Affiliation:
1Department of Neuroscience and Behavioral Medicine, University of Santo Tomas Hospital, Manila 1015, Philippines
2Department of Internal Medicine, San Pedro Hospital of Davao City, Inc., Davao City 8000, Philippines
Email: lionheart_sda@live.com
ORCID: https://orcid.org/0000-0003-4271-8435
Affiliation:
1Department of Neuroscience and Behavioral Medicine, University of Santo Tomas Hospital, Manila 1015, Philippines
3Department of Internal Medicine, Chinese General Hospital and Medical Center, Manila 1014, Philippines
Email: jklokin@ust.edu.ph
Explor Neuroprot Ther. 2023;3:398–408 DOI: https://doi.org/10.37349/ent.2023.00057
Received: April 09, 2023 Accepted: August 25, 2023 Published: October 31, 2023
Academic Editor: Silvia Fischer, Justus-Liebig-University, Germany
The article belongs to the special issue Therapeutic Targets for Neuroprotection in Ischemic Stroke
Aim: Stroke is the second most common cause of mortality and disability worldwide with ischemic strokes being the predominant type. The advent of neuroprotectants brought hope of improved outcomes and quality of life, but current guidelines, despite numerous trials, have no strong recommendation advising their use. This meta-analysis aims to evaluate the degree of effect and safety of the neuroprotectants cytidine-5’-diphosphocholine (CDP-choline), cerebrolysin, edaravone, and MLC601, in the recovery of patients with cerebral infarcts.
Methods: An extensive literature search, through the databases of PubMed, PMC, Cochrane, and Ovid, was done with the keywords “CDP-choline”, “cerebrolysin”, “MLC601”, and “edaravone” each combined with the term “acute ischemic stroke”. Eligible studies included randomized controlled trials of these neuroprotectants administered to patients with acute ischemic strokes. A total of 2,025 studies were found, and after the application of screening criteria, 24 studies were eligible for analysis.
Results: The analysis showed that the functional outcome of patients with acute ischemic strokes improved significantly when receiving neuroprotectants versus placebo supported by an odds ratio = 0.29 (0.09–0.50) with a confidence interval of 95%. The P-values are 0.0022 for the one-tailed test, and 0.0030 for the two-tailed test which express the significant improvement of functional outcomes in patients with acute ischemic strokes taking neuroprotectants.
Conclusions: This study thus supports the use of neuroprotectants in patients with acute ischemic strokes to improve long-term functional outcomes and ultimately quality of life.
Despite the number of advances in treatment and care, stroke remains the second most common cause of mortality and disability worldwide, while in the Philippines, cerebrovascular disease is third among all causes of death [1–3]. Based on type, strokes are divided into ischemic and hemorrhagic strokes with ischemic strokes occurring more frequently of the two. In the Philippines, ischemic strokes make up 63% of all stroke types [4]. In the most recent years, the field of stroke medicine has theoretically achieved the pinnacle of treatment of ischemic strokes by way of thrombolysis and mechanical thrombectomy. Currently, these are the cornerstones in the acute management of ischemic stroke with Class I Level A recommendation [5]. Yet, despite such miraculous advances in stroke care, a great majority of patients are unable to receive such treatments due to delayed arrival to capable facilities or lack of able facilities at all. Considering both developed and underdeveloped countries, 65% to up to 85% of patients present beyond the golden period for recombinant tissue plasminogen activator (rtPA) which is 4.5 h [6–9]. After thrombolysis and mechanical thrombectomy, the standard of stroke care is severely lacking in regard to functional recovery with long-term goals relying mostly on secondary prevention strategies against stroke recurrence through the use of statins, anti-platelets, anticoagulants, and control of risk factors, and physical rehabilitation to improve functionality [5].
