Affiliation:
1Department of Pathophysiology, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-3606-5215
Affiliation:
2Faculty of Medicine, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0003-2718-5191
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-7742-1346
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-6898-1149
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-8145-7931
Affiliation:
3Department of Dental Therapy, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
Email: levkiv@tdmu.edu.ua
ORCID: https://orcid.org/0000-0001-7327-051X
Affiliation:
4Department of Dental Surgery, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0002-1247-2430
Affiliation:
4Department of Dental Surgery, I. Horbachevsky Ternopil National Medical University, 46000 Ternopil, Ukraine
ORCID: https://orcid.org/0000-0001-9671-8049
Explor Med. 2023;4:942–955 DOI: https://doi.org/10.37349/emed.2023.00186
Received: June 24, 2023 Accepted: September 11, 2023 Published: December 11, 2023
Academic Editor: Gaetano Isola, University of Catania, Italy
The article belongs to the special issue Gut Microbiota Derived Metabolites and Chronic Inflammatory Diseases
Aim: The aim is to analyze the microbiome of gingival sulcus and periodontal pockets of patients with periodontal disease associated with systemic diseases.
Methods: A microbiological study was conducted to analyze the microflora of the periodontal pockets in patients with different systemic pathologies and periodontal diseases. Plaque samples were collected from the gingival sulcus and periodontal pockets, and they were subsequently cultured on nutrient medium and glass plates.
Results: The microbiota of the gingival sulcus and periodontal pockets in patients with associated systemic diseases in combination with periodontal disease was studied. The frequency of detecting the qualitative composition of the microbiota in the periodontal niche of patients with periodontal diseases and systemic diseases was determined. The research paper outlined groups of microorganisms isolated from periodontal pockets of patients with periodontal and systemic diseases.
Conclusions: The degree of colonization by microorganisms differed slightly, while the frequency of detection of specific populations of opportunistic bacteria increased in chronic generalized periodontitis compared to chronic catarrhal gingivitis.
Diseases of periodontal tissues constitute a significant problem in modern society due to their widespread and progressive course as they accompany tooth loss and compromised function of the maxillofacial system, decreasing patients’ life quality [1, 2]. The main etiological and pathogenic factors of periodontal diseases are the aggressive microbes and their by-products [3, 4]. Periodontopathogenic oral microflora is the initial mechanism of the development of chronic inflammation in periodontal tissues. The hyperproductive growth of pathogenic microorganisms characterizes the dysbiosis of different endo-ecological niches of the oral cavity, leading to and supporting the severity and course of inflammatory-destructive processes in the periodontium. In the pathogenesis of inflammatory periodontal diseases, there is an interaction of two pathogenetic mechanisms: the influence of anaerobic microflora and the immunological reactivity of the human body. Periodontopathogenic species of bacteria differ from others in being highly adhesive, invasive, and toxic concerning periodontal tissues. Periodontopathogenic microorganisms have a wide range of pathogenic factors, which allows them to induce a prolonged inflammatory process. Bacteria can produce enzymes that inactivate antibiotics, which complicates treatment.
The researchers recognized that the course of the pathological process in the periodontium depends not only on the virulence of microorganisms in the oral cavity but also on the body’s immune resistance [5]. Therefore, the state of the immune system and the resistance of periodontal tissues to bacterial invasion are crucial in the modern concept of the etiology and pathogenesis of periodontal diseases [6]. Herpesvirus infections may impede the antibacterial host defense and alter periodontal cells to predispose bacterial adherence and invasion [7]. One of the problems of modern clinical dentistry is the increase in the incidence of chronic inflammatory pathology of the periodontium, which develops because of secondary immune deficiency [8].
General reasons that determine the resistance of periodontal tissues to pathogenic influences are also important [9]. The relationship between systemic and periodontal pathology is apparent. Systemic pathology is a risk indicator or risk factor in periodontal diseases [10, 11].
Systemic disorders can impact various systems in the body, including the circulatory, respiratory, digestive, immune, and endocrine systems. They often involve complex interactions between different organs and tissues. Many systemic disorders are chronic, persisting over time, and may require ongoing management or treatment. Some systemic conditions have identifiable risk factors, such as genetics, lifestyle factors, environmental exposures, and infections. Diagnosing systemic diseases involves a combination of medical history, physical examination, laboratory tests, and sometimes specialized procedures.
