Affiliation:
1Graduate School of Human Science and Environment, University of Hyogo, Himeji, Hyogo 670-0092, Japan
2Research Institute for Food and Nutritional Sciences, University of Hyogo, Himeji, Hyogo 670-0092, Japan
Email: komuratomomi@gmail.com
ORCID: https://orcid.org/0000-0003-1133-4041
Affiliation:
1Graduate School of Human Science and Environment, University of Hyogo, Himeji, Hyogo 670-0092, Japan
2Research Institute for Food and Nutritional Sciences, University of Hyogo, Himeji, Hyogo 670-0092, Japan
ORCID: https://orcid.org/0009-0008-4885-0377
Affiliation:
3Faculty of Food and Nutrition Science, Tezukayama Gakuin University, Sakai, Osaka 590-0113, Japan
ORCID: https://orcid.org/0000-0001-9453-0739
Explor Neurosci. 2024;3:80–102 DOI: https://doi.org/10.37349/en.2024.00038
Received: July 12, 2023 Accepted: January 19, 2024 Published: April 07, 2024
Academic Editor: Marcello Iriti, Milan State University, Italy
Pesticides are used to ensure the mass production and quality of foods, depending on the environment where they are grown. Trace amounts of pesticides are ingested through diet and high ratios of its components have been detected in humans. Neonicotinoid insecticides are nicotine analogs that disrupt neurons, induce neural excitation, and cause behavioral abnormalities and chronic toxicity. The herbicide glyphosate causes behavioral disorders due to abnormalities in the balance of intestinal microflora. These abnormalities can be found in the F2-generation and beyond. Glyphosate decreases the number and size of experimental animal fetuses, possibly through abnormal deoxyribonucleic acid methylation in parental germ cells, resulting in transgenerational toxicity. It also causes the death of dopamine neurons, which are believed to be involved in the development of Parkinson’s disease (PD). The intestinal microflora is considerably altered by ingesting pesticides used in crops. Lactic acid bacteria and some other intestinal bacteria have gut-regulating and immunomodulatory effects that have recently been implicated in neurological disorders, such as depression and dementia. Therefore, a healthy diet should be traced back to crops. An agriculture-medicine partnership linking “agriculture” and “preventive medicine” has recently been considered important based on the hypothesis that agriculture and health sectors should collaborate to create a healthy environment for producing healthy food. Although food considerations tend to focus on the functionality of vegetable and fruit components, that of environmental bacteria should also be considered.
Over the last 100 years, improvements in public health and medical care have remarkably increased the average life expectancy, which continues to rise [1]. However, the limit of human life is considered to be approximately 120 years, regardless of diet or lifestyle [2]. In addition, increasing the lifespan is one of the purposes of public health; however, the number of patients with age-related diseases such as dementia is increasing [3]. This is due to a gap between the physiological lifespan (the period from birth to death) and a healthy lifespan (the period during which a person can live independently): the calculated global average lifespan is 73.3 years, with a healthy life expectancy of 63.7 years [4]. In 2020, it was shown that Japan, one of the countries with the longest lifespans, had an average life expectancy of 84.3 years, with a healthy lifespan of 74.1 years [4]. Therefore, both the world and Japan have a gap of approximately 10 years between the average lifespan and the healthy lifespan [4]. During this gap, the quality of life declines, and individuals cannot live independently, resulting in a requirement for medical and/or nursing care [5]. Consequently, social insurance systems for medical and nursing care services are burdened by heavy costs [5]. Furthermore, as the population ages and the birthrate declines, a scarcity of younger generations to support older adults might become a reality, and healthy older people may become responsible for caring for their peers who are experiencing a decline in activities of daily living. Consequently, it would be desirable to match the health span with the lifespan by delaying senescence. This approach would improve various types of functional deterioration associated with aging, increase the quality of life, and reduce medical and nursing care costs [6]. Therefore, people should take responsibility for extending their own healthy lifespans by maintaining health through awareness of their own well-being. The focus on healthy life expectancy diminishes once the need for long-term care arises. Central nervous system diseases contribute to the demand for such care; particularly, dementia and stroke alone account for over 40% of individuals requiring long-term care [7]. Recently, an increasing number of reports have shown a relationship between central nervous system diseases and the microbiota composition in the gut.
This article focuses on dietary habits, with a particular emphasis on agricultural products. Human consumption of agricultural products is commonplace, and several studies have investigated the impact of pesticides used in their cultivation on human health. Recent findings have indicated that pesticides can have implications for neurological functions, potentially leading to developmental disorders in children and age-related neurodegenerative diseases [8, 9]. Concerns have also emerged regarding the potential alteration of intestinal microflora and associated health implications, even in the next generations not directly exposed to these pesticides. These pesticides may exert negative effects on various aspects of the human body [10]. Therefore, this paper aims to provide a comprehensive review of the effects of major pesticides used worldwide on neurological function, gut microbiota, and potential implications for future generations.
