Affiliation:
Department of Dietetics and Applied Nutrition, Amity Medical School, Amity University, Gurugram, Haryana 122413, India
ORCID: https://orcid.org/0000-0002-4700-4792
Affiliation:
Department of Dietetics and Applied Nutrition, Amity Medical School, Amity University, Gurugram, Haryana 122413, India
Email: medhananjaysharma@gmail.com
ORCID: https://orcid.org/0000-0003-3264-4188
Explor Neurosci. 2024;3:103–129 DOI: https://doi.org/10.37349/en.2024.00039
Received: January 31, 2024 Accepted: March 05, 2024 Published: April 07, 2024
Academic Editor: Dirk M. Hermann, University of Duisburg-Essen, Germany
The article belongs to the special issue Enteric Neuro-Gliopathies: Ready for Prime Time?
Background: The main objective of the study was to carry out a systematic literature review to investigate the beneficial role of antioxidants in obesity and diabetes and the association of antioxidants in neuro-gliopathies and gut microbiome on antioxidant production and enteric nervous system (ENS) protection.
Methods: A literature search was done electronically on 8 June 2022 in the databases Google Scholar, and PubMed, reviewing all the articles published in English. There were no limitations for the study (region, or any time frame). The study included randomized controlled trials (RCTs) and observational studies on a human subject, primarily focusing on information such as a change in body weight, body mass index (BMI), waist-to-height ratio (WHtR), waist-to-hip ratio (WHR), fasting blood glucose level, glycated haemoglobin (HbA1c), and other parameters that connected with diabetes and obesity. The search was also conducted for neuro-gliopathies and gut microbiome.
Results: The beginning database search picked out a total of 2,428 articles, 1,310 in PubMed, 876 in Google Scholar, and 242 records from other sources. A total of 2,040 (total duplicates 388) was found after removing the duplicated articles, and after reading the title and abstracts were further decreased to 139 full-text articles. These 139 studies went for full-text analysis, which resulted in the exclusion of 123 studies and generated a final 16 articles included for systemic analysis.
Discussion: This literature search of present studies shows the interconnection between antioxidant intake among obese and diabetes neuro-gliopathies. The findings indicate both obese and diabetic patients have a minimum content of antioxidants, especially carotenoids, retinol, ascorbic acid, tocopherol, magnesium, and zinc. While few research illustrated that ingestion of the abovementioned antioxidants was lowered among diabetes and obese subjects in contrast with their normal-weight population, this was not endorsed by every study.
Antioxidants are the chemical compounds present endogenously as a normal defense mechanism of the host cell or can be taken from the diet. Some examples of enzymatic antioxidants include superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), glutathione S-transferase (GST) and the non-enzymatic antioxidants like reduced glutathione (GSH), carotenoids, flavonoids, lipoic acids, and vitamin A, C and E. GST converts reactive electrophilic species to hydrophilic forms and easily excretable products as a result of their conjugation with GSH. Vitamin C and E are involved in the termination of the lipid peroxidation process. The flavonoids help in scavenging free radicals, and some proteins act as antioxidants such as peroxiredoxin, thioredoxin (TXN), and glutaredoxins [1]. Emerging studies reports indicate that natural antioxidants can modulate oxidative stress (OS) and improve immune function in obesity and diabetes [2]. So, the supplementation of lipoic acid, zinc, carnitine, cinnamon, green tea, and the vitamins C and E, and some evidence for omega-3 polyunsaturated fatty acids, coenzyme Q10 (CoQ10), green coffee, resveratrol, or lycopene [3].
Current findings indicate that inadequate serum levels of a few antioxidants may be associated with excess fat content in the body [4]. Many research have reported that overweight and obese people have a low level of antioxidants in their blood compared with the general population, and there is an inverse relation between antioxidant concentration and body mass index (BMI) [5–7]. The ingestion of high fruits and vegetables is linked with a lower prevalence of obesity and related symptoms and co-morbidities such as diabetes [8]. Many studies suggest that obese people may have to take a large number of antioxidants to accomplish the lipids in the plasma as general-weight people [9].
Obesity and diabetes are becoming the twin epidemic crises globally. Several epidemiological studies reveal that parallel escalation of both metabolic disorders. Recent years have seen an increasing body of work on the following metabolic defects by these disorders that link the impaired tissue perfusion, sleep disturbance, altered vitamin D and micronutrients, and gastrointestinal (GI) tract issues [10]. Diabetic neuropathy also affects the enteric nervous system (ENS) which promotes severe changes in digestive functionality such as motor, secretory/absorptive, and vascular changes [11]. Particularly, the ENS is a part of the autonomic nervous system that comprises a neuronal network distributed across the GI tract all together with enteric glial cells (EGCs) [12, 13]. The EGCs are the non-neuronal cells that constitute the major part of ENS and are involved in physiological activities and homeostatic maintenance of the GI tract [13].
Enteric glia plays an important role as neuroprotective in ENS by secreting neuroprotective compounds such as antioxidant-reduced GSH. The GSH synthesis enzymes in the myenteric plexus with L-buthionine sulfoximine inhibitor impact neuronal survival and inflammation. Both neurons and enteric glia possess the cellular machinery necessary for GSH synthesis [14].
As discussed EGCs provide metabolic support to the ENS neurons, and during gut motility protect their axons. It also noticed that in certain pathophysiological conditions, the EGCs might transdifferentiate into ENS neurons. Recent research revealed that EGCs possess immunological aspects and help patients with several immunological diseases of the gut [15]. Though EGCs are non-immune cells and are capable of producing a variety of immunological responses such as cytokines and chemokines in the response to pathological stimuli, such as EGCs express Toll-like receptors (TLRs) including TLR2, TLR3, and TLR4 [16, 17]. EGC secretes interferon-γ (IFN-γ), interleukin-1β (IL-1β), IL-6, and C-C motif ligand 2 (CCL2) activated by microbial products such as lipopolysaccharides. EGCs generally respond to pro-inflammatory cytokines derived from macrophages and CD4+ T cells. For example, IL-1β triggers the regulation of IL-6 and CCL2 in EGCs. Likewise, IFN-γ and lipopolysaccharides (LPS) co-stimulation of EGCs releases the IL-1β and IL-18 [18]. Excess OS causes neurodegeneration and gut motility and how antioxidants supplementation mitigate these effects have been illustrated in Figure 1.
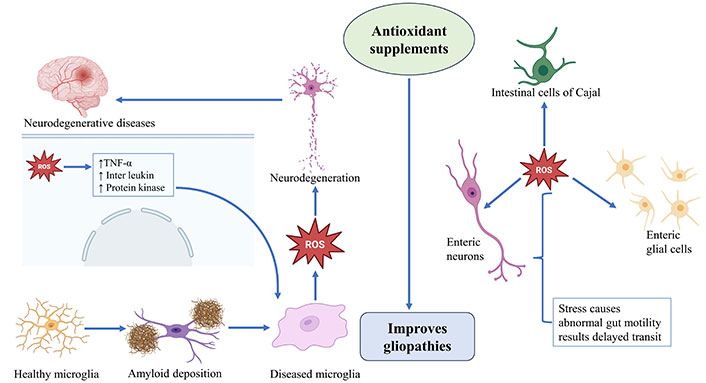
Excess OS causes neurodegeneration and gut motility. TNF-α: tumor necrosis factor-α; ROS: reactive oxygen species. The image was created with the help of Biorender.com
The GSH has complex metabolic and biochemical fates and is a cofactor for the many enzymes that function in modifying the obesity and diabetes responses. The GSH depletion may cause to increase the energy metabolism and reduce adipose accretion, while the elevated GSH peroxidase activity induces insulin resistance [19, 20].
