Affiliation:
UMR PNCA, Université Paris-Saclay, AgroParisTech, INRAE, 91120 Palaiseau, France
Email: anne.blais@agroparistech.fr
ORCID: https://orcid.org/0000-0002-0897-7612
Explor Med. 2025;6:1001330 DOI: https://doi.org/10.37349/emed.2025.1001330
Received: January 31, 2025 Accepted: May 09, 2025 Published: June 09, 2025
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
The article belongs to the special issue Gut Microbiota Derived Metabolites and Chronic Inflammatory Diseases
Lactoferrin (LF), an iron-binding protein, is found in mammalian milk. LF is also secreted by different cell phenotypes. LF shows a wide range of biological activities, as many preclinical and clinical studies indicate that LF and its derived peptides have many biological functions in host defence, including not only antibacterial, but also antiviral, antifungal, and antiparasitic effects. These results raise the view that these compounds might affect the composition of the intestinal microbiota. LF is generally recognized as safe (GRAS). This protein has been shown in experimental studies to exert beneficial effects on intestinal inflammation. This review will target the beneficial effects of oral LF supplements on the intestinal ecosystem during inflammation and highlight the mechanisms by which LF may contribute to reducing inflammatory flare, and present perspectives for future research.
Lactoferrin (LF) is a component of the milk whey protein of most mammals. LF, is a globular iron-binding glycoprotein that belongs to the transferrin family. LF is found in human breast milk at relatively high concentrations (2–3 g/L) [1, 2]. Bovine and human LF share sequence homology (69% amino acid sequence homology) and structure homology as judged from their three-dimensional structures [3]. LF is also found in many other fluids. Many cells within human tissues and organs can produce LF and presence of LF has been confirmed in kidneys, lungs, gallbladder, pancreas, intestine, liver, prostate, and cells of the immune system and in fluids such as saliva, tears, sperm, cerebrospinal fluid, urine, bronchial secretion, synovial fluid, umbilical cord blood, blood plasma, and vagina discharge [3]. LF, in the circulation of healthy individuals, is found in concentrations ranging between 2 and 7 µg/mL [4]. Intact bovine milk-derived LF (bLF) can be absorbed by intestinal epithelial cells and enter the systemic circulation in mice and rats [5, 6]. Intact bLF can be recovered in the duodenal (~9.5%) and in the colonic luminal fluids (~7.2%) [7].
LF mass from milk and colostrum is in the range of 83–87 kDa, while in neutrophils, LF mass is about 87–91 kDa [8]. Among the different physiological functions of LF, as will be presented in the following paragraphs, one of the most important is a direct antimicrobial role. In addition, LF shows effects on the intestinal epithelial cells. Furthermore, the LF’s ability to modulate immune response and to protect against viral infections and septic shock has been described [9–14].
bLF appears to be safe for human consumption. Indeed, this compound was approved in 2012 by the European Food Safety Authority (EFSA) as a novel food ingredient [15]. Earlier, in 2001, bLF was considered generally recognized as safe (GRAS). In fact, bLF is GRAS for use as a food additive and dietary supplement in infants and adults by the Food and Drug Administration (FDA) [16]. bLF is currently added to several foods, including infant formulas, yoghurts, beverages, and dietary supplements [17, 18]. The aim of the present review is to present the experimental and clinical arguments that suggest that LF can be beneficial in situations of inflammatory flare, and to present some directions for future research in that field.