The balance of intestinal microflora is highly dependent on diet and eating habits [11]. However, an imbalanced composition of the human intestinal microbiota has been linked to age-related diseases such as dementia [12]. Therefore, the effects of the environment on agricultural products that are frequently consumed by humans should be considered.
The major methods of agricultural product cultivation are conventional, organic, and natural. These methods include the use of agricultural chemicals and synthesized fertilizers, organic fertilizers without agricultural chemicals, and no agricultural chemicals or fertilizers. Pesticides in conventional agriculture can reduce crop diseases and insect damage, as well as maintain quality and production. However, the amount of pesticides and chemical fertilizers applied must be within the range specified by the laws of individual countries and should not pose harm to humans [13].
Various microorganisms live in the soil of agricultural lands and play crucial roles in degrading nitrogen components and producing compost [14]. Plants grow by absorbing nutrients from compost through their root systems [15]. However, pesticides that suppress plant pathogens also kill microorganisms, leading to a shortage of nutrients; therefore, chemical fertilizers are required to produce crops [16]. The components of pesticides and chemical fertilizers are also absorbed through plant roots and can be found in fruits and vegetables; therefore, methods to detect pesticides in fruits and vegetables using sensing and other technologies are currently under development [17, 18]. For example, surface-enhanced Raman scattering, a highly sensitive sensing method that amplifies biomolecule signals on metal surfaces by a million times or more, enables the detection of minute amounts of pesticides on the surface of fruits within minutes [19].
Synthetic chemical pesticides were initially developed mainly in Europe and the USA during the 1950s, and organochlorine pesticides, such as dichlorodiphenyltrichloroethane (DDT) and benzene hexachloride, as well as organophosphorus pesticides such as parathion, were imported into Japan, where they led to major developments in the agrochemical industry [20]. However, organochlorine pesticides are highly toxic, take a long time to decompose in nature, and can become concentrated in the food chain, causing great harm to the environment [21]. Therefore, natural ecosystems are being destroyed worldwide due to their widespread application [13]. Furthermore, the calculated environmental and social costs of the effects of pesticides on public health in the USA, increased pest control costs due to the development of pesticide resistance, and the loss of honeybees are enormous [22].
The American biologist Rachael Carson warned of the dangers of organochlorine pesticides such as DDT in 1962 [23]. Although DDT is no longer used or produced in developed countries such as Japan, malaria, which is spread by anopheles mosquitoes, is problematic in African, Asian, and Central and South American countries. Therefore, DDT is still applied in these countries with restrictions because there are no economically feasible alternatives that are as effective in preventing the spread of these mosquitoes [24]. However, these chemicals continue to cause pollution on a global scale, and trace amounts are still being detected in humans [25, 26].
Although pesticide companies have supposedly developed synthetic chemical pesticides with reduced toxicity to humans and less environmental harm [21], epidemiological studies indicate that these pesticides still have an impact on human health (Table 1). Fungicides, for example, are employed to combat bacteria and fungi. Some fungicides inhibit the biosynthesis of various body tissues, including the nucleus, cell wall, and cell membrane, as well as the respiratory system of pathogens, while others possess multiple mechanisms of action against pathogens [27]. However, these fungicides may also affect non-target microorganisms. Comprehensive information about the types of fungicides, their modes of action, and associated health concerns can be found in the literature [27] and should be reviewed there.