As per the World Health Organization (WHO), obesity is a state of agglomeration of ample fat in adipose tissues so that normal functioning of health can be deprived [21]. Medically, obesity may explain the adult having a BMI greater than 30, but “the definition is not sufficient because it does not fully explain the regional dispensation of fat within the body such as abdominal/visceral vs. subcutaneous. Individuals who have similar total body fat and BMI do not mean they have similar abdominal fat. Abdominal fat agglomeration linked with increased risk of non-communicable diseases”.
Abdominal obesity (AO) is linked with an increased inunction of free fatty acids (FFAs) from the visceral fat depot and metabolic overactivity (such as insulin resistance) [22]. The overflow of FFAs to various organs may be caused by hyperlipolysis of hypertrophied intra-abdominal adipocytes. These conditions may lead to impairing liver function that may cause overproduction of liver glucose and insulin resistance. The liver insulin resistance may reduce the degradation of apolipoprotein B and increase the production of lipoprotein rich in triglycerides (TG) [23]. In obese patients, the intrusion of macrophages occurs in adipose tissues, which may result in inflammation. Other factors may include IL-6 and TNF-α which may increase the inflammation markers of C-reactive protein in plasma [23].
The heavy fat mass may bring many difficulties such as slow locomotion and tiredness in a small distance walking, as back, hip, knee, ankle, and foot pain. On the knee joint, the tendons, and cartilage fascia all are affected because of increased body weight, which may result in osteoarthritis. An abnormal increase in body weight may also invite not only insulin resistance but also metabolic syndrome which are the major factors for cardiovascular diseases (CVDs) [24].
The recent finding suggests that increased body fat may increase the OS that can be assessed by urinary F-2 isoprostanes [25]; hence, the formed free radicals are the important etiology and carry forward the obesity-related comorbidities [26], and many studies show that antioxidant may have safeguarded effect on such conditions [27].
The past few decades and recent studies indicate an increase in obesity and diabetes which represent a major medical problem worldwide. As per the data available from the WHO, 422 million people anguished from diabetes. In 2019, 1.5 million death were directly reported from diabetes [28], 1.9 billion were overweight and more than 650 million adults were suffering from obesity [21]. Several reports present both insulin resistance and impaired insulin release as the main factors for the onset of diabetes [type 2 diabetes mellitus (T2DM)]. In obesity, insulin resistance occurs due to the permanent increase of plasma-FFAs and primary utilization of these lipids by the muscle that induces attenuation of glucose uptake. The increased blood glucose level is compensated by a rise in insulin secretion by the pancreas, and the drop-down in insulin secretion takes place as a late phenomenon [29].
In diabetes, it has been implicated that there is an imbalance between antioxidant scavenging activity and the formation of ROS. ROS are the end product produced during protein glycation and as a result of advanced glycation end-product receptor binding, which impairs the insulin signaling pathway and produces cytotoxicity in islets of Langerhans (especially in β-cells). However, these effects can be minimized by the increased intake of antioxidants. Many findings indicate that the intake of increased antioxidants is effective in minimizing the adverse effect of the disease [30]. The association between unhealthy diets and OS and the interaction of antioxidants and immunity has been described in Figure 2.
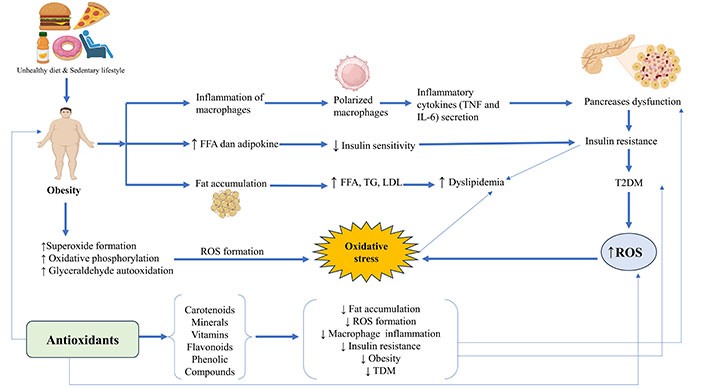
Unhealthy diets and OS and the interaction of antioxidants and immunity. LDL: low-density lipoprotein. The image was created with the help of Biorender.com
Taking into consideration the adverse impacts of diabetes and obesity and being overweight on human health, this study intends on groups of overweight/obese and diabetes subjects to find out the impact of serum/plasma antioxidant concentration and to determine further the influences of antioxidant intervention on demographic characteristics of these subjects. Therefore, we involved observational studies and randomized controlled trials (RCTs), primarily focusing on entities such as weight change, BMI change, waist-to-hip ratio (WHR), waist-to-height ratio (WHtR), fasting blood glucose level, glycated haemoglobin (HbA1c), and other parameters related to diabetes and obesity. The main objective of this systematic review is to find out the relationship between antioxidants in obesity and diabetes. And also, to find the association of antioxidants in neuro-gliopathies.
The systematic review was carried out and summarized as per the recommendations governed by the preferred reporting items for systematic reviews and meta-analysis—PRISMA [31]. The systematic literature review phase wise study has been described in Figure 3.
A broad search strategy was evolved to systematically recognize the studies on the action of antioxidants in diabetes and obesity or mitigate their effect using keywords and controlled vocabulary.
A literature search was done digitally on 8 June 2022 in the databases Google Scholar, and PubMed, reviewing all the articles published in English. There were no limitations to the study (such as study design, region, or any time frame).
The search term for Google Scholar are “antioxidants and obesity”, “vitamin C and obesity”, “vitamin E and obesity”, “tocopherol and obesity”, “carotenoids and obesity”, “ascorbic acid and obesity”, “mineral antioxidants and obesity”, “zinc and obesity”, “magnesium and obesity”, “selenium and obesity”, “antioxidants and diabetes”, “antioxidant and diabetes mellitus”, “antioxidant and blood glucose”, “antioxidant and type 2 diabetes”, “plant-based antioxidants” and “obesity and diabetes”.
The final search for PubMed was ((vitamin C) and (diabetes)) and (obesity), (antioxidant) and (“diabetes and obesity”), (vitamin E) and (diabetes and obesity), (carotenoid) and (diabetes and obesity), (mineral) and (“diabetes and obesity”), (zinc) and (“diabetes and obesity”), (magnesium) and (“diabetes and obesity”), (selenium) and (“diabetes and obesity”), (plant-based antioxidant) and (“diabetes and obesity”).
The study included if they reach the mentioned criteria:
Inclusion/exclusion criteria were based on screening of the titles, keywords, and abstracts of the primary search.
We included all types of studies reporting the role of antioxidants, minerals, and vitamins having an antioxidant effect on patients suffering from obesity and diabetes.
The impact of the gut microbiome on antioxidant production and ENS protection.
Only included human studies.
Studies written in English were eligible to be included. Only full-text articles are included.
There is no limitation in the type of study.
The studies were excluded if they encountered the mentioned criteria:
Studies written in languages other than English.
The studies on an experimental model like rats/mice.
We have excluded the data that represents or talks about lifestyle factors, antioxidants’ effect on other diseases rather than diabetes and obesity, the plant extract effects, and those that do not meet our requirement of title.
The beginning database search picked out an aggregate of 2,428 articles, 1,310 in PubMed, 876 in Google Scholar, and 242 records from other sources. A total of 2,040 (total duplicates 388) was found after removing the duplicated articles, and after reading the title and abstracts were further decreased to 139 full-text articles. These 139 studies went for full-text analysis, which resulted in the exclusion of 123 studies and generated a final 16 articles included for systemic analysis, as depicted in the flowchart presented in Figure 4 and the bar graph in Figure 5.
