included in the final statistical model | Age, platelet count, serum AST/√ALT ratio and albumin accounted for 93.9% of the risk of incident HCC among individuals with NAFLD-cirrhosis |
| De Vincentis et al., 2022 [49] | The UKBB database was used to assess prospectively incident cirrhosis, decompensated liver disease, HCC, and/or liver transplantation among 266,687 recruited individuals followed during a median 9-year time | PRS-HFC based on polymorphisms in PNPLA3, TM6SF2, MBOAT7, and GCKR improved diagnostic accuracy and PPV for severe liver disease among those classified as at intermediate-high risk with NFS, FIB-4, APRI, or Forns. Risk stratification and prediction were either not or were poorly affected by unfavorable genetics in subjects not having metabolic risk factors | To the ends of identifying severe incident CLD, common genetic variants provide additional prognostic information which is not captured by validated clinical/biochemical parameters |
| Fujiwara et al., 2022 [50] | Derivation set = 48 patients previously submitted to curative HCC ablation Tissue validation set 1 = 106 HCC-naive individuals Tissue validation set 2 = 59 previously submitted to curative HCC resection Serum validation set = 59 HCC-naive | A 133-gene signature, (PLS)-NAFLD predicted incident HCC over a 15-year follow-up High-risk PLS-NAFLD was associated with specific immune cell phenotypes in fibrotic portal tracts along with impaired metabolic regulators PLS-NAFLD was bioinformatically translated into a four-protein secretome signature, PLSec-NAFLD, which was validated in an independent cohort of HCC-naive patients with NAFLD and cirrhosis. Combination of PLSec-NAFLD with a previously defined index (the etiology-agnostic PLSec-AFP) further improved HCC risk stratification | This proof-of-concept study developed and validated PLS/PLSec-NAFLD. Given that they predict long-term HCC risk and estimate effects of therapeutic interventions in patients with NAFLD, these signatures may potentially improve the poor outcome of NAFLD-HCC and disclose novel avenues for HCC chemoprevention |
| Jambulingam et al., 2023 [51] | All 189 patients consecutive new referrals for NAFLD services between 2011 and 2019 were enrolled, 58.7% of whom were submitted to liver biopsy | The fast fibrosis progressors were identified by a combination of metabolites and lipoproteins (AUROC 0.788, 95% CI: 0.703–0.874, P < 0.001) better than with noninvasive markers | The combination of metabolites and lipids may help in the risk-stratification of fast fibrosis progression among NAFLD patients |
| Chen et al., 2023 [52] | NAFLD, defined as otherwise unexplained raised ALT, was assessed in a total of 54,773 individuals belonging to 2 independent: study populations: the MGI (7,893 individuals) and the UKBB (46,880 individuals) cohorts | PNPLA3-rs738409 genotype and diabetes identified patients with FIB-4, 1.3–2.67, currently considered indeterminate risk for NAFLD, who exhibited a risk of cirrhosis similar to those with FIB-4, 2.67, who are considered high-risk | PNPLA3 genotyping improves prognostication of liver outcomes compared to common judgement based on clinical and laboratory assessment |
| Liu et al., 2023 [53] | 550 Chinese with biopsy-proven NAFLD | The combination of serum BAs with WC, DBP, ALT, or HOMA-IR identified mild fibrosis, in either sex, irrespective of obesity with AUROCs 0.80, 0.88, 0.75 and 0.78 in the training set (n = 385), and 0.69, 0.80, 0.61 and 0.69 in the testing set (n = 165), respectively. Interestingly, these AUROCs were more accurately than those yielded by FIB-4, NFS, and Hepamet fibrosis score | Mild fibrosis is accurately identified non-invasively with analysis of secondary BA levels combined with anthropometric and hepato-metabolic biomarkers |
ALT: alanine transaminase; AUROC/AUC: area under the curve; AFP: alpha-fetoprotein; APRI: aspartate transaminase-to-platelet ratio index; AST: aspartate transaminase; PLSec: prognostic liver secretome signature; BAs: bile acids; BMI: body mass index; CLD: chronic liver disease; DBP: diastolic blood pressure; HOMA-IR: homeostatic model assessment for insulin resistance; MGI: Michigan genomics initiative; PLS: prognostic liver signature; PPV: positive predictive value; PRS-HFC: polygenic risk score-hepatic fat content; UKBB: United Kingdom Biobank; WC: waist circumference
These studies are commented as follows. Dongiovanni et al. [46] conducted a methodologically robust study combining data of at-risk probands as well as people from the general population, in which a Mendelian randomization analysis using risk alleles in PNPLA3, TM6SF2, GCKR and MBOAT7, and a polygenic risk score for intra-hepatic fat were utilized. Data support the notion that, probably, it is hepatic steatosis per se that causes incident liver fibrosis, irrespective of inflammatory changes, and that genetic polymorphisms associated with increased intra-hepatic fat content increase the risk of hepatic fibrosis, to the degree predicted by their steatogenic effects [46]. This study indirectly suggests that severity of steatosis and its duration might be utilized for stratifying the risk of fibrosing chronic liver disease, at least among those carriers of “at risk” alleles in PNPLA3, TM6SF2, GCKR and MBOAT7.