Effects of pesticides on health and epigenetics
| Pesticide | Risks to human health | Epigenetics | |||||
|---|---|---|---|---|---|---|---|
| Type | Reagents | Action | Diseases and health hazards | References | Epigenetic actions | Animal type | References |
| Organochlorine | Carbon tetrachloride Chlordane DDT DDE (metabolite of DDT) Dieldrin Heptachlor Hexachlorocyclohexanemethoxychlor Vinclozoin | Insecticide | Growth Premature birth Abortion Prostate cancer Leukemia PD Thyroid disorders Amyotrophic lateral sclerosis | [28–38] | DNA methylation (methoxychlor, vinclozoin, DDT, and DDE) Histone modification (dieldrin) | In vitro (mesencephalic, dopaminergic neuronal, and cells) Rat Mouse embryo Human | [39–45] |
| Organophosphorus | Acephate Chlorpyrifos Coumaphos Diazinon Dichlorvos Fonofos Parathion Methyl parathion Malathion Phosmet Triazophos | Insecticide | Brain and neurological developmental abnormalities in infants Deintellectualization Increased incidence of attention deficit hyperactivity disorder Lung cancer Lymphoma Leukemia Asthma PD Alzheimer’s disease | [46–55] | microRNA expression (dichlorvos and triazophos) | In vitro (porcine kidney and epithelial cells) Zebrafish | [56, 57] |
| Weeding | Carcinogenesis Reproductive dysfunction Endocrine disruption Allergic rhinitis | [58–61] | DNA methylation Histone modification microRNA expression | Japanese medaka (oryzias latipes) In vitro (PBMC and mammalian stem cell) Rats Mouse | [62–67] | ||
| Carbamate | Glyphosate Aldicarb Carbaryl Methiocarb Pirimicarb Mancozeb Maneb Thiocarbamate Propoxur | Weeding | Neurotoxicity Thyroid disease Developmental abnormalities | [68–70] | Histone modifications (propoxur) | In vitro (gastric cells) | [71] |
| Pyrethroids | Cyhalothrin Cypermethrin Deltamethrin Permethrin | Insecticide | Endocrine disruption | [72, 73] | DNA methylation | Human (blood) | [74] |
| Neonicotinoids | Clothianidin Imidacloprid Thiamethoxam | Insecticide | Abnormal neurodevelopment | [75] | Histone modifications | Mouse | [76] |
| Bipyridinium | Paraquat | Weeding | Neurotoxicity PD | [77] | Histone modifications | In vitro [immortalized rat, mesencephalic dopaminergic cells (N27 cells)] | [45, 78] |
DDE: dichlorodiphenyldichloroethylene; PBMC: peripheral blood mononuclear cells; PD: Parkinson’s disease
Agricultural chemical companies have been developing synthetic chemical pesticides that are less toxic to humans and have a lower negative environmental impact [79]. The major products are neonicotinoid pesticides, which are nicotinic paralogs, and the herbicide glyphosate [80, 81]. Furthermore, the application of these agrochemicals in conventional agriculture is considered the main cause of deteriorating biodiversity [82]. High rates of these substances have been detected in the urine of newborns, children, and adults [83–87], indicating that humans ingest low concentrations of these pesticides and/or chemical fertilizers through diets. Some study findings do not support the safety and security of conventional agricultural products [88–90]; these findings are comprehensively discussed below.
Insects can be classified as harmful or beneficial. Incidents of colony-collapse disorder in which beneficial honeybees suddenly disappear, leaving behind the queen and larvae, are occurring worldwide [91]. Consequently, this renders honeybee colonies impossible to maintain and leads to large-scale deaths in and around hives. Neonicotinoids might be the cause of this phenomenon [92–94]. If so, neonicotinoids negatively impact not only pests but also beneficial insects, and this could harm the ecosystem (Figure 1). Neonicotinoids are highly selective and neurotoxic to insects but have low acute toxicity in humans [95]; therefore, they have been applied worldwide since the early 1990s.
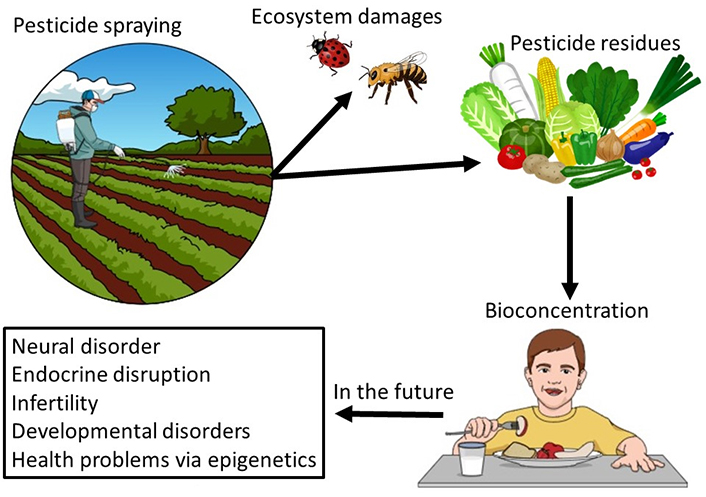
Effects of pesticides on the environment and humans. The application of pesticides to agricultural lands has resulted in the mass mortality of beneficial insects, such as honeybees and ladybugs, in their natural habitat. Crops grown under pesticide application can be infiltrated by pesticides through the roots into leaves, stems, and fruits. Pesticides also adhere to plant surfaces, and the ingestion of these chemicals by humans can cause various health complications
Acetylcholine is a neurotransmitter released from neurons, including parasympathetic and motor neurons. It is responsible for transmitting electrical signals from the synapses to dendrites to transmit information from one neuron to another. Nicotine can activate acetylcholine receptors because of its similarity to acetylcholine. Therefore, it can induce a cellular response similar to that of acetylcholine [96]. Neonicotinoids bind to nicotinic acetylcholine receptors (nAChRs) in the nervous systems of animals because their chemical structure is similar to that of nicotine [97]. Importantly, nAChRs regulate the central and peripheral nervous systems of insects and mammals, respectively, and bind to acetylcholine, a type of neurotransmitter [97]. Such binding stimulation is transmitted as a signal, which is essential for diverse physiological functions, such as memory, learning, regulation of the autonomic nervous system, and signaling to muscles [98]. Furthermore, nAChRs are also involved in the formation of neuronal synapses and networks during mammalian development [99]. However, since neonicotinoids cannot be easily degraded after binding to nAChRs, they become toxic by continuously distracting neurons (Figure 2) [100]. Humans and insects share a basic structure comprising five subunits, although it slightly differs among species, causing changes in neonicotinoid binding [101]. Neonicotinoids bind more easily to insects than human nAChRs and are considered less lethal to humans [102]. However, whether neonicotinoids definitely do not negatively affect the human brain or physiological functions should be considered.