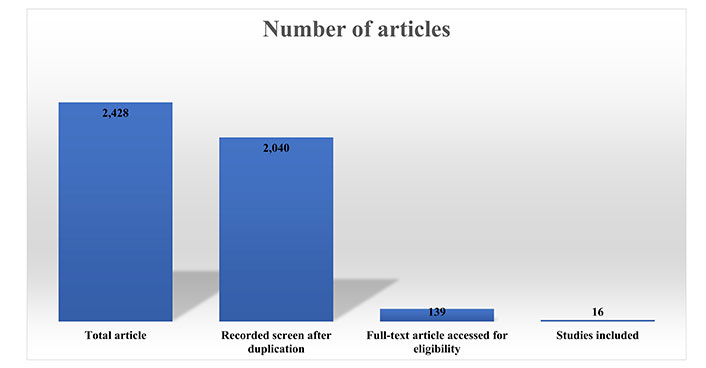
The bar diagram of the systematic review, inclusion, and exclusion studies in the review
An overview of the 16 articles, that encounter the general inclusion criteria, and the detail of the studies such as author(s) & publication year, the aim of the study, country, study design/analysis, descriptions, outcomes, and findings that explicitly deal with the role of an antioxidant in diabetes and obesity is provided in Table 1.
Study characteristics for selected studies
| Author (publication year) | Aims of the study | Country | Study design/analysis | Descriptions | Outcomes | Findings |
|---|---|---|---|---|---|---|
| Canas et al. [32], 2017 | To assess the effect of mixed carotenoids on central obesity and adipokines in children | Florida | A RCT | In this study, 20 children were included having a mean age of 10.5 years old ± 0.4 years old, BMI more than 90th percentile, and supplemented with MCS (contains 2,000 IU β-carotene, 10 mg lutein; 2 mg zeaxanthin, 500 µg of α-carotene, and 10 mg of γ-tocopherol). The primary results were found to change in β-carotene concentration, BMI z-score, and, abdominal fat whereas the secondary outcomes were found in insulin resistance markers and adipokines. | The result shows that there was an inverse correlation of β-carotene with BMI z-score, WHtR, visceral and subcutaneous adipose tissue. | The increased concentration of β-carotene reflects a decrease in BMI z-score, WHtR, and visceral and subcutaneous adipose tissue. MCS suggests an eminent role in obese children. |
| Coyne et al. [33], 2009 | Association between serum carotenoid concentration and metabolic syndrome | Australia | A cross-sectional study | The information was collected from the international diabetes federation 2005, conducted over 1,523 adults with a mean age of 25 years and more from urban areas suffering from metabolic syndrome. The anthropometric data analysis, blood glucose, and lipid levels were determined along with serum carotene levels. | The persons with a metabolic syndrome found significantly lower (P < 0.05) concentrations of mean serum α-carotene, β-carotene, and the sum of all mentioned carotenoids. | The differences were noticed in former and non-smokers but not found in the present smokers. It concluded that the low level of serum α-carotene, β-carotene, and the sum of the included carotenoids was linked with metabolic syndrome. |
| Daniels et al. [34], 2014 | To find out the association between excess consumption of fruits and vegetables increases serum carotenoid, and also activities of lecithin cholesterol acyltransferase (LCAT) and paraoxonase-1 (PON-1) in T2DM. | UK | A RCT | In this study, 8 obese T2DM subjects were randomized to a one or more than six portion per day fruits and vegetable diet were consumed for eight weeks. Fasting and postprandial blood glucose were noted. The serum carotenoids, HDL2 and HDL3 were measured by HPLC, the PON-1, HDL2, and HDL3 by a spectrophotometric and LCAT, HDL2 and HDL3 by fluorometric assay. | When comparing the group having more than six and one portions of fruit and vegetables, it was found that carotenoids increased in serum, along with HDL2 and HDL3 (α-carotene, β-cryptoxanthin, lutein, and lycopene having P values were 0.008, 0.042, 0.012, and 0.016), LCAT and PON-1 in HDL3 (P = 0.044 and 0.006, respectively). | It concluded that increased consumption of fruit and vegetables increased the carotenoid concentration and also influenced the enzyme intermingled with antioxidant properties of HDL, and these changes may help to increase the cardioprotective properties of this lipoprotein. |
| García et al. [35], 2012 | To evaluate the concentration of vitamin A, ascorbic acid, and zinc linked with adiposity and leptin in women | Mexico | A cross-sectional study | In this study, 580 Mexican women (mean aged 37 years old ± 7.5 years old) from the rural area of the said region were evaluated. Anthropometric measurement [such as weight, height, waist circumference (WC), and HC], and fasting blood samples (such as glucose, zinc, leptin, lipid profile, and vitamin A, C, and E) was taken for analysis. | The prevalences of overweight and obesity were found at 36% and 44% (with BMI > 25 kg/m2 and BMI > 30 kg/m2), whereas the prevalences of zinc, ascorbic acid, and tocopherol were alike in these patients but no vitamin A deficiency was found in them. It was noticed that vitamin C was inversely linked with BMI, WHR, and leptin (P < 0.05), whereas retinol was directly linked with leptin (P < 0.05). The leptin concentration was linked with lower vitamin C and zinc concentration in obese women (P < 0.05) and vitamin A concentration was higher in non-obese women (P ˂ 0.01). Whereas tocopherol was not linked with any obesity markers. | The concentration of zinc, retinol, and ascorbic acid were linked with adiposity, obesity, and leptin in women. |
| Harari et al. [36], 2020 | To assess whether tissue carotenoid concentration has an inverse association among obese and insulin-resistant adults | Australia | Cohorts studied | In this study, 80 participants were included between 2008 and 2013 in Sydney with a mean age of 51.4 years old ± 1.1 years old. The retinol, lutein, lycopene, α-carotene, zeta-carotene, β-carotene, phytofluene, and phytoene were assessed by HPLC. Body composition was assessed by Dual-energy X-ray absorptiometry. Insulin resistance in 64 participants was measured by a two-step hyperinsulinemic-euglycemic clamp. In sixty patients MRI was used to measure the extra fat in the pancreas and liver. The carotenoid and retinol were measured by periumbilical subcutaneous fat biopsy in the tissue (n = 16). | Zeta-carotene was three times more in adipose tissue in contrast with serum while lycopene and lutein made up 21 percent and 20 percent of serum carotenoids. Liver (P ≤ 0.028) and adipose tissue (P = 0.023), but not muscle (P ≥ 0.16), insulin resistance was inversely associated with the serum carotenoids. | It concludes that there was a favorable relation between serum carotenoids and adipose tissues and metabolic health in humans. |
| Hekmat et al. [37], 2014 | To assess the relationship between gestational women and fat-soluble antioxidants | Iran | A case-control study | In this study, 82 pregnant women were included for the study among which 41 were suffering from GDM and 41 were healthy women with a 32 weeks gestational age. Five ml venous blood was collected and sent for retinol and α-tocopherol analyzed by chromatography, and the data were calculated with chi-square (χ2) and t-test. | The serum retinol in the non-experimental group was 0.59 mg/dL which was quite higher than the GDM group (0.46 mg/dL; with P = 0.01). The mean α-tocopherol in gestational women was 6.21 mg/dL and in the non-experimental group was 6.92 mg/dL (P < 0.05). | It was concluded that the retinol was remarkably lower in diabetic pregnant women compared with the control group, and this is due to decreased antioxidant concentration in the GDM group. |
| Islam et al. [38], 2013 | To evaluate whether serum zinc concentration linked to diabetes and prediabetes | Bangladesh | A cross-sectional study | In this study, 280 patients were included, among which 51 percent were normal, 13 percent had prediabetes and 36 percent had diabetes. | Mean serum zinc concentration was found low in prediabetic in contrast to normal (65 ppb/L) and diabetic participants (33 ppb/L). Multiple linear regression shows lower zinc levels in prediabetes than in those with normal blood glucose levels. Linear regression for HOMA parameters did not show any statistically significant relation between zinc concentration, insulin resistance (with P = 0.08), and β-cell function (with P = 0.07). | Low serum zinc is linked with insulin resistance along with an increase in BMI. |
| Larsen et al. [39], 2014 | To determine whether ascorbic acid intake may cause a change in WC and body weight, among obese people linked with a genetic predisposition | Denmark | A prospective cohort study | In this study, 7,569 participants were included. They summarized 50 obesity-linked SNPs in four genetic scores and their score for BMI, WC, and WHR with which the SNPs connected. The linear regression evaluated the link between vitamin C consumption and annual change in body weight (ΔBW) or WCs (ΔWC). | The result indicates no remarkable connections between dietary vitamin C and ΔBW or ΔWC. The risk allele of the fourteen WHR-connected SNPs was connected with a ΔWC of 0.039 cm annually (with P = 0.02, at 95% confidence interval: 0.005 to 0.073) per 100 mg every day higher vitamin C intake. | The study does not assist any connection between vitamin C and body weight or WC, but a diet with a high ascorbic acid may be weakly connected with higher WC gain. |