Interestingly, although biomarkers are available to identify those fibrotic NAFLD forms, measurement of liver stiffness appears to be more accurate than non-invasive, blood-based scores in ruling out advanced fibrosis. Illustrating this notion, Labenz et al. [47] found that measurement of liver stiffness was able to identify advanced fibrosis more accurately than NFS, FIB-4, and APRI.
Ioannou et al. [48] in their study specifically addressing those variables that might predict the risk of incident HCC among those with NAFLD-cirrhosis found that four out of seven predictors, selected out of 25 potential variables, accounted for most of the observed HCC risk in NAFLD-cirrhosis [48]. This study portends an improved benefit of prediction models risk-based screening strategy over the screen-all approach [48]. Moreover, these simple models are available as web-based tools (www.hccrisk.com) and can be used in individual patients or by healthcare systems or, also, to select high-risk patients to enroll in RCTs.
More recently, De Vincentis et al. [49] reported that, compared to validated clinical and biochemical parameters, the combination of common genetic variants improved the ability to predict severe liver disease both in the setting of general population and in at high risk NAFLD subjects, particularly among those individuals exhibiting metabolic risk factors. This study encourages the utilization of combined PRS-HFC with non-invasive clinical fibrosis scores to identify those individuals who, being at risk of incident severe liver disease, are most at need of aggressive treatment approaches as well as intensive follow-up schedules.
Fujiwara et al. [50] adopted a complex study design. In short, these authors first defined the signature in their derivation set for recurrent HCC. Next, this signature was validated in tissue validation set 1 and tissue validation set 2. Finally, PLS-NAFLD was translated to a serum-protein panel, PLSec-NAFLD, using their computational algorithm, TexSeC, which was externally validated in serum validation set. These authors discovered a 133-gene signature, PLS-NAFLD which was able to predict incident HCC over a 15-year follow-up. High-risk PLS-NAFLD was associated with specific immune cell phenotypes in fibrotic portal tracts along with impaired metabolic regulators. PLS-NAFLD was after validated in independent cohorts of patients with NAFLD who were either HCC naive or HCC. PLS-NAFLD was bioinformatically translated into a four-protein secretome signature, PLSec-NAFLD, which was validated in an independent cohort of HCC-naive patients with NAFLD and cirrhosis. Combination of PLSec-NAFLD with a previously defined index (the etiology-agnostic PLSec-AFP) further improved HCC risk stratification. Therefore, PLS-NAFLD can be used for RCTs and also as a surrogate end-point in studies of HCC chemoprevention [49]. Despite its high academic standard, findings from this study seems to have scarce chances to enter clinical arena by now.
Jambulingam et al. [51] reported that those individuals exhibiting a fast fibrosis progression could accurately be identified through a specific combination of metabolites and lipoproteins which performed better than noninvasive markers. Clearly, those 14 metabolites, that at multivariate analysis were significantly associated with fibrosis progression, are not universally available at hospital laboratories and this study, presently, seems to have high academic importance more than true clinical relevance.
Chen et al. [52] reported that prognostication of liver outcomes may be improved with PNPLA3 genotyping which suggests a more liberal use of this test in clinical practice and in research.
A recent study has followed an innovative approach to predict mild liver fibrosis through analysis of BA serum profile with ultra-performance liquid chromatography coupled with tandem mass spectrometry. To this end Liu et al. [53] found that the combination of serum BAs with widely available clinical and laboratory parameters identified mild fibrosis more accurately than other validated scores of common use [52]. Although this study elegantly shows that assessment of secondary BA levels combined with anthropometric and hepato-metabolic biomarkers accurately identifies mild fibrosis non-invasively, the requirement of sophisticated laboratory machinery hampers a universal diffusion of this strategy.
The technical details of those studies commented above are summarized in Table 1.
Hong et al. [54] speculated that nonalcoholic fatty pancreas (NAFPD) and NAFLD might be risk factors for breast cancer. By evaluating 961 breast cancer patients and 1,006 non-cancer controls, these authors found that, at multivariate analysis, NAFLD, NAFPD, and serum uric acid were independently associated with breast cancer [54]. On these grounds, the authors established a risk assessment model whose rising scores were associated with sharply increasing OR of breast cancer indicating that incident breast cancer may accurately be predicted with the proposed scoring system [54].
Conceptually, it is uncertain whether it is correct to risk-stratify NAFLD patients utilizing the same risk scores that are validated in the general population. Predictably, such algorithms tend to underscore cardiovascular risk in NAFLD given that they fail to specifically address multiple pro-atherogenic pathogenic NAFLD features, such as subclinical inflammation, insulin resistance, hypertriglyceridemia and amount of intrahepatic fat content [55, 56]. In agreement with this prediction, Wu et al. [57] in their study retrospectively evaluating 10,453 individuals (3,519 with and 6,934 without NAFLD) followed for 116 months and exhibiting 957 clinical and 752 subclinical CVD events, have shown that ultrasonographic assessment of steatosis severity, non-invasive liver fibrosis scores and apolipoprotein profiles predicted the 10-year risk of CVD more accurately than the conventional CVD risk scores [57].