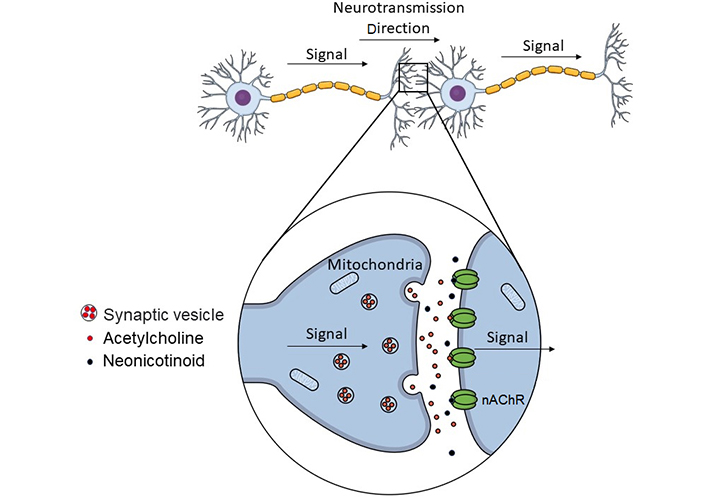
Binding of pesticide components to acetylcholine receptors and abnormal neurotransmission. When a signal is transmitted to a neuron, acetylcholine is released from the nerve ending, binds to the acetylcholine receptor of the next neuron, and the signal is transmitted. Pesticide components bind to the acetylcholine receptor and switch on neurotransmission, even in the absence of acetylcholine, causing abnormal excitation
Neonicotinoid (acetamiprid) metabolites have been detected at higher concentrations in the urine of low-weight infants than in normal-weight infants, as well as in the urine of premature neonates in Japan [87]. Low birth weight is a risk factor for developmental and health disorders, such as autism and diabetes, respectively [103]. Therefore, neonicotinoid exposure might be a factor in these disorders. Furthermore, neonicotinoids can pass through the human placenta to a fetus [104, 105]; therefore, they might affect humans and other mammals [106, 107].
Neonicotinoids are unlikely to pass through the blood-brain barrier and migrate into the brain [95]. However, their binding to nAChRs in neuronal cells derived from the rat cerebellum induces neuroexcitability and behavioral abnormalities [106, 107]. The effects of neonicotinoids at doses equivalent to those considered non-toxic to pregnant, breastfeeding maternal, and young mice (3 and 10 weeks of age) were evaluated using elevated plus-maze tests and other techniques. The results showed that neonicotinoids and their metabolites are transferred from mothers to offspring that exhibit anxiety-like, aggressive, and sexual behaviors [108–110].
Human environments have significantly changed compared with the past due to the spread of public health and the development of improved medical and agricultural technologies. Although the global average lifespan is increasing, chronic states, such as lifestyle-related diseases and allergies, are common [111]. Genetic and environmental factors are notably involved in allergic diseases such as asthma [112].
The hygiene hypothesis was generated based on the finding that improved sanitation reduces the probability of infection with pathogens during infancy, which is associated with disease onset [113–115]. However, exposure to various bacteria at an early age, when the immune system is incomplete, reduces the incidence of allergic diseases later in life [113]. This theory was based on the observation that among children raised with many siblings: younger brothers and sisters are less susceptible to allergic diseases, whereas the eldest children and those with no siblings are more susceptible to allergic diseases [113]. This is because the youngest children are more likely to be exposed to bacteria from other siblings from an early age, which strengthens their immune system [113]. Notably, the sanitary conditions were worse, and the standard of living was significantly lower in East Germany than in West Germany in 1989 [116]. However, the incidence of hay fever was 3-fold to 4-fold higher in West Germany. One reason for this is believed to be the large proportion of working women in East Germany and the fact that many infants were left in daycare centers, which provided more exposure to environmental microorganisms [116–118].