| Manning et al. [40], 2004 | To determine whether higher ingestion of tocopherol may be associated with insulin resistance among the overweight | New Zealand | Randomized clinical trial | Eighty overweight (BMI > 27 kg/m2) arbitrarily selected to obtain 800 IU per day of tocopherol and placebo for 3 months, and after that further 1,200 IU per day of tocopherol dose was increased for three months. | The result shows that plasma peroxidation decreased by 27% in the first three months and by 29% at 6 months. At three months it was noticed that the fasting glucose level and insulin concentration were significantly reduced but these things were not happening with six months of supplementation. Throughout the study, the alanine transaminase concentration was declining. | The author revealed that tocopherol supplementation helps to reduce OS, and the risk of insulin resistance and improves hepatocellular function. |
| Mansego et al. [41], 2015 | To determine the nutrigenetic impact of tocopherol, catechol-O-methyltransferase (COMT), and TXN gene polymorphism on WC | Spain | A case-controlled trial | A total of 738 participants were included among which 246 were abdominally obese and 492 were non-abdominal obese. A valid procedure was used to assess anthropometric, biochemical, OS, and antioxidant intake. DNA was isolated from white blood cells and seven polymorphism genotypic genes taking part in OS were assessed by the SNPlex system. The consequences of c-793T were more than C polymorphism. The TXN was evaluated by reporter assay. | The AO group had increased 8-oxo-2-deoxyguanosine levels and took in less retinol and tocopherol compared to the non-AO group. Logistic regression analysis shows that TXN and COMT were linked with WC and AO. These polymorphisms were more firmly linked with variations in WC in subjects with low tocopherol ingestion. | WC is linked both with dietary tocopherol ingestion and genetic variants of COMT and TXN proposing the presence of a complex nutrigenetic pathway that regulates AO. |
| Mummidi et al. [42], 2021 | To understand the impact of serum carotenoid on the pediatric metabolic index [such as insulin sensitivity (IS)] | Mexico | A cross-sectional study | Five hundred eighty children were involved in the study. Serum β-cryptoxanthin and lycopene concentrations were checked among them by using the UPLC-photodiode array method to check their heritability and association with cardiometabolic risk traits. They used response surface methodology to govern the 2-way interaction of carotenoids and pediatric metabolic index on the Matsuda IS index. | The concentration of β-cryptoxanthin and lycopene were [h2 = 0.58, P = 1 × 10–7 and h2 = 0.98, P = 7 × 10–18]. The study found a negative phenotypic correlation (P ≤ 0.05) between β-cryptoxanthin and five cardiometabolic risk traits (such as BMI, WC, triglycerides, fat mass, fasting glucose of –0.22, –0.25, –0.18, –0.23, –0.09 and positive correlation with HDL (0.29), whereas the lycopene showed an inverse association with fasting glucose –0.08 and conclusive association with HDL (0.18). | Response surface methodology showed increased α and β-carotenoids had maximum effect on IS index compared with β-cryptoxanthin or lycopene. |
| Östh et al. [43], 2014 | To evaluate the β-carotene concentration reduced in obesity and how it is linked with T2DM | Sweden | A cross-sectional study | In this study total, of 55 female patients were included among which 43 were non-diabetic and 12 were T2DM, and subcutaneous adipose tissues were obtained from these patients undergoing elective surgery. Subjects were split into 4 groups BMI < 23, 23 ≤ BMI < 28, to BMI ≥ 28, and T2DM. | The β-carotene concentration was found 50 percent less in adipocytes from the obese and diabetes group than in the other groups. The triacylglycerol was found in 92 ± 1 percent of adipocytes in the lean group whereas 99 ± 2 percent in the diabetic obese group (P < 0.05). | It concluded that β-carotene concentration was lowered in the obese subject and its lower concentration indicates adipocytes from T2DM subjects reflect obesity. |
| Sanchez-Lugo et al. [44], 1997 | To determine any significant relationship between ingestion of tocopherol and ascorbic acid and IS, in Hispanic, non-Hispanic, and African Americans of both sexes | California, Colorado, Texas | A cross-sectional study | In this study, 1,151 participants were included from Hispanic, African American, and non-Hispanic. The tocopherol and ascorbic acid concentrations were evaluated with IS and insulin concentration. IS was assessed by insulin modification, minimal model analysis, and frequent intravenous glucose tolerance test, and nutrient consumption was measured by food frequency questionnaire methods. | The Pearson correlation coefficient for vitamins C and E in corresponds to IS were r = 0.07 (with P = 0.02) and r = 0.07 (with P = 0.01). After monitoring BMI and total energy intake, these associations were not statistically significant. | The findings did not produce any remarkable relation between improved IS and intake of vitamins E and C. |
| Takagi et al. [45], 2020 | To assess the higher dietary ingestions of carotenoid-rich vegetables helps in lowering visceral adiposity among Japanese | Japan | Randomized clinical trial | In this study, 28 Japanese men aged (40 years old to 65 years old) with a BMI of more than 25 kg/m2 were included in 8-week-long randomized clinical trials. Four dietary groups were made with variations in lycopene and lutein concentration. | The result shows that everyday beverage consumption may increase the carotenoids in plasma level and reduce the visceral fat in all groups significantly. The WC was significantly lowered in the group that had high lycopene with low lutein, and the CoQ10 oxidation rate was significantly reduced in each group. Only in low lycopene with low lutein group had to differ in their gene expression profile that indicates the effect of carotenoids on genetic profile. | It concluded that dietary intake of carotenoid-dense vegetables helps in the reduction of intra-abdominal visceral fat. |
| Toprak et al. [46], 2017 | Determining the magnesium supplementation for the obese and pre-diabetes patients having mild to moderate CKD problems helps to improve their metabolic profile. | Turkey | A randomized control trial | In this study, 118 pre-diabetic and obese patients suffering from hypomagnesemia with a calculated GFR rate between 90 ml/min and 30 ml/min per 1.73 m2 were taken in the study. Fifty-seven patients were taking 365 mg of magnesium orally and 61 groups were placebo once daily for 3 months. | The result shows that the group having magnesium compared with placebo had significantly decreased insulin resistance (–24.5 vs. –8.2 percent, with P = 0.007), insulin (–29.6 vs. –2.66 percent, P ˂ 0.001), HbA1c (–6.6 vs. –0.16 percent, P ˂ 0.001), WCs (–4.8 vs. 0.55 percent, P ˂ 0.001), whereas magnesium (0.21 mg/dL ± 0.18 mg/dL vs. –0.04 mg/dL ± 0.05 mg/dL, P ˂ 0.001) and albumin (0.91 vs. –2.91 percent, with P = 0.007) were remarkably increased. The metabolic syndrome also decreased pre-diabetes (–17.5 vs. –9.8 percent, P = 0.140) and obesity (–15.7 vs. –8.2 percent, with P = 0.131). | It concluded that magnesium supplementation in CKD patients suffering from hypomagnesemia along with obesity and diabetes helps to improve metabolic status. |
| Wilson et al. [47], 2017 | To evaluate the vitamin C concentration (linked with obesity, glycemic control, and smoking) among prediabetes and diabetes. | New Zealand | A cross-sectional study | In the study, 89 participants were involved among those which NGT, pre-diabetes, and T2DM with managed diet and or with metformin. | The plasma ascorbic acid concentration was remarkably less in T2DM compared with NGT was 41.2 µmol/L and 57.4 µmol/L. In the prediabetes and T2DM groups, it was noticed that there was a high amount of vitamin C (˂ 11.0 µmol/L) was noted. | It concluded that the vitamin C requirement was greater in adults/patients with a history of prediabetes, T2DM, obesity, and smokers. |
HDL2: high-density lipoprotein 2; HPLC: high-performance liquid chromatography; HC: hip circumference; GDM: gestational diabetes mellitus; HOMA: homeostatic model assessment; SNPs: single nucleotide polymorphisms; UPLC: ultra-performance liquid chromatography; CKD: chronic kidney disease; GFR: glomerular filtration rate; NGT: normal glucose tolerance; MCS: mixed carotenoid supplementation
Most of the studies found cross-sectional studies [33, 35, 38, 42–44, 47], randomized control trials [32, 34, 40, 45, 46], case control [37, 41], and cohort study [36, 39]. The bar diagram is illustrated in Figure 6.