As LF hydrolysis is minimal in infant intestinal content, oral administration of LF has been shown to improve growth and renewal of the intestinal epithelium of infants during development [19]. Experimental studies show the positive effects of LF on the intestine. LF modulates numerous parameters involved in the physiology of the intestinal epithelium. The modifications of such parameters include an increase in the intestinal epithelial differentiation, as judged from the measurement of the brush-border-associated enzymatic activities and amino acid transporters, an increase in the mouse jejunal villus height, and a decrease in epithelial cell spontaneous apoptosis [20, 21]. In Caco-2 cells, bLF decreases the expression of Cdck2 and increases the expression of TAF1, which regulates cellular proliferation and differentiation [20]. In rat pups, bLF accelerates intestinal maturation, increases expression of tight junction (TJ) proteins, of goblet cell marker mucins, and of several enzymes associated with the intestinal brush border membranes. However, LF ingestion was more efficient for increasing the expression of TJ proteins in the colon than in the ileum [6]. LF is internalized through clathrin-mediated endocytosis, and after entering inside the nucleus, LF increases thymidine incorporation in crypt cells and regulates the transcription of many genes [22]. Moreover, metabolic approaches indicated that higher plasma LF concentration is correlated to higher amino acid concentration, in particular essential ones, that favor protein synthesis, supporting an enhanced growth and maturation of the intestine and a potential therapeutic interest in post-natal development [6]. LF possesses antibacterial and immunomodulatory properties that play a role in the protection of the epithelial barrier. LF plays key roles in the regulation of the intestinal mucosal immune system [23, 24]. The intestinal epithelial cells include not only absorptive cells but also cells that participate in the immune activities, such as those involved in cytokines secretion [25, 26].
LF can be administered by different routes: oral, intravenous, or local. LF digestion in the stomach by pepsin is limited. This limited digestion releases bioactive peptides, called collectively lactoferricin, which have been characterized for their antibiotic capacities [27]. LF-derived peptides have been studied for their biochemical properties of lactoferricin peptides may interact with lipidic compounds and negatively charged surfaces of both Gram-negative and Gram-positive bacteria, as well as with fungi, viruses, and parasites thus explaining presumably partly antibacterial, antifungal, antiviral, and antiparasitic activities of these peptides [28, 29]. LF was shown four decades ago to be not fully degraded by the exocrine enzymatic pancreatic protease activities [30], thus raising the view that a part of the ingested LF may remain in intact form in the small intestine luminal fluid. In newborns, LF is absorbed, but in adults, the LF its bioavailability is very low. However, LF can be absorbed in intact form by epithelial cells and enter the systemic circulation, but the exact percentage of LF that is absorbed remains unknown [31, 32]. As free LF has a half-life of about 12–60 min in blood, many approaches to optimize oral delivery of LF are under development [33].
The presence of LF in the intestinal fluid and in blood coincides with effects of this protein on several tissues, including notably small and large intestine. The physiological effects of LF on target cells have been shown to involve the binding to specific receptors. The target cells equipped with receptors to LF include intestinal epithelial cells [33, 34], lymphocytes [35], and macrophages [36].
The small and large intestines are well known to be inhabited by a complex mixture of microbes, among which bacteria have been the subject of most studies. The concentrations of bacteria increase from the proximal part of the small intestine to the distal part of the large intestine. In the large intestine, the bacterial concentration is in the range of 109–1012 colony-forming unit (CFU) per g of content. The spectacular increase in the concentration of bacteria in the distal small and large intestine is notably due to a much slower transit of the intestinal content in the large intestine than in the small intestine, allowing intense metabolism of the available substrates supplied by the host [37]. This microbial population includes not only bacteria, but also other microorganisms such as archaea, viruses, and fungi. Regarding protozoans in the intestine, these eukaryotic microorganisms are usually not considered members of the intestinal microbiota. They represent a heterogeneous group of living organisms, with several of them being classified as parasites [38].
As previously reported, LF and LF-derived lactoferricin have been demonstrated in numerous studies to exert antibacterial, antiviral, and antifungal, but also antiparasitic effects and to stimulate the immune response of intestinal epithelial cells. These effects are related to the effects of LF on specific immune response regulation [39–41]. Notably, LF can modulate cytokines such as interleukins (IL-1b, IL-6, IL-10, and IL-18) and tumor necrosis factor alpha (TNF-α) production by intestinal immune cells [42, 43].