Epidemiological studies have found that children raised in farm households, where they have more contact with domestic animals, have fewer allergies and asthmatic diseases than those raised in urban areas [119, 120]. Lipopolysaccharide in airborne bacteria boosts the immune system, reducing susceptibility to allergic diseases [121]. Therefore, simply taking a walk in a natural environment with soil and water is sufficient to boost the immune system compared with urban areas. Furthermore, exposure to environmentally harmless or beneficial microorganisms at an early age can promote immune system development [122].
The old friends hypothesis considers “good” bacterial species that have lived in the intestinal tract, skin, nasal cavity, and oral cavity over long periods of human history and benefitted humans as “old friends” [123]. This theory states that the overuse of antibiotics kills beneficial bacteria in the intestines, causing an imbalanced immune system [123]. Moreover, excessive body washing eliminates beneficial bacteria that play important roles in the barrier function, which protects the skin from external enemies [124]. Subsequently, the immune system becomes hypersensitive in an environment where beneficial bacteria are lacking. Furthermore, the loss of exposure to bacteria from the birth canal due to cesarean sections and/or antibiotics administered at an early age inhibits the establishment of bacterial species that should be “old friends” [123]. Therefore, the immune system does not mature appropriately or has disrupted homeostasis that would normally be maintained by old friends, resulting in an excessive response to substances that should not be attacked [123–127]. This action has irreversible consequences, including food allergies, inflammatory colorectal diseases (such as Crohn’s disease), autoimmune diseases (such as type 1 diabetes), and psychiatric disorders (such as autism) [123–127].
These hypotheses have implied that diverse microorganisms in the environment, soil, and air protect humans from infections and enhance immunity. Therefore, re-evaluating a hygienic environment is important in the context of protecting good physical and mental health by reaffirming the importance of “old friends” who strengthen and train the human immune system. In this way, encountering various microorganisms from a young age might be an asset to human health. The above mentioned commensal bacteria are important as “old friends” and symbiotic bacteria. Recently, accumulating evidence shows that pesticides influence our health by affecting these microorganisms, as summarized below.
Glyphosate is the most widely used herbicide worldwide due to its effectiveness and low toxicity [128]. It inhibits 5-enolpyruvylshikimic acid-3-phosphate synthase (EPSPS), which is essential for the biosynthesis of the amino acids phenylalanine, tyrosine, and tryptophan through the shikimic acid pathway [128, 129]. Notably, this pathway is plant-specific and is considered safe for humans; however, it is also found in plants, bacteria, fungi, and prokaryotes, consequently affecting soil and human gut bacteria [129, 130]. Glyphosate action is non-selective in plants, although its effects vary among bacterial species [131]. Adding glyphosate to intestinal bacteria models in vitro causes bad bacteria, such as clostridia and salmonella, to thrive while concurrently diminishing many good lactic acid bacteria and other bacteria [131]. Fecal samples from rats administered with glyphosate from gestational day 6 to 13 weeks post-partum showed imbalanced intestinal microflora [132]. Among human gut bacteria, approximately 54% of species and 20% of total gut bacteria have EPSPS, and glyphosate, which inhibits this enzyme, may cause damage to human gut bacteria [133]. The gut microbiota plays an important role in human health through immune and metabolic interactions [134]. A fluctuating composition of the intestinal microflora and dysbiosis, and an excess or diminished abundance of specific bacteria, can cause defects in the human intestinal tract, systemic immunity, and metabolism; consequently, these might be involved in various pathological states, such as autoimmune diseases, rheumatoid arthritis, autism, and multiple sclerosis [135–138].
The number of children with autism, attention deficit hyperactivity, and other neurodevelopmental disorders has increased rapidly in the USA and Japan [139, 140]. Increased glyphosate application to corn and soybean crops between 1995 and 2010 in the USA positively correlated with an increase in the incidence of autism in public schools, suggesting that glyphosate was a contributor [141]. Similarly, triplets comprising two boys and one girl delivered through cesarean section had autism and epilepsy, respectively, and high levels of glyphosate were found in their urine and metabolites, indicating increased intestinal clostridia [142]. However, changing their diet to organic products decreased glyphosate levels and alleviated neurological symptoms [142]. Epidemiological findings have notably identified an association between increased intestinal clostridia and the development of autism, indicating a relationship with disrupted intestinal microbiota due to glyphosate [143]. Hundreds of bacteria types exist within the Clostridium genus, and while the genus does not only include bad bacteria such as Clostridium botulinum and Clostridium tetanus, it also comprises good bacteria that suppress runaway immunity; therefore, the Clostridium genus is not totally detrimental [144].