Obesity and related complications [32, 35, 39, 41, 44], diabetes and related complications [34, 37, 38, 42, 44], and the studies included both diabetes and obesity and related complications [33, 36, 40, 43, 46, 47]. Most of the studies included antioxidants such as carotenoids [32–34, 36, 42, 43, 45], vitamin C [35, 39, 44, 47], vitamin E [37, 40, 41, 44], zinc [35, 38], vitamin A [35, 38] and one study on magnesium [46].
The study on outcome measures include BMI [32, 33, 35, 39, 40, 42–47], WHR/WC [32, 33, 35, 39, 41, 46, 47], abdominal fat/visceral fat [32, 36, 42, 47]. Lipid level including HDL, very LDL (VLDL) or LDL [33–35, 42, 43, 47], body composition was assessed by dual X-ray absorptiometry [36], blood glucose level [33–35, 37, 42], insulin resistance [32, 36, 40, 46], HbA1c [38, 46, 47], HOMA [38, 43] and IS by minimal model analysis [44].
The participants of the study were diverse. The study only included smokers [33], pregnant women [37], CVD [34], and CKD [46].
The share of women and men in the study also differed. Three studies included only women [35, 37, 43], two studies included only children [32, 42], and another one, only men [45]. In the remaining ten studies, representatives of both sexes were recruited [33, 34, 36, 38, 39, 40, 41, 44, 46, 47].
There are three studies from Florida [32], two studies from Australia [33, 36], UK [34], two studies from Mexico [35, 42], Iran [37], Bangladesh [38], Denmark [39], two studies from New Zealand [40, 47], Spain [41], Sweden [43], mixed studies from California, Colorado and Texas [44], Japan [45] and Turkey [46] are included in this paper. The bar diagram is illustrated in Figure 7. The articles published year-wise have been illustrated in the bar diagram Figure 8.
Studies interlink the antioxidants and neuro-gliopathies in the gut and brain in diabesity has been described elaborately in Table 2, and the antioxidant supplements suppresses the OS and neuro-gliopathies has been elaborated in the Table 3.
Studies interlink the antioxidants and neuro-gliopathies in the gut and brain in diabesity
| Antioxidants and related substances | Cases | Action | Functional role | References |
|---|---|---|---|---|
| Probiotics (Lactobacillus salivarius AP-32 probiotics) | 6-Hydroxydopamin-induces Parkinson’s diseases | Parkinson’s disease associated with gut dysbiosis | The supplementation of L. salivarius AP-32 enhanced directly or indirectly the host enzyme activity | [48] |
| GSH | Colitis | Prevents enteric neuron death | Heterogeneous role of GSH in myenteric plexus of ENS and during GI inflammation | [49] |
| Polyphenols | Brain-neuromodulation | Gut microbiota composition is altered by the ingestion of natural bioactive molecules such as polyphenols | The idea strength that maintaining a healthy microbiome by modulating the bioactive molecules in diet is essential for a healthy brain | [50] |
| Bioactive peptides | Gut microbiota and neurodegenerative diseases | Dysbacteriosis of gut microbiota observed in the etiology of neurodegenerative diseases | The bioactive compounds modulate the gut microbiota as a novel strategy to control and reduce neurodegenerative diseases | [51] |
| Luteolin (flavonoids) | Alzheimer’s disease | Brain glucose regulation, anti-inflammatory and gut microbiota liver-brain axis | The study shows that the intake of luteolin enhances the brain’s insulin resistance and neuroinflammation | [52] |
| Flavonoids | Diet-induced obesity associated with low-grade gut inflammation | Gut microbiota changes and intestinal inflammation in obesity | The flavonoids showed a protective effect against obesity associated with low-grade gut inflammation | [53] |
| Flavonoids | Inflammatory bowel diseases | Flavonoids protect against IBD by modulating enter-hormones such as glucagon-like peptide 1 (GLP-1), GLP-2, dipeptidyl peptidase-4 inhibitor, ghrelin, and cholecystokinin | Flavonoids showed a protective role against IBD | [54] |
| Anthocyanin | Neurodegenerative diseases | The neurodegenerative diseases through the microbial intestinal-brain axis | The study suggested that the microbial-intestinal-brain axis would be a novel mechanism by the protective effect of anthocyanin in neurodegenerative diseases | [55] |
| Ferulic acid (FA) | Neurodegenerative diseases | FA esterases are the critical enzymes of the gut microbiota that facilitate the release of FA from feruloylated sugar ester conjugates and influence systematic health | FA and other antioxidants help to combat the neurodegenerative processes | [56] |
| Flavonoids | Neuroinflammation and neurodegenerative diseases | Bridging the gut and brain | Neuroprotective and neuroinflammation effect of flavonoids on Alzheimer’s disease, Parkinson’s disease, and pain | [57] |
| Curcumin | Parkinson’s disease | Reduces the free radical oxygen production by the total fraction of NADPH-containing associates | Membrane stabilizing and protective effect | [58] |
IBD: inflammatory bowel diseases; NADPH: nicotinamide adenine dinucleotide phosphate hydrogen
Antioxidants in suppressing the OS and neuro-gliopathies
| Antioxidants | Case | Action | Functional role | References |
|---|---|---|---|---|
| Carotenoids | Neurodegenerative diseases for instance Huntington’s disease, Parkinson’s disease, amyotrophic lateral sclerosis, epilepsy, schizophrenia, multiple sclerosis, neuropathic pain, and Alzheimer’s disease | Suppresses the oxidative damages | Anti-inflammatory, antioxidants | [59] |
| Curcumin | Alzheimer’s disease therapy | Amyloid β peptide (Aβ) treated human neuroblastoma IMR-32 cells | Amyloid-disaggregating properties, antioxidant, anti-inflammatory | [60] |
| Dementia therapy | Increased the Aβ-degrading enzymes | Neuroprotective, suppresses TNF, formation of Aβ plaque, and also protects the brain cells from noxious agents | [61] | |
| Fatty acids (DHA) | Enhances non-amyloidogenic amyloid precursor protein (APP) processing | It modulates membrane fluidity and reduces the OS | The increased membrane fluidity and non-amyloidogenic processing of APP enhanced the secretion of sAAPα in Alzheimer’s disease HEK293 cells. This increased secretion of sAAPα protects against apoptosis | [62] |
Epigallocatechin-3-gallate Extracted from Camelia sinensis | Reduces the risk of Alzheimer’s disease and age-related dementia | Stabilizes the mitochondrial function such as adenosine triphosphate (ATP) | It regulates the p-glycoprotein. It also shows antiapoptotic and antioxidative activities that directly inhibit Aβ plaque formation | [61] |
| Thioflavones | Neuroprotective agents | Reduces OS | It activates the anti-apoptotic cell survival proteins of PI3K/Akt and ERK1/2 pathway in neuroblastoma cell lines | [63] |