Neuroprotective agents have been developed and used over the years in the hope of being the panacea for neurological diseases, especially stroke. These medications were designed to improve neuronal survival by their mechanisms of action which include reducing neuronal injury through improving neuronal membrane integrity for cytidine-5’-diphosphocholine (CDP-choline), reducing oxidative stress and inflammation by scavenging reactive oxygen species and other substances causing oxidative stress for edaravone, and, with the newer drugs cerebrolysin and MLC601, by promoting neuronal regeneration and synaptogenesis [10–13]. Among the neuroprotective agents available today in Asia, CDP-choline, cerebrolysin, edaravone, and MLC601 are the most studied in relation to short and long-term stroke recovery. In spite of the pharmacological potential and number of studies involving neuroprotectants, guidelines do not highly recommend the use of such agents classifying them at Class 3 Level A strength of evidence [5].
CDP-choline is also known commercially as citicoline (Figure 1). This is a naturally occurring compound which is a precursor of the neurotransmitter, acetylcholine, and drives the production of phosphatidylcholine which is a component of the cell membrane of the neuron. The active component of CDP-choline works as an antioxidant and anti-inflammatory and influences and modulates the neurotransmitter balance in the central nervous system [10].
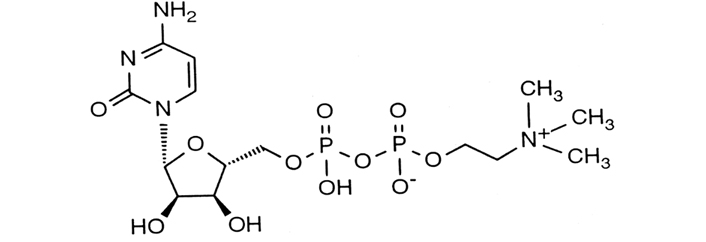
CDP-choline molecular structure with a CAS number of 987-78-0
Note. Adapted from “The role of citicoline in neuroprotection and neurorepair in ischemic stroke,” by Álvarez-Sabín J, Román GC. Brain Sci. 2013;3:1395–414 (https://doi.org/10.3390/brainsci3031395). CC BY.
Cerebrolysin (Figure 2) is a peptide-based nootropic drug derived from porcine brain proteins, composed of low-molecular weight peptides and free amino acids which can penetrate the blood-brain-barrier and act centrally as a neuroprotective agent with anti-inflammatory and antioxidant properties, and neurorestorative properties by activating brain derived neurotropic factor and nerve growth factor to activate neuronal stem cells to mature into neurons that can replace those damaged by stroke. This drug also improves energy metabolism and enhances synaptic plasticity [11].
Edaravone is an organic molecule with a pyrazolone structure, chemical formula C10H10N2O, and its chemical name is 3-methyl-1-phenyl-2-pyrazolin-5-one (Figure 3). It is a reactive oxygen species scavenger and, thus, has antioxidant and anti-inflammatory effects and gives it its neuroprotective properties as well as the ability to stabilize and protect the blood-brain-barrier [12].
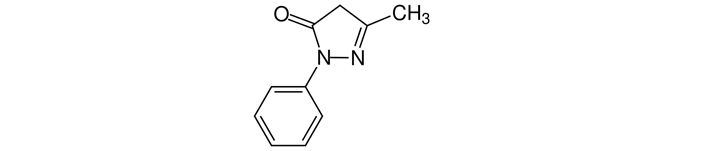
Edaravone molecular structure with a CAS number 89-25-8
Note. Adapted from “Edaravone,” by Wikipedia (https://en.wikipedia.org/wiki/Edaravone). CC0.
MLC601 is a combination of plant extracts from the plants Astragalus membranaceus, Ligusticum wallichii, Panax ginseng, Panax notoginseng, and Pueraria lobata which all have antioxidant, anti-inflammatory, neuro-, and cardio-vascular protective properties. Studies of this drug have shown both neuroprotective and neurorestorative capabilities [13].
The primary goal of this study is to revisit the major trials for each of these neuroprotectants with the aim of highlighting the gravity of the benefits of these medications as a class in the overall functional recovery of patients with ischemic strokes, and thus help validate their utilization in stroke management.
The primary objective of this study is to evaluate the degree of effect of the pharmacologic neuroprotectants CDP-choline, cerebrolysin, edaravone, and MLC601 in the functional recovery of patients with ischemic strokes. The secondary objective is to describe the safety of administering neuroprotectants in ischemic strokes.