An epidemiological study found significant correlations between the development of autism and organophosphorus pesticides and glyphosate in 2,961 children with autism in the USA [145]. Glyphosate exposure disrupts the balance of intestinal microflora and causes behavioral abnormalities in experimental animals; therefore, the possibility that glyphosate has a negative effect on brain development cannot be denied [146]. Despite the conventional view that only genetic factors are responsible for developmental disorders such as autism, environmental factors are also likely to play major roles [147]. The American Academy of Pediatrics issued an official statement in 2012 that pesticides increase childhood cancer and negatively affect brain development [9]. In addition, non-toxic doses of glyphosate administered to female rats during gestation and lactation result in increased oxidative stress markers in the brains of rat pups, as well as irregular acetylcholine and glutamate metabolism and impaired learning ability [148]. Therefore, based on scientific evidence to date, the International Federation of Gynecology and Obstetrics issued a global recommendation to cease the usage of glyphosate because of its suspected association with neurodevelopmental and other disorders such as cerebral palsy caused by methylmercury poisoning. Therefore, according to this precautionary principle, avoiding glyphosate since it can pass through the placenta is a social responsibility [149].
Epigenetics refers to a system that controls and transmits gene expression without altering its DNA sequence [150]. The main mechanisms involved include DNA methylation, histone protein modification, and miRNA expression [150]. This concerns carcinogenesis, developmental origins of health and disease (DOHaD), and its impact on the next generation [151]. The DOHaD theory states that epigenetic changes from fertilization to development affect health and disease in adulthood, and this theory has recently received attention. However, abnormal DNA methylation in the germ cells of parents exposed to toxic chemicals with endocrine-disrupting effects (Figure 1), including pesticides, dioxins, bisphenol A (a plastic material), and polychlorinated biphenyls, are passed down through generations of grandchildren and great-grandchildren who were not directly exposed to these agents [10]. This phenomenon is known as transgenerational toxicity. This phenomenon is known as transgenerational toxicity: the effects of various pesticides on epigenetics are listed in Table 1.
For example, F0-generation female pregnant rats that were transiently exposed to glyphosate (25 mg/kg body weight per day) and F0 or F1 rats that were directly exposed essentially manifested no pathological effects. However, prostate, kidney, and ovarian diseases, as well as obesity and congenital disabilities, manifested in the F2 and F3 generations [152]. Sperms from F1 to F3 males revealed more DNA methylation mutations than those in unexposed controls, which were considered to be responsible for the failure of the next generation [152]. The effects of glyphosate on the next generation were investigated by mating normal male rats with the female offspring of maternal rats that had been exposed to glyphosate (2 mg/kg body weight per day) from gestation to lactation [153]. The results revealed no effects in F1, although the numbers and sizes of fetuses were reduced in F2, even at low doses of glyphosate [153]. Uteri from F1 female rat pups exposed to glyphosate during development expressed more genes for estrogen receptor-alpha and abnormal DNA methylation in the regulatory region of these receptors [65]. Therefore, abnormalities in F2-generation fetuses are speculated to be due to glyphosate-causing DNA methylation abnormalities.
Female hormone receptors play an important role in the uterus and require appropriate amounts of hormones; however, their abnormal expression causes polycystic ovary syndrome, endometriosis, and unexplained infertility in humans (Figure 1) [154–156]. Abnormal mammary glands developed in male pups born to rats whose mothers had been administered with glyphosate from gestation to lactation [157]. Moreover, glyphosate altered the abundance of estrogen receptor-alpha transcript variants through hypermethylation of estrogen receptor promoters using DNA from these mammary tissues. Furthermore, glyphosate causes mutations in DNA methylation in plants (Arabidopsis thaliana, common bean, soybean, and rice) [158–160].
The mechanism through which glyphosate causes mutations in DNA methylation has not yet been clarified; however, since substances with environmental hormone effects cause mutations in DNA methylation, glyphosate might be involved [161]. Many pesticides have environmental hormone effects [162]. Reactive oxygen species (ROS) might also be involved as they disrupt DNA methylation [163]. Generally, DNA methylation has a substantial impact because it is responsible for the regulation of gene expression [164]. In addition, methylated DNA is usually passed on within a cell and also after it divides. The fact that DNA methylation mutations occur in germ cells also suggests that genomic imprinting can be passed on to the next generation and beyond. Although experimental animal findings can be applicable to humans, opinions differ as to whether mutated DNA methylation causes transgenerational effects in humans [165, 166]. As mentioned above, pesticides such as glyphosate modify the intestinal microbiota, possibly resulting in indirect effects on the next generation by causing changes in epigenetic effects. Current pesticide toxicity tests include next-generation reproduction; however, effects up to three generations have not been investigated. Therefore, future mandatory tests of pesticide toxicity should include epigenetic effects such as DNA methylation.