| Ginsenosides (GRg1 and GRg3, extracted from Ginseng) | Neuroprotective effect in Alzheimer’s diseases | Suppresses the Aβ associated neurotoxicity as linked with ROS | Ginsenosides regulate acetylcholine receptor channels, as these channels are linked with learning and memory. GRg1 significantly suppresses the Aβ associated neurotoxicity. GRg3 significantly reduces the amyloid-β40 and amyloid β42 level in the treatment of the brain of the transgenic mice. GRg3 also protects against glutamate-induced neurotoxicity | [61] |
| Quercetin (flavonoids) | Oxidative stress and apoptosis | Reduces inflammatory parameters and OS | Supplementation of quercetin decreases the neuronal damage, scavenges the free radicals by PCB, and prevents PCB-induced PCB and OS in rat brains | [64] |
| Retinoic acid (RA) | Proteosome inhibition in neurodegenerative diseases | Cell death in SH-SY5Y cells via AKT pathway | RA treatment of cultured neuroblastoma cells under which proteasome inhibition results in the accumulation of ubiquitinated protein loses its ability to kill the cells and thus shows the protective role in neurodegenerative diseases | [65] |
| Vitamin C | Neurodegenerative diseases | Maintain the cellular integrity and apoptosis in mitochondria | Antioxidants and neuroprotective activities in neuropathic pain | [59] |
| Vitamin E | Neurodegenerative diseases in neuropathic pain | Maintain the cellular integrity and apoptosis in mitochondria | Antioxidant properties in Alzheimer’s diseases | [59, 66] |
| Resveratrol/Y27632/NAD+/ZVAD-FMK | Synapto-protective role | Kinase/Caspase | Y27632 and NAD+ showed a strong synaptoprotective role in the neuronal culture of mice brains, whereas resveratrol and ZVAD-FMK failed to show neuroprotective function | [67] |
DHA: docosahexaenoic acid; GRg1: ginsenoside Rg1; NAD+: nicotinamide adenine dinucleotide; AKT: protein kinase B; sAAPα: soluble α-amyloid precursor protein; PI3K: phosphoinositide 3-kinases; ERK1/2: extracellular signal-regulated kinase 1/2; PCB: polychlorinated biphenyls
The GI manifestation of diabetes is very common and a source of discomfort and motility and almost every part of GI tract from the esophagus to the rectum causes a variety of symptoms such as heartburn, nausea, vomiting, diarrhea, abdominal pain, and constipation. So it is important to understand the underlying mechanism of diabetic gastroenteropathy [68]. Over recent years, the data regarding diabetic gastroenteropathy has expanding and the role of ENS and autonomic neuropathy causing GI disturbance caused by the intestinal cells of Cajal and neurotransmission in diabetics [68]. The enteric neurons are located at the different regions of the intestine and display the different susceptibilities to diabetic damage and insulin treatment, which highlight the neuronal microenvironment in the pathogenesis of diabetic neuropathy. Shift in the balancing of free radicals leads to OS in the gut which in turn leads to enteric neuropathy in diabetes [69].
The human gut consists of over 100 trillion microorganisms including 1,000 different species of bacteria, that play a crucial role pathophysiologically as well as physiologically in the host. An imbalance of GI ecosystem leads to contribute the development of several diseases such as Alzheimer’s disease, depression, and diabetes [70]. A study shows the connection between early disruption of the microbiome leads the GI distract function and increases the susceptibility of autism [71]. An altered microbiome leads to immune system dysregulation, inflammation, OS, metabolic and methylation abnormalities as well as GI distress [71]. In contrast to glial cells the central nervous system from the oxidative damage by synthesizing oxidants [72]. The OS and associated brain diseases, the antioxidants rescue the neuronal cells from OS by neutralizing the ROS [73].
Seven studies (4 cross-sectional studies and 3 randomized control trials) examined the connection between dietary or plasma carotenoids and diabetes and obesity. Serum carotenoid such as β-carotenoid are known as indicators of fruit and vegetable ingestion and many results show that a higher intake of these products is effective against obesity [8] and its correlated problems such as diabetes and CVDs. Both the studies observational and randomized control trial studies followed the design either measurement of dietary consumption and serum concentration were evaluated and associated with BMI, WC, WHtR, or other measures were associated between serum carotenoid concentration and measures of adiposity. Canas et al. [32] investigated that increased concentration of β-carotene reflects a reduction in BMI z-score, WHtR, and visceral and subcutaneous adipose tissue. The MCS (contains 2,000 IU β-carotene and 500 µg of α-carotene, 10 mg of lutein, 2 mg zeaxanthin, and 10 mg of γ-tocopherol). The MCS brought about –0.19 ± 0.13 changes in BMI z-score with a P value of 0.024, 3% ± 2% deviation in WC, and 0.03 ± 0.03 reduction in the WHtR with P value of 0.039 at six months. β-Carotene was found in inverse relation with BMI z-score, WHtR, visceral, and subcutaneous adipose tissue at baseline with P values 0.003, 0.017, 0.023, and 0.045 [32].
Coyne et al. [33] summarized that the low level of serum carotene (such as α and β), and the sum of these carotenoids (such as α-carotene, lycopene, β-cryptoxanthin, zeaxanthin, and β-carotene) was linked with metabolic ailments. The person with metabolic syndrome has found significantly lower (P < 0.05) serum concentrations of α (with P = 0.25), and β-carotene (with P = 0.15) along with the sum of five carotenoids (P = 0.41). In current smokers, there was found no remarkable variation in carotenoid concentration and metabolic ailments, but in former and who did not smoke the α and β-carotene were found significantly lower with metabolic syndrome [33].
Another study reported by Daniels et al. [34] that excess ingestion of coloring fruits and green vegetables is linked with carotenoid concentration and also influences the enzyme-linked antioxidant properties of HDL. The group having ≥ 6 portions of colored fruits and green vegetables every day was found increased serum carotenoid, HDL2, and HDL3 (α-carotene, β-cryptoxanthin, lycopene, and lutein having P values 0.008, 0.042, 0.016, 0.042 and 0.012) the activities of LCAT and PON-1 in HDL3 (with P value 0.044 and 0.006). The study furnished mechanistic support for increased consumption of coloring fruits and green vegetables is effective in reducing the prevalences of diabetes and its associated CVD [34].