The following databases were utilized for extensive literature search: PubMed (1976 to June 2020); PMC (January 1980 to June 2020); Ovid (1982 to June 2020) and Cochrane (1980 to 2020). A comprehensive search strategy was used with the following terms “CDP-choline”, “cerebrolysin”, “MLC601”, and “edaravone” each combined with the term “acute ischemic stroke”. The search strategy was adapted for each database in order to achieve more sensitivity and improve the quality of yielded studies. The references of relevant publications found by the search were screened for further studies which may be included in the analysis.
Eligible studies included randomized controlled trials (RCTs) involving CDP-choline, cerebrolysin, edaravone, and MLC601 administered to human patients with acute ischemic strokes. Publication status did not affect eligibility, and only studies in English were included to avoid any alteration or inaccuracy of the data when translated.
RCTs with patients of any age or sex with acute ischemic stroke confirmed by neuroimaging [i.e. computed tomography (CT) scan or magnetic resonance imaging (MRI)] were included. RCTs of patients with intracerebral hemorrhage, traumatic brain injury, and subarachnoid hemorrhage were excluded.
RCTs involving the compounds CDP-choline, cerebrolysin, edaravone, and MLC601, administered at any dose or in any form for any period of time, pitted against a placebo or the combination of a neuroprotectant and standard of care against standard of care alone were included. Studies which compared multiple neuroprotectants against each other or against a placebo were excluded.
In the included RCTs, the primary outcome evaluated was functional status (e.g., Barthel Index score, modified Rankin Scale, Glasgow Outcome Scale, or trialists’ own definition) at the end of a follow-up period which lasted at least 14 days. Secondary outcomes to be measured are the occurrence of death and the frequency of adverse events due to the neuroprotective agent.
The authors reviewed the abstracts of articles retrieved in the search. Full-length papers for any possible abstract that met the inclusion criteria were obtained. The review of all retrieved papers to identify the trials that met the inclusion criteria for the study was likewise done.
Independent extraction of the efficacy data from eligible trials was performed. Discrepancies were resolved via discussion between the authors and by referencing the original report. The data extracted from the eligible studies were organized in table form on Microsoft Excel with the main author of the study, the year it was completed, the study population, the sample size, the mean baseline National Institutes of Health Stroke Scale (NIHSS), time of medication initiation, dose and route of medication, and follow-up period.
Dichotomous statistical meta-analytic methods were used, and the Mantel-Haenszel method was applied for the estimation of odd ratio (OR) in fixed effects and DerSimonian-Laird for random effects to calculate the global treatment effect. Stata version 16.1 by StataCorp LLC and Microsoft Excel 2019 were used to encode and process the data statistically.
Literature search using the PubMed, PMC, Ovid, and Cochrane online search engines yielded a total of 2,025 studies with 297 studies for cerebrolysin, 542 for CDP-choline, 1,109 for edaravone, and 77 for MLC601. Of these initial search outcomes, 24 studies were retained and further scrutinized for inclusion in the analysis. After application of the inclusion and exclusion criteria, 24 studies were selected for evaluation. After further deliberation on the details of these 24 studies, the number of included studies was reduced to 15 with 5 studies for cerebrolysin, 4 studies for CDP-choline, 3 studies for edaravone, and 3 studies for MLC601 which were included in the final statistical analysis. A flow diagram describing this process is presented in Figure 4. A summary of the included studies is presented in Table 1.