Insecticides are designed to target the nervous system of insects. Therefore, these products may also have neurotoxic effects on non-target animals, including humans and other mammals. The association between adult neurodegenerative diseases and pesticide exposure is believed to result from a combination of environmental factors and genetic susceptibility. Although aging is unquestionably the most significant risk factor, low-dose and long-term exposure to pesticides plays an additional role in the development of these diseases [8].
PD is a neurodegenerative disorder characterized by the accumulation of α-synuclein in the brain, leading to a reduction in the number of neurons. Patients with PD experience tremors, slow motion, and imbalance as neurons controlling motion in the midbrain become disrupted. The causes of PD are multifaceted, involving factors such as aging, sex, and genetic predisposition, compounded by environmental influences like pesticide exposure [167]. An association between glyphosate and the development of PD has been suggested. For example, findings in China and Brazil have shown that PD develops weeks to months after accidental dermal exposure to large doses of glyphosate products [168, 169]. Furthermore, a patient in Japan had PD 4 years after recovering from glyphosate poisoning [170].
Although a causal relationship between glyphosate and PD is unclear, excess ROS are involved in the development of this disease, and glyphosate exerts neurotoxic effects due to ROS generation [171]. Furthermore, exposing mice to glyphosate causes the death of dopaminergic neurons, which are believed to be involved in the development of PD [172]. PD is not associated with glyphosate alone but also with exposure to pesticides, such as paraquat and rotenone [173]. Furthermore, PD was recognized in France as an occupational illness of agricultural workers in 2012 [174].
Alzheimer’s disease is the most frequent type of dementia. The accumulation of amyloid-β and tau proteins in the brain causes memory impairment. Although 70% of the risk associated with the incidence of Alzheimer’s disease is due to genetic factors, other causes include obesity, smoking, hypertension, and diabetes mellitus [175]. It is reported that in addition to these factors, exposure to certain pesticides, particularly chronic exposure to organophosphorus, contributes to the risk of Alzheimer’s disease incidence [54, 176]. Dementia and pesticide exposure are related to genetic susceptibility, with a suggestion that people with mutations in certain genes, such as enzymes that detoxify pesticides, are susceptible to exposure to pesticides [54].
Farm laborers and pesticide spreaders who have been involved in agricultural work for long periods could be exposed to long-term pesticide exposure, although at low concentrations. It has been reported that chronic exposure to low concentrations of organophosphorus among farm workers could cause negative influences on brain function, including changes in attention, speaking, vision, memory, and emotion, as well as the incidence of illnesses such as depression [177]. Sheep farmers also routinely use low concentrations of organophosphorus to remove parasites from their sheep; however, this concentration has also been shown to impair neurological function [178]. Therefore, long-term exposure to organophosphorus, even at low concentrations, can have severe implications not only for farmers but also for workers in other industries.
This study focused on the insecticide neonicotinoid and the herbicide glyphosate. However, the variety of organophosphates, pyrethroids, herbicides such as glufosinate and paraquat, and bactericides is wide. Even if their respective exposure levels are low and within standards, the effects of combined exposure should be considered. However, the safety standards for pesticides are based on toxicity tests of individual pesticides rather than their combined effects [179]. Experimental animals exposed to low doses of six pesticides and an acceptable daily intake reportedly developed impaired liver and other organ functions [180].
Interestingly, smart agriculture that integrates agriculture and information technology has been promoted. This concept includes the aerial application of pesticides using drones [181] that are allegedly safer since they spray at lower altitudes than airplanes. However, a lower on-board capacity requires highly concentrated pesticides that are not immune to aerial spread. Many homes and public facilities in Japan are located near farmlands, which raises concerns regarding the spread of pesticides. Aerial pesticide spraying is considered dangerous and is entirely prohibited in principle in the European Union [182]. Furthermore, inhaled pesticides are dangerous because they directly enter the bloodstream and are circulated throughout the body [183]. Current pesticide toxicity tests include acute inhalation toxicity studies of experimental animal models. However, the effects of chronic inhalation by adults and inhalation by children have not been investigated. Therefore, while drones are useful for surveying the growth of agricultural products, promoting the aerial application of pesticides using drones could be dangerous, and the possibility of drone crashes indicates a need for careful consideration of their uses and associated risks.