Similarly, Harari et al. [36] reported that there was a favorable relation between adipose tissues, metabolic health, and serum carotenoids level. Carotenoids found an inverse association with TG (r value was –0.22, and P = 0.051), and TG and serum retinol tended positively linked (r value was 0.22, and P = 0.051). ζ-Carotene in adipose tissue was found negatively associated with TG and phytofluene, and total carotenoid was found inverse association with serum TG. A total carotenoid found an inverse association with the HOMA-estimated insulin resistance (IR, HOMA-IR) and fasting insulin. ζ-Carotene shows an inverse relation with HbA1c (r value was –0.23, and P = 0.069) [36].
Similarly, one more study by Mummidi et al. [42] reported that the response surface methodology showed increased α and β-carotenoids had a maximum effect on IS index compared with β-cryptoxanthin or lycopene. The concentration of β-cryptoxanthin and lycopene were [h2 = 0.58, P = 1 × 10–7 and h2 = 0.98, P = 7 × 10–18]. The study found a negative phenotypic correlation (P ≤ 0.05) between β-cryptoxanthin and five cardiometabolic risk factors (such as BMI, WC, fat mass, fasting glucose of –0.22, –0.25, –0.18, –0.23, –0.09 and positive correlation with HDL (0.29), whereas the lycopene showed a positive association with HDL (0.18) and negative association with fasting blood glucose (–0.08) [42].
Another study reported by Östh et al. [43] that β-carotene concentration was lowered in the obese subject, and its lower concentration indicates adipocytes from T2DM subjects cause obesity. The β-carotene concentration was found 50 percent lower in adipocytes from the obese and diabetes group than in the other groups. Triacylglycerol was found in 92% ± 1% of adipocytes in the lean group whereas 99% ± 2% in the diabetic obese group (P < 0.05) [43].
One more randomized clinical trial study reported by Takagi et al. [45] concluded that dietary intake of carotenoid-rich vegetables helps in the reduction of intra-abdominal visceral fat. The result shows that the daily beverage intake raises the carotenoids in plasma level and reduces the visceral fat in all groups significantly. The WC was significantly lowered in the group that had high lycopene with low lutein, whereas the CoQ10 oxidation rate was significantly reduced in overall groups. Only in low lycopene with low lutein group had to differ in their gene expression profile that indicates the effect of carotenoids on genetic profile [45].
The most common carotenoids such as β-carotene, lutein, crocin, lycopene, zeaxanthin, and curcumin have antioxidants and anti-inflammatory properties in the management of neurodegenerative disorders [74, 75].
People with diabetes may face enteric neuropathy caused by a decrease in the number of neurons in the ENS. A treatment strategy that can be used is antioxidants. Antioxidants help to promote redox balancing and decrease enteric neuron death. So, quercetin-loaded microcapsules (QLMs) are antioxidants with meticulous release in the gut. These QLMs promote controlled release and higher bioavailability of quercetin a good antioxidant with neuroprotective. A study was conducted on the effect of QLMs on enteric innervation and the oxidative status of the ileum of diabetic rats. The study showed a reduction in the carbonyl content and increased molecular weight of antioxidants (DQ10: diabetic groups treated with QLM at a dose of 10 mg/kg; and DQ100: diabetic groups treated with QLM at a dose of 100 mg/kg). The group treated with QLMs at the dose of 10 mg/kg had a significant result on the nitrergic neurons that reduce oxidative damage in diabetes [76].
Three cross-sectional studies and one prospective cohort study reported the connection between ascorbic acid and obesity and diabetes. Many studies revealed that a low content of ascorbic acid might be a risk factor for several disease mortality. One prospective cohort study by Larsen et al. [39] reported that there was no relation between ascorbic acid and body weight or WC, but ascorbic acid-rich diet may be weakly correlated with higher WC gain to the people genetically sensible to an increased WHtR. The result indicates no remarkable relation between dietary ascorbic acid and deviation in body weight or WC. An allele of the 14 waists to hip ratio associated SNPs was associated with an annual change in WC of 0.039 cm annually (P = 0.02, 95% confidence interval: 0.005 to 0.073) per 100 mg per day higher ascorbic acid consumption. However, the annual change in WC only remained borderline significant after adaptation for the annual change in body weight [39].
One more cross-sectional study by Wilson et al. [47] found out that the ascorbic acid requirement was greater in adults/patients with a history of prediabetes, type-II diabetes, obesity, and smokers. The results showed that the plasma ascorbic acid concentration was significantly less in T2DM (41.2 µmol/L) compared with NGT (57.4 µmol/L). In the prediabetes and T2DM groups, it was noticed that there was a high amount of vitamin C (< 11.0 µmol/L) was noted. The BMI, fasting glucose, dietary ascorbic acid intake, and smoking history (having P values of 0.001, 0.001, 0.032, and 0.003) were independent predictor of ascorbic acid serum concentration [47]. García et al. [35] and Sanchez-Lugo et al. [44] reported the combined effect of ascorbic acid along with retinol, tocopherol, and zinc that will be described in the below section.
Neuropathic pain is the more common painful condition that happens after a lesion or an insult to the somatosensory nerve system. Recent studies showed that treatment with vitamin C showed a positive effect [1]. One study showed, that in diabetic peripheral neuropathy, the mean visual analog score was significant in the treatment group (5.54 ± 0.81 vs. 6.72 ± 0.90; P < 0.0001) at 12 weeks [1].
One randomized clinical trial, two case-control, and one cross-sectional study reported the connection between tocopherol and obesity and diabetes. Tocopherol is a natural antioxidant and plays a beneficial role against ROS in the body’s defense system. The low level of vitamin E is directly associated with increased central adiposity and related health problems such as diabetes CVD and many more. One randomized clinical trial by Manning et al. [40] reported that tocopherol enhances OS, insulin resistance, and hepatic cellular function in an overweight subject. The supplementation of 800 IU/day of tocopherol and placebo for three months, and after that further 1,200 IU/day of tocopherol dose was increased for three months. The result shows that plasma peroxidation decreased by 27% in the first three months and by 29% at 6 months. At three months it was noticed that the fasting glucose level and insulin concentration were significantly reduced but these things were not happening with six months of supplementation. Throughout the study, the alanine transaminase concentration was declining. The vitamin E supplementation was not significant in the subjects receiving the doses and their WC (P = 0.82) and BMI (P = 0.87), but the insulin concentrations and fasting plasma glucose were significantly reduced (r = 0.235, P = 0.04) [40].
Another case-control trial by Mansego et al. [41] reported that WC is linked with dietary tocopherol intake in the obese subject. In the AO group, it was found that 8-oxo-2’-deoxyguanosine was at higher levels, and were taking lower retinol and tocopherol compared to the non-AO group. Logistic regression analysis indicates that TXN and COMT were linked with WC and AO. Moreover, these polymorphisms were more firmly linked with changes in WC in subjects having a lower intake of tocopherol. WC is linked both with dietary tocopherol ingestion and genetic variants of COMT and TXN proposing the presence of a complex nutrigenetic pathway that regulates AO [41]. Hekmat et al. [37] and Sanchez-Lugo et al. [44] reported the combined effect of tocopherol along with retinol, and ascorbic acid that will be described in the below section.
A study was conducted on the ileum of diabetic rats to investigate the effect of vitamin E (1 g/kg body weight) supplementation on myosin-V and neuronal nitric oxide synthase immunoreactive myenteric neurons. The diabetic group treated with vitamin E showed increased size of nitrergic neurons, and the neuronal density was found higher by 27% in a nor-glycemic treated vitamin-E group than the nor-glycemic group (P < 0.05). The result showed that vitamin E elicited a neuroprotective and neurotrophic effect on the natural aging process [77].