Summary of included studies evaluating the effectivity of neuroprotectants in ischemic strokes
| Source | No. of patients | Age (mean) | Baseline NIHSS (mean) or stroke severity | Initiation of medications | Dose | Route | Follow-up period |
|---|---|---|---|---|---|---|---|
Muresanu et al. (2016) [14] | 208 | 64.0 | 10.4 | Within 24 h to 72 h after the onset of stroke | 30 mL cerebrolysin in PNSS once a day for 21 days | IV infusion | 90 days |
Heiss et al. (2012) [15] | 908 | 65.3 | 16.4 | Within 12 h after the onset of stroke | 30 mL cerebrolysin in PNSS once a day for 10 days | IV infusion | 90 days |
Gharagozli et al. (2017) [16] | 100 | 67.8 | 11.1 | Within 18 h after the onset of stroke | 30 mL cerebrolysin in PNSS once a day for 7 days then 10 mL until day 30 | IV infusion | 30 days |
Stan et al. (2017) [17] | 60 | 64.3 | 10.0 | Within 24 h to 48 h after the onset of stroke | 30 mL cerebrolysin in PNSS once a day for 10 days | IV infusion | 30 days |
Lang et al. (2013) [18] | 119 | 66.3 | 11.65 | Within 30 min from rtPA administration | 30 mL cerebrolysin in PNSS once a day for 10 days | IV infusion | 90 days |
Clark et al. (1999) [19] | 394 | 70.5 | 13.0 | Within 24 h after the onset of stroke | 500 mg CDP-choline once daily for 6 weeks | Oral intake | 6 weeks |
Clark et al. (1997) [20] | 259 | 67.8 | 12.85 | Within 24 hours after stroke onset | 500 mg, 1,000 mg, and 2,000 mg CDP-choline daily in 1–2 doses daily for 6 weeks | Oral intake | 12 weeks |
Dávalos et al. (2012) [21] | 2,298 | 72.9 | 15.0 | Within 24 h after the onset of stroke | 1,000 mg CDP-choline IV in PNSS twice a day for 3 days then 1,000 mg tab twice a day for a total of 6 weeks | IV infusion for 3 days then oral intake for a total of 6 weeks | 90 days |
Clark et al. (2001) [22] | 899 | 67.5 | 14.2 | Within 24 h after the onset of stroke | 1,000 mg CDP-choline twice daily for 6 weeks | Oral intake | 6 weeks |
Sun et al. (2019) [23] | 130 | 51.9 | 22.2 | During admission | 30 mg edaravone in PNSS twice daily for 14 days | IV infusion | 14 days |
Sharma et al. (2011) [24] | 50 | 57.1 | 10.3 | Within 6 h to 72 h after the onset of stroke | 30 mg edaravone in PNSS twice daily for 14 days | IV infusion | 90 days |
EAISG (2003) [25] | 250 | 66.2 | Mild to moderate | Within 72 h after the onset of stroke | 30 mg edaravone in PNSS twice daily for 14 days | IV infusion | 12 months |
Chen et al. (2013) [26] | 1,100 | 61.4 | 8.7 | Within 72 h after the onset of stroke | 4 capsules MLC601 3 times daily for 3 months | Oral intake | 3 months |
Venketasubramanian et al. (2017) [27] | 880 | 61.8 | 8.6 | Within 72 h after the onset of stroke | 4 capsules MLC601 3 times daily for 3 months | Oral intake | 2 years |
Harandi et al. (2011) [28] | 150 | 65.3 | Mild to moderate | Within 1 month after the onset of stroke | 4 capsules MLC601 3 times daily for 3 months | Oral intake | 12 months |
PNSS: plain normal saline solution; IV: intravenous; EAISG: Edaravone Acute Infarction Study Group
The 15 studies included in the final analysis were all blinded, randomized, placebo-controlled trials with a neuroprotectant pitted against a placebo control with patients receiving the standard of care for acute ischemic infarct which included any or all of the following: anti-platelet medications, 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors, rtPA, and physical therapy or rehabilitation. Of the included studies the oldest was published in 1999 and the most recent in 2019. The inconsistency index (I2) was used to measure heterogeneity of the studies included resulting in an index of 70%.
A total of 7,828 participants were noted in the 15 studies included with all patients above the age of 18 years diagnosed with an acute ischemic stroke ranging from mild to moderate severity based on the baseline NIHSS scores. In all the studies, initiation was started within 72 h of symptom onset except for one that started treatment within 90 days from symptom onset. Follow-up was continued for at least 14 days from the initiation of treatment and as long as 24 months thereafter with most studies having a final follow-up at 90 days. All studies were done pitting a neuroprotectant against a placebo with all participants receiving the standard of care appropriate for their stroke during the study period. Safety was evaluated in all studies as well.