More recently, it is becoming increasingly clear that metabolomic signature plays a key role in precision medicine approaches in NAFLD arena [58]. For example, Martínez-Arranz et al. [59] by evaluating metabolome serum profile in 1,154 individuals with biopsy-proven NAFLD, and from four mouse models of NAFLD with impaired very low-density lipoprotein (VLDL)-triglyceride (TG) secretion, and one with normal VLDL-TG secretion, were able to identify three different metabolic subtypes: A, B, and C, occurring with variable prevalence (47%, 27%, and 26%, respectively). While the percent occurrence of NASH and fibrosis was comparable across these subtypes, serum concentrations and rate of secretion of VLDL-TG were lower among subtype A than subtypes B and C, and so was the 10-year high risk of CVD, measured with the Framingham risk score, and the frequency of patatin-like phospholipase domain-containing protein 3 NAFLD risk allele. Collectively, data demonstrate that metabolomic signatures, mirroring known CVD and genetic risk factors, offer clinically relevant information to stratify the risk of CVD in NAFLD with such metabolomic features [59]. Finally, it should not be neglected that cardiovascular risk assessment is of vital importance in determining the entire natural history of NAFLD and that biomarkers of fibrosis predict cardiovascular risk in these patients [60, 61].
Those studies illustrated in the present review have convincingly shown that comprehensive understanding of the various aspects (hepatic, cardio-metabolic and cancer) may enable physicians and researchers to risk-stratify and accurately characterize the multilayered prognosis of individuals with NAFLD.
Data support the notion that simple non-invasive scores, such as FIB-4, may be utilized for conducting an effective risk-stratification of NAFLD in clinical practice by accurately predicting the risks of all-cause mortality, liver-associated clinical events, major adverse cardiovascular events, HCC, and CKD [62] (Figure 1).
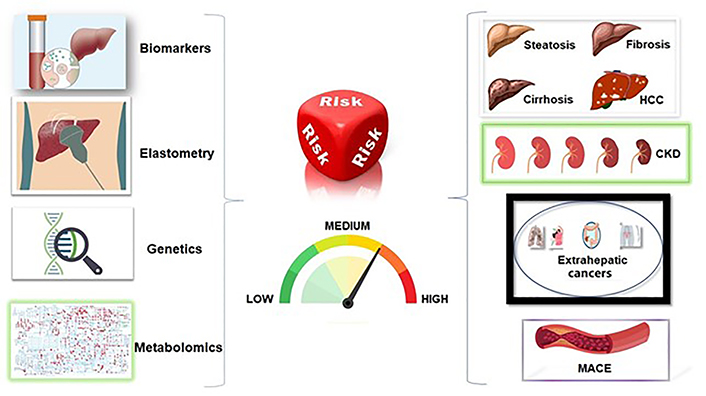
Risk stratification in NAFLD. The left-hand column illustrates the tools to use for risk assessment in the individual patient with NAFLD. Collectively, these diagnostic techniques will allow categorizing patients into various sub-groups with respect to the odds of hepatic events, extra-hepatic cancers and nephro-cardiovascular risks illustrated in the right-hand part of this graphical abstract. MACE: major adverse cardiovascular events
However, such as discussed above, utilization of more sophisticated approaches, also including genetic testing and metabolomic signatures might be necessary to better characterize certain patient subsets, particularly those individuals falling within the “grey zones” with uncertain clinical outcomes. Clearly, these more sophisticated approaches are not universally available, and presently, their use is limited to the fields of research.
Thanks to the risk-stratification strategy, we may now aim at more precision and personalized medicine approaches both in diagnostic guidelines, referral patterns, and follow-up protocols adopted in clinical practice. Additionally, such precision medicine approaches will prove invaluable also in RCTs evaluating HCC chemoprevention strategies and when investigating innovative pharmacological approaches in NAFLD arena.
APRI: aspartate transaminase-to-platelet ratio index
AUROC/AUC: area under the curve
BAs: bile acids
CI: confidence interval
CKD: chronic kidney disease
CVD: cardiovascular disease
F3: fibrosis stage 3
FIB-4: fibrosis 4
HCC: hepatocellular carcinoma
HR: hazard ratio
LSM: liver stiffness measurement
MetS: metabolic syndrome
NAFLD: nonalcoholic fatty liver disease
NASH: nonalcoholic steatohepatitis
NFS: nonalcoholic fatty liver disease fibrosis score
PLS: prognostic liver signature
PLSec: prognostic liver secretome signature
RCTs: randomized clinical trials
T2D: type 2 diabetes
TG: triglyceride
VLDL: very low-density lipoprotein
AL: Conceptualization, Data curation, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Amar Tebaibia ... Nadia Oumnia
Cristina Felicani ... Pietro Andreone
Rudy El Asmar ... Samer AlMasri
Ralf Weiskirchen
Vincenzo Giorgio Mirante