One randomized control trial reported the association between magnesium and obesity and diabetes. Magnesium is an important trace element linked with cellular enzymatic activity, especially glycolysis, and stimulates adenosine triphosphatase. Toprak et al. [46] reported that magnesium supplementation in CKD (suffering from hypomagnesemia) patients with obesity and diabetes help to improve their metabolic status. The result shows that the group having magnesium compared with placebo had significantly decreased insulin resistance (–24.5 vs. –8.2 percent, P = 0.007), insulin (–29.6 vs. –2.66 percent, P < 0.001), HbA1c (–6.6 vs. –0.16 percent, P < 0.001), WC (–4.8 vs. 0.55 percent, P < 0.001), whereas magnesium (0.21 mg/dL ± 0.18 mg/dL vs. –0.04 mg/dL ± 0.05 mg/dL, P < 0.001) and albumin (0.91% vs. –2.91%, P = 0.007) were remarkably increased [46].
A peripheral nerve injury is a very common complication during trauma that requires a functional recovery and regeneration. So, it is necessary to find a suitable material that would promote peripheral nerve regeneration due to the shortage of capacity for peripheral nerve regeneration. So, that’s why magnesium attracted with good biocompatibility and appropriate degradability to increase attention during the past years [78].
A cross-sectional study by García et al. [35] investigated that a combination of zinc, retinol, and ascorbic acid were linked with adiposity, obesity, and leptin concentration in women. The prevalences of overweight and obesity were found at 36% and 44% (with BMI > 25 kg/m2 and BMI > 30 kg/m2), whereas the prevalences of zinc, ascorbic acid, and tocopherol were similar in these patients but no vitamin A deficiency was found in them. It was noticed that ascorbic acid was inversely linked with BMI, WHtR, and leptin (P < 0.05), whereas retinol was directly linked with leptin (P < 0.05). The leptin concentration was linked with lower zinc and ascorbic acid concentration in obese women (P < 0.05) and vitamin A concentration was higher in non-obese women (P < 0.01). Whereas tocopherol was not associated with any obesity markers [35]. Sanchez-Lugo et al. [44] concluded that there was no significant relation between improved IS and intake of tocopherol and ascorbic acid. The Pearson correlation coefficient for vitamin C and E about IS was r value of 0.07 (P = 0.02) and an r value of 0.07 (P = 0.01) respectively [44].
Hirschsprung disease is a severe disease of ENS development caused by the nonfunctioning of ENS precursor migration into distal bowel aganglionosis (DBA). Depletion of vitamin A causes DBA in serum retinol-binding-protein-deficient (Rbp4–/–) mice. RA decreases the phosphatase and tensin homolog (Pten) accumulation in migrating cells. This Pten overexpression decreases the ENS precursor migration. So, the study showed the deficiency of vitamin A deficiency is a no-genetic risk factor that causes the Hirschsprung disease’s penetrance and expressivity [79].
One more case-control study by Hekmat et al. [37] investigated that retinol was remarkably lower in diabetic pregnant women in comparison with the control group, and this is due to lowered antioxidant concentration in GDM women.
Zinc is another essential micronutrient that has a beneficial role in various physiological processes such as tissue repair, cellular immunity, and cell development [35]. And, few studies reported that zinc is linked with the degree of obesity [80]. A low plasma zinc level is linked with excess fat deposition and reduces lean mass accrual [81]. Islam et al. [38] reported that low serum zinc highlighted the increase in insulin resistance with increasing BMI. Serum zinc concentration was less in prediabetic to normal (65 ppb/L) compared with diabetic (33 ppb/L). Multiple linear regression shows lower zinc levels in prediabetes than in those with normal blood glucose levels. Linear regression for HOMA parameters did not show any statistical connection between zinc level, insulin resistance (P = 0.08), and β-cell function (P = 0.07) [38]. The copper and vitamin B12 deficiency associated with myeloneuropathy mimicking subacute without cognitive dysfunction [82].
A study was conducted on myenteric in the jejunum of diabetic rats to evaluate the synergetic effect of the association of vitamin C and tocopherol. The rats from the normoglycemic treated with ascorbic acid and α-tocopherol and the diabetic treated with ascorbic acid and α-tocopherol group were supplemented with 1 g ascorbic acid/L in water and 1% α-tocopherol in chow. The supplementation of these helps to prevent the cell loss of myenteric neurons expressing HuC/D and TrkA equally. They observed a reduction of calcitonin gene related peptide (CGRP) nerve fiber varicosities and prevention of increased cell body size of vasoactive intestinal polypeptide (VIP) neurons (P < 0.05) [83].
In conclusion, this literature search of present studies shows the interconnection between antioxidant intake among obese and diabetes. The enteric glia and neurons both express the cellular mechanism for GSH synthesis and glial GSH synthesis is necessary for neuronal survival in isolated ENS. The GSH has complex metabolic and biochemical fates and is a cofactor for the many enzymes that function in modifying the obesity and diabetes responses. The GSH depletion may cause to increase the energy metabolism and reduce adipose accretion, while the elevated GSH peroxidase activity induces insulin resistance. Over recent years, the data regarding diabetic gastroenteropathy is expanding, and the role of ENS and autonomic neuropathy causing GI disturbance caused by the intestinal cells of Cajal and neurotransmission in diabetics. The enteric neurons are located at the different regions of the intestine and display the different susceptibilities to diabetic damage and insulin treatment, that highlight the neuronal microenvironment in the pathogenesis of diabetic neuropathy. Shift in the balancing of free radicals leads OS in the gut which in turn leads to enteric neuropathy in diabetes. Emerging studies reports indicate that natural antioxidants can modulate OS and improve immune function in obesity and diabetes. The findings indicate both obese and diabetic patients have a minimum content of antioxidants, especially carotenoids, retinol, ascorbic acid, tocopherol, magnesium, and zinc. While few research illustrated that ingestion of the abovementioned antioxidants was lowered among diabetes and obese subjects in contrast with their normal-weight population, this was not endorsed by every study. However, most of the studies including observational and randomized control trials reported the positive effect of antioxidant intake and its management on obesity and diabetes subjects as immunity boosters.
AO: abdominal obesity
BMI: body mass index
CKD: chronic kidney disease
COMT: catechol-O-methyltransferase
CoQ10: coenzyme Q10
CVDs: cardiovascular diseases
EGCs: enteric glial cells
ENS: enteric nervous system
FFAs: free fatty acids
GDM: gestational diabetes mellitus
GI: gastrointestinal
GSH: glutathione
HbA1c: glycated haemoglobin
HDL2: high-density lipoprotein 2
HOMA: homeostatic model assessment
IL-1β: interleukin-1β
IS: insulin sensitivity
LCAT: lecithin cholesterol acyltransferase
LDL: low-density lipoprotein
MCS: mixed carotenoid supplementation
NGT: normal glucose tolerance
OS: oxidative stress
PON-1: paraoxonase-1
QLMs: quercetin-loaded microcapsules
RA: retinoic acid
RCTs: randomized controlled trials
ROS: reactive oxygen species
SNPs: single nucleotide polymorphisms
T2DM: type 2 diabetes mellitus
TG: triglycerides
TLRs: Toll-like receptors
TNF-α: tumor necrosis factor-α
TXN: thioredoxin
WC: waist circumference
WHR: waist-to-hip ratio
WHtR: waist-to-height ratio
LS and DS: Conceptualization, Formal analysis, Writing—original draft, Writing—review & editing. LS: Supervision. Both authors wrote, read, approved the study for publication, and provided their critical feedback and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All datasets for this study are included in the manuscript.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4666
Download: 50
Times Cited: 0
Ravi Philip Rajkumar
Gabrio Bassotti
Momin Ahmed ... Brandon Lucke-Wold