The composition of the intestinal microflora is mainly influenced by diet [184, 185]. Particularly, western diets that are high in carbohydrates and fats but low in fermentable fiber content have been implicated in the increased incidence of non-communicable chronic diseases, including neuropsychiatric disorders, metabolic disorders, and the increased prevalence of obesity [186].
Changes in the intestinal microbiota of maternal mice fed with a high-fat diet resulted in abnormal intestinal microbiota and socially dysfunctional offspring [187]. Furthermore, social dysfunction was evident in subsequent generations, raising concerns that these effects are multigenerational. Moreover, abnormal intestinal bacteria have also been associated with lifestyle-related diseases such as allergies, obesity, and diabetes mellitus [188], which might be influenced by the quality and quantity of agricultural products consumed in the diet, as well as by food-derived microbes.
A healthy diet needs to be traced back to crops, and the agriculture-medicine partnership linking agriculture with preventive medicine is considered important [189]. A previous study showed that daily exposure to organophosphorus pesticides decreases the amount of acetic acid, a short-chain fatty acid produced by intestinal bacteria in humans [190]. In that study, urine and stool samples were collected from 38 volunteers, and exposure levels were evaluated by measuring pesticide metabolites and other parameters to assess their effects on the gut microbiota and metabolite concentrations. This study revealed statistically significant negative correlations between the concentrations of urinary dialkyl phosphate, a marker of organophosphorus pesticides, and those of acetic and lactic acid in stools [190]. Although the mechanism of action is not yet understood, routine exposure to organophosphorus insecticides might contribute to intestinal immune regulation and other factors.
Some intestinal bacteria and lactobacilli have intestinal regulating and immunomodulatory effects and affect cranial nerve diseases, such as depression and dementia [8]. When food is considered, the focus tends to be directed toward the functionality of vegetable and fruit components such as polyphenol antioxidants. However, the functionality of environmental bacteria is also important.
Caenorhabditis elegans (C. elegans) is an alternative model organism that has been used in genetics experiments since 1974 [191]. It is a non-parasitic, free-living soil nematode that primarily feeds on bacteria. Most nematodes are hermaphrodites and have short lifespans of approximately 3 weeks at 25℃, making them easy to rear. Molecular biological techniques, including gene analysis, have been established, and many genes homologous to those in mammals have been discovered. Consequently, C. elegans has become a valuable host model for evaluating pesticide toxicity or epigenetic experiments. Pesticides are known to affect nematode locomotion, feeding behavior, brood size, growth, longevity, and cell death [192, 193]. Therefore, it is crucial to quantify the percentage of neurons exhibiting neurodegenerative disorders after exposure to pesticides [194]. C. elegans undergoes generational changes approximately every 3 days after hatching, allowing for the acquisition of the next and third generations in an early cycle. The transmission of epigenetic conditions from the nematode gut to the germline enhances stress resistance in the next generation of nematodes [195]. In addition, models of Alzheimer’s disease with a human amyloid-β synthesis gene and PD models with green fluorescent protein inserted into the synuclein have been developed [196, 197]. We previously found that lactic acid bacteria treatments inhibit amyloid-β in some C. elegans models of Alzheimer’s disease [198]. However, no references exist regarding the effects of pesticide exposure on the next generation or neurodegenerative disease and microbiomes using the nematode. Therefore, in the future, the use of nematodes could reveal the possibility of epigenetic transmission of the abovementioned influences of pesticides.
Pesticides are used to ensure increased food production and adequate quality, depending on the environment in which the food is grown. However, it should be noted that considering and carefully evaluating the toxicity and health hazards associated with various pesticides is important for human health and that of future generations. Therefore, it is necessary to conduct research from an integrated perspective, accumulate data in each field, and revise rules for pesticide use and evaluation methods. Lastly, to consider agricultural and health improvements, it may be important to clarify the functionality of various bacteria existing in the environment and nature. Research on pesticides has tended to focus on environmental issues and direct health effects on the human body; however, this review introduced indirect health effects through their impacts on bacteria and other microorganisms, as well as epigenetic effects as a mechanism of these impacts. Therefore, considering these indirect effects will be necessary in the future.
C. elegans: Caenorhabditis elegans
DDT: dichlorodiphenyltrichloroethane
nAChRs: nicotinic acetylcholine receptors
PD: Parkinson’s disease
ROS: reactive oxygen species
Thanks are due to Editage (www.editage.com) for English language editing.
TK: Writing—original draft. MY and YN: writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This article was supported in part by a Grant-in-Aid for Scientific Research [22K11781] from the Japan Society for the Promotion of Science. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