The measure of the primary endpoint was varied among the studies but the common factor measured was the change in the functionality of the participants during the study period using validated, widely utilized, and objective scales (e.g., NIHSS, modified Rankin Scale, Barthel Index score, Fugl-Meyer Assessment). Secondary endpoints measured in these studies were related to either other forms of functional outcome measures or safety outcomes (e.g., adverse events, serious adverse events, death rates).
This study provides a quantitative summary of valid and high-quality clinical studies performed in qualified centers. The summary of the analysis is presented in a forest plot in Figure 5. Analytical results show that the outcome of patients with acute ischemic strokes improved significantly when receiving neuroprotectants versus placebo [OR = 0.29 (0.09–0.50); confidence interval (CI) = 95%]. The significance of the efficacy of neuroprotectants in stroke recovery is further supported by the P-values calculated at 0.0022 for the one-tailed test and 0.0030 for the two-tailed test; both being < 0.005.
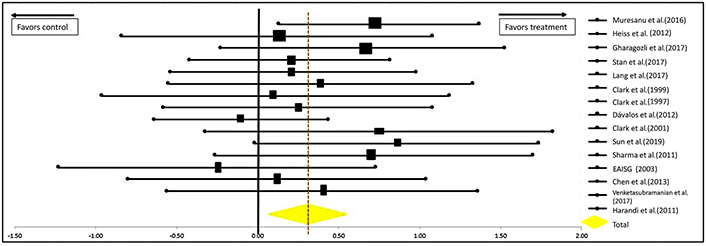
Forest plot from meta-analysis of the effect of neuroprotectants on ischemic stroke recovery showing estimates of the effect size with 95% CI. The relative weight for each trial is indicated by the size of the black square. The dashed line represents the overall pooled estimate. The solid black line represents the confidence interval for each of the included studies
Another analysis was done excluding the study by Dávalos et al. [21] for CDP-choline. This is the International Citicoline Trial on acUte Stroke (ICTUS) trial, the largest study for CDP-choline to date which propelled CDP-choline into popularity when it was first introduced in the market. But despite this, experts deduced that the results of this study were heavily influenced by the peak in the use of rtPAs for acute cerebral infarcts. Thus, to reduce the influence of rtPA in the analysis of our data, we recalculated the data excluding this study and the results can be seen in Figure 6. The significance of the efficacy of neuroprotectants in stroke recovery remains significantly in favor of their use as the P-values are 0.0018 for the one-tailed test and 0.0036 for the two-tailed test [OR = 0.32 (0.11–0.53); CI = 95%].
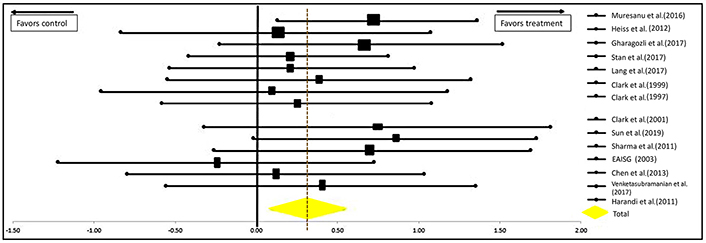
Forest plot from meta-analysis of the effect of neuroprotectants on ischemic stroke recovery excluding the study of Dávalos et al. [21] showing estimates of the effect size with 95% CI. The relative weight for each trial is indicated by the size of the black square. The dashed line represents the overall pooled estimate. The solid black line represents the confidence interval for each of the included studies
Neuroprotectants as a class, despite the number of well-planned, high-quality RCTs done, have remained in the grey area of medications physicians can offer to stroke patients who hope to recover from their disabilities. Most guidelines do not support the administration of such medications, yet in some trials, patients have shown some degree of improvement in both functionality and quality of life. In this era of thrombolysis and thrombectomy, procedures which are considered the current gold standard of ischemic stroke treatment, it would seem that neuroprotectants may not have a role in stroke treatment. Nevertheless, when considering the delay in time to first consult in highly urbanized countries for patients arriving within 2 h from symptom onset, less than 30% receive rtPA; and only 15% of patients arriving within 6 h from symptom onset undergo mechanical thrombectomy [29, 30]. Data from developing countries were far less encouraging as the cost of treatment, lack of medications, and limited expertise constrain the capability of physicians to offer the gold standard of treatment. Therefore, the desire to offer more alternatives to improve the probability and degree of recovery from stroke is paramount.