The functional role of LF in gastrointestinal inflammation is mainly attributed to its capacity to bind to iron, thus inhibiting the invasion or adhesion of bacteria. However, other mechanisms of action have been shown, LF can bind to LF receptors in intestinal mucosa and lymphatic cells to modulate the antimicrobial effects of LF, but also to specific receptors in bacteria [44] and to bacterial cell walls, leading to the disruption of bacteria integrity and accordingly contributing to bacterial death. Direct interaction with Gram-negative bacteria leading to cell membrane damage has been reported [45, 46]. Bovine LF retards the growth of the pathogenic bacteria Clostridium difficile in a model of bacterial infection [47]. This result is of major importance considering the high incidence of Clostridium difficile infection in individuals with inflammatory bowel diseases (IBDs). However, we do not know if specific bacteria can degrade LF, a situation which would render bacteria more resistant to the antibacterial effects of LF.
Concerning the antiviral effect of LF, this protein has been shown to be active on both DNA- and RNA-viruses, including human rotaviruses [48, 49], which represent a major cause of diarrhea in children younger than five years [50]. The fungicidal activity of LF has been shown against various Candida species, including Candida albicans, a common fungus found within the human intestinal microbiota [51–53]. Candida albicans exacerbates intestinal inflammation in mice [54]. Lastly, the antiparasitic effect of LF has been demonstrated in vitro against the malaria parasite Plasmodium falciparum [55], which is involved in complex interaction with the gut microbes [56]. Lastly, microbicidal effects of LF-derived peptides have been demonstrated against the parasite Entamoeba histolytica [57]. This parasite infects humans primarily within the intestinal tract [58], provoking intestinal inflammation and ulceration in infected patients [59].
LF is also active on the host intestinal epithelial cells. In rodents, a model, as well as in an in vitro study with intestinal epithelial cells, LF has been shown to efficiently increase intestinal cell proliferation and differentiation [20]. Interestingly, LF ingestion by the mother during gestation and lactation was shown to promote early pup development in a rodent model [6]. This latter effect coincided in pups with modification of intestinal epithelial physiology. In fact, increased small intestine epithelial cell differentiation as well as increased colon barrier function are recorded after LF supplementation. The increased epithelial cell differentiation was associated with increased expression of genes coding for the tight-junction proteins. In addition, in such a situation, LF supplementation was associated with higher plasma amino acid concentrations, maybe because of increased expression of amino acid transporters. This latter hypothesis needs to be experimentally tested to confirm or invalidate it.
IBDs are chronic inflammatory disorders affecting the gastrointestinal tract, with the two main forms being ulcerative colitis (UC) and Crohn’s disease. IBD etiology is not fully understood, but the interactions among mucosal immune cells, barrier function, and commensal enteric flora involve both genetic and environmental factors (including dietary parameters) that presumably play significant roles [60]. IBD induced an increase in intestinal permeability, reduction of TJ protein expression, and overproduction of proinflammatory cytokines, followed by accumulation and activation of immune cells. LF may be of interest in individuals prone to IBDs since this protein acts as a protector of the intestinal barrier [61]. The disease activity coincides with increased secretion of pro-inflammatory cytokines, including notably TNF-α, IL-1, IL-6, and IL-8. These molecules play a crucial role in mediating the response to infection [62]. Many studies reported that LF orally administered can bind to LF receptors on intestinal cells and gut-associated lymphatic tissue to modulate cytokine production. Such binding is associated with the stimulation of the synthesis of anti-inflammatory cytokines, including IL-4 and IL-10, these proteins being considered potent anti-inflammatory and immunomodulating compounds involved in the protection of the mucosa against infections and inflammation [63, 64].
bLF oral administration in animal colitis model can diminish signs of intestinal inflammation, probably partly by modulating the immune system and by reducing the pro-inflammatory cytokine production and secretion in the colonic tissue. Furthermore, LF can increase the expression of TJ proteins [65–67]. LF has also been shown to activate the mitogen-activated protein kinase (MAPK) to promote cells proliferation and differentiation [68]. In addition, bLF and its derived peptides, when given orally, reduce signs of experimentally induced colitis in mice when given orally [69]. Indeed, in this latter experimental work, bLF reduces the presence of occult blood in feces and the number of TNF-α-producing cells in the distal colon. Oral delivery of a Lactococcus lactis strain secreting LF-derived peptides can diminish the development of colitis in mice [70]. Furthermore, in the model of colitis induced by dextran sulfate sodium (DSS), bLF was found to reduce inflammation and, accordingly, the impairment of colonic epithelial barrier function. These effects were paralleled by changes in the composition of the flora, such changes being hypothesized to improve the intestinal barrier regeneration [71].
The effects of LF supplementation have also been tested in the model of endotoxemia provoked by administration of lipopolysaccharide (LPS). This model is characterized by a systemic inflammatory response [72]. LPSs are glycolipids that are components of the bacterial surface. LPS produced by Gram-negative bacteria can be partly transferred from the luminal intestinal fluid to the bloodstream [73]. Within the intestine, LPS systemic administration has been shown to increase intestinal permeability, epithelial cell apoptosis and shedding, while provoking villus shortening and overall diarrhea [74–77]. These effects of LPS are associated with several intestinal dysfunctions, such as decreased mucosal oxygen consumption and amino acid absorption [78, 79]. LF ingestion diminishes the systemic inflammation induced by LPS and reduces the associated intestinal damages in different in vivo experimental models [80–83].
Conversely, a recent study has shown that LF deficiency further aggravates LPS-induced inflammation [84]. From a mechanistic point of view, absorbed bLF or bLF peptides can bind to LPS, preventing systemic excessive LPS signaling. Such effect results in the inhibition of the nuclear transcription factor kappa B (NF-κB). Such inhibition then reduces TNF-α secretion by TLR4 (monocytes/macrophages/dendritic cells) and then finally limits the interaction of TNF-α with the TNRF1 (tumor necrosis factor receptor 1) [85]. Such interaction would reduce enterocyte apoptosis and shedding, as well as TJ damage. LF supplementation, when performed before LPS administration, has been recently shown to reduce the circulating concentration of the inflammatory cytokine TNF-α, to prevent the increase intestinal permeability, and to maintain the morphology of the intestinal mucosa when compared with controls without LF [14]. Moreover, this study reported a good correlation between blood TNF-α level and intestinal permeability, suggesting a direct anti-inflammatory action of LF in the intestine and that the sensitivity of the jejunum and the colon to the beneficial effect of LF against LPS challenge is not similar [14]. LF is also able to inhibit intestinal dysfunction in inflammation by modulating the MAPK pathway, which is essential to maintain intestinal integrity and to reduce production of proinflammatory factors [86]. Another anti-inflammatory effect of LF is related to its ability to reduce production of reactive oxygen species by granulocytes [87]. The ability of LF to protect the intestinal tract against systemic inflammation may involve not only an anti-inflammatory effect but also a direct protective effect on epithelial cells’ growth, differentiation, and TJ formation. Figure 1 schematically shows the main beneficial effects of LF.
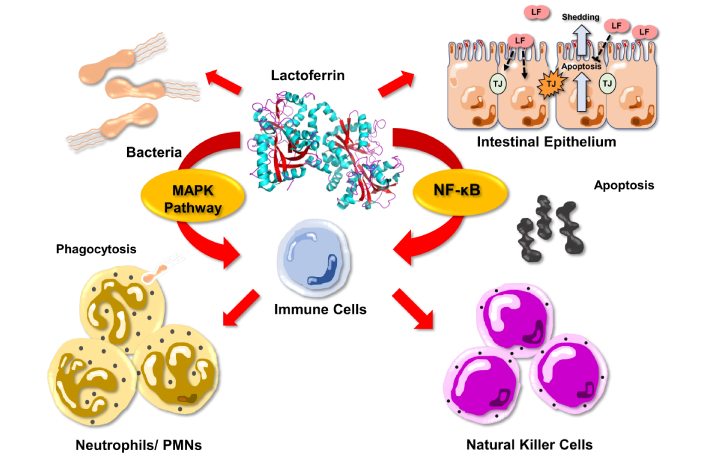
Schematic presentation of lactoferrin effects on intestinal inflammation. LF: lactoferrin; TJ: tight junction; MAPK: mitogen-activated protein kinase; NF-κB: nuclear transcription factor kappa B
Incidentally, but importantly, the amount of LF in feces, which represents neutrophil infiltration in case of intestinal inflammation, is used in addition to calprotectin, as a fecal marker of inflammation [88]. This is because the amounts of LF released by neutrophils have been shown to correlate with the severity of inflammation in the gastrointestinal tract [89]. In that latter case, it is worth to note that LF release from neutrophils is from endogenous but not dietary origin. It is possible that in a healthy situation, a part of LF in the fecal material originates from the diet. In any case, the presence of LF in feces suggests that this protein is not extensively degraded by the intestinal microbiota. Evaluation of the resistance of LF to the different proteases and peptidases equipping the bacteria within the large intestine luminal fluid needs further work.