The present meta-analysis offers data on the effect of CDP-choline, cerebrolysin, edaravone, and MLC601 in the treatment of acute ischemic stroke compared to placebo. This is the first meta-analysis which considers these medications as a class all together, and provides statistical evidence that as a class, they do provide significant benefits in the functional recovery of patients suffering from acute ischemic strokes. The analysis shows a significant improvement in the functional outcomes of patients receiving neuroprotectants and this is reflected in the forest plot and P-values. Based on this new data, neuroprotectants as a class should be considered when planning the holistic treatment of ischemic stroke patients, especially in patients who were not eligible or fortunate enough to receive the gold standard of treatment. The ultimate goal in stroke treatment is to return the patient to a functional state where a good quality of life can be attained. It is the belief of the authors that neuroprotectants can increase the viability of this outcome. With regard to the safety of administering the medications, all the studies included in the analysis reported the rates of adverse events, serious adverse events, and mortality and it was shown that all four molecules can be administered safely to patients with ischemic strokes and that only minor, tolerable, and non-consequential adverse events may occur in a small number of patients.
As of the making of this meta-analysis, there are no other meta-analyses that compare these four specific agents or any other drugs in this class nor were there any considering them as one drug-class and statistically evaluating the effectiveness of this class of drugs in the functional recovery of patients with acute ischemic strokes. A systematic review of neuroprotective agents in acute ischemic stroke by Goenka et al. [31], published in 2019 included the four agents in this meta-analysis among other drugs which have been applied in the treatment of ischemic strokes to improve functionality and reduce mortality, and the authors of the study stated that they were unable to statistically analyze the drugs they reviewed due to the presence of remarkable heterogeneity in reporting the study outcomes. They concluded that neuroprotectants, despite encouraging results in pre-clinical studies, do not show significant benefits in actual human trials. They did mention two drugs namely edaravone and MLC601, which were included in this meta-analysis, had potential if more well-designed randomized control studies with long-term follow-up were done. Thismeta-analysis does acknowledge the heterogenicity of the data since the measure of heterogeneity (I2 = 75%) in this study is moderate to high and this is attributed to the varying outcome measures and treatment protocols in the included studies, and inclusion of rtPA in the standard therapy. As with the other authors, it is also recommend that more randomized control trials be done for neuroprotectants to truly establish their ultimate potential, especially for the patient who could not receive the gold standard of treatment.
It can be concluded that, with the current data provided by the high-quality studies included in this meta-analysis, the use of neuroprotective agents (i.e. CDP-choline, cerebrolysin, edaravone, and MLC601) has a significant effect in influencing the functional outcomes of patients with ischemic strokes and should be considered in the holistic approach to treating patients with acute ischemic strokes. Administration of these agents to ischemic stroke patients is safe with only minor side effects expected in a very small number of patients. More uniform trials are warranted to isolate the true effects of neuroprotectants in patients with ischemic stroke who have not received thrombolysis or thrombectomy as part of the treatment in the acute phase of their stroke.
CDP-choline: cytidine-5’-diphosphocholine
CI: confidence interval
EAISG: Edaravone Acute Infarction Study Group
NIHSS: National Institutes of Health Stroke Scale
OR: odd ratio
RCTs: randomized controlled trials
rtPA: recombinant tissue plasminogen activator
The authors of this study acknowledge the contributions of our statistician Mr. Nathaniel Ellano who provided us with the data presented in our meta-analysis.
SDBAZ: Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing. JKL: Conceptualization, Data curation, Formal analysis, Project administration, Supervision, Writing—review & editing. All authors read, reviewed and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The data and materials obtained for this meta-analysis were obtained online using search engines and the research databases mentioned in the methodology. The articles referenced in this paper can be accessed freely by searching for them on PubMed, PMC, Ovid, Cochrane, or Google Scholar.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Said Hachimi-Idrissi
Yang Yang ... Yi Li
Lidija Radenovic
Renata Murguiondo-Pérez ... Antonio Ibarra
Poppy Kristina Sasmita ... Bernadus Bernardino Bramantyo