Although there is a set of data indicating a beneficial role of bLF oral ingestion (usually 10 g per kg of diet) for reducing the signs and severity of colitis in several experimental models, we need clinical trials with volunteers prone to chronic intestinal mucosa inflammation are obviously required to test if such dose may prove to be active for the reduction of the severity of inflammation. Obviously, LF per se cannot substitute for pharmacological treatments in patients with different pathologies resulting in chronic intestinal inflammation, but LF supplementation may prove to be effective in patients as adjunctive therapy in future randomized-controlled clinical trials. More information is needed on the precise dispatching of ingested LF between the intestinal luminal fluid and the bloodstream according to the dose of LF used. Such results are necessary for our understanding of the origin of LF, either from the intestinal content or bloodstream, for the observed effects. Notably, the measurement of the concentrations of LF after dietary supplementation in the small and large intestine, respectively, would be very helpful. Indeed, if, as shown in several experimental studies, LF is partly resistant to protease activities in the gastrointestinal tract, it can be expected that a part of LF will be transferred through the ileocecal junction from the small to the large intestine. Measurement of the resistance of LF to protease and peptidase activities from the intestinal bacteria present in the large intestine luminal fluid also needs to be tested. Randomized controlled clinical studies with increasing doses of oral LF and assay of LF in feces and blood in healthy subjects will be obviously instructive to answer this question. As a significant part of orally ingested LF is degraded within the gastrointestinal tract, new formulations such as encapsulation and nanoparticular carriers that can reduce LF degradation within the gastrointestinal luminal fluid and presumably enhance its absorption may prove to be helpful.
We also need to get more information on the impact of LF in oral supplements and at increasing doses on the microbiota composition and metabolic activity. Indeed, if the available results clearly indicate antimicrobial effects of LF, we do not know the effects of increasing the amounts of oral LF on the fecal microbiota composition. The efficiency of LF for modulating growth and virulence of pathogenic bacteria, fungi, viral, and parasite infections could be usefully tested in animal models.
The encouraging results obtained after oral supplementation with LF in situations of experimental intestinal inflammation should allow utilization of LF in clinical trials with volunteers prone to chronic intestinal inflammation or in remission phase. Progress in the identification of LF-derived peptides and their biochemical characteristics (heat resistance, resistance to bacterial proteases) and physiological characteristics (absorption through the intestinal epithelium, dose-effect curves on pathophysiological parameters) is another important goal. This finding would help development of LF peptides efficient in situations of inflammatory states, whose efficacy will be first tested in experimental studies to provide insights for clinical studies. Moreover, as bioavailability of orally administered LF is low oral formulation resistant to gastric digestion is also needed.
Finally, although LF has been recognized as safe by the American and European authorities, possible side effects of LF supplementation need to be considered and investigated in the different human subpopulations, such as infants and the elderly, to fully evaluate the potential of LF in situations of intestinal inflammation.
Indeed, it is only fair to recognize that most of the knowledge on the effects of LF on the small and large intestine mostly originates from preclinical experimental studies.
bLF: bovine milk-derived lactoferrin
GRAS: generally recognized as safe
IBDs: inflammatory bowel diseases
IL: interleukin
LF: lactoferrin
LPS: lipopolysaccharide
MAPK: mitogen-activated protein kinase
TJ: tight junction
TNF-α: tumor necrosis factor alpha
AB: Conceptualization, Visualization, Writing—original draft, Writing—review & editing.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Olha Denefil ... Khrystyna Loza
Weinan Du ... Juxiu Li
Alejandra Vargas ... David A. Johnson
Shin Takasawa ... Maiko Takeda
Zeneng Wang ... Robert Koeth