The endocrine system is one of the most significant internal factors affecting the immune response. Multifunctional relationships between the gastrointestinal, immune, and endocrine systems are extremely important in the etiology and pathogenesis of periodontal disease [12]. In the structure of endocrine pathology, one of the leading roles belongs to thyroid gland disorders, especially primary hypothyroidism. Hypofunction of the thyroid gland affects the functional state of many organs and systems, particularly, the tissues of the dento-alveolar complex, the metabolism of bone tissue, and the state of the immune system. Destruction of immunological parameters and the development of a secondary immunodeficiency in the course of hypothyroidism were established [13]. However, the connection between the changes in the oral cavity microbiota due to systemic diseases and the development of periodontal tissue diseases has not been sufficiently studied yet [14].
Many taxonomic refinements have developed as split-offs from previously established genera and species. Classical culture and molecular methods have advantages and drawbacks. It makes sense to recognize their respective attributes to gain the most from studies on periodontal microflora. A few techniques, such as Bergey’s method, 16S ribosomal RNA (rRNA) sequencing, and next generation sequencing (NGS), can be used to identify bacterial taxa.
Bergey’s manual of systematic bacteriology [15] was used as the early standard for identification. It is the leading resource for determining the identity of prokaryotic organisms, emphasizing bacterial species using every characterizing aspect. The advantages of this method are the assessment of living microorganisms, the ability to recognize viable cells in a sample, the easiness of quantitating cells in a piece, and its high sensitivity with appropriate media [16].
The use of molecular techniques for identifying bacteria in periodontology is relatively recent. 16S rRNA sequencing is a molecular biology technique targeting the 16S rRNA gene. This gene is present in all bacteria and contains variable regions that can be used for taxonomic classification. It provides high taxonomic resolution at the genus and species levels, making it suitable for identifying and characterizing individual microbial species within a sample. 16S rRNA sequencing involves PCR amplification of the 16S rRNA gene region, followed by DNA sequencing of the amplicons. The obtained sequences are compared to reference databases to assign taxonomic classifications to the microbes present in a sample.
NGS is a high-throughput sequencing technology that has revolutionized the field of genomics and metagenomics. It enables the rapid and cost-effective sequencing of large volumes of DNA or RNA. This method can be applied to various types of sequencing, including whole-genome sequencing, metagenomic sequencing, transcriptome analysis, and more. It is not limited to 16S rRNA genes and can sequence entire genomes or other genetic elements. NGS generates vast amounts of sequencing data, providing a comprehensive view of the genetic information present in a sample. It allows for a deeper understanding of community microbial diversity and functional potential within a community. NGS can provide both taxonomic information (similar to 16S rRNA sequencing) and functional information by sequencing the entire genetic content of organisms in a sample.
The microbiocenosis of the gingival sulcus and periodontal pockets was investigated to achieve the research goals. In total, 539 patients were examined in the main group. Among them, 83 patients with primary hypothyroidism, 62 patients with chronic colitis, 102 patients with chronic pancreatitis, 112 patients with peptic ulcer disease of the stomach and duodenum, 60 patients with gastroduodenal pathology associated with Helicobacter pylori, and 120 patients with combined pathology of various parts of the gastrointestinal tract and periodontal diseases. Such inclusion and exclusion criteria for patients in a survey were employed, as shown in Table 1.
Inclusion and exclusion criteria of patients into a survey
| Inclusion criteria | Exclusion criteria |
|---|---|
| A compensated form of a systemic disease, no more than ten years. | Decompensated form of a systemic disease, more than ten years in combination with another systemic illness. |
| Patients treated with a pharmacotherapeutic method. | Surgical interventions as a method of treatment of a systemic disease. |
| The age of the patient is from 18 years to 60 years. | The patient’s age is over 60 years. |
| Patients with systemic pathology and the presence of teeth. | Patients with systemic pathology and absence of teeth. |
Patients included in the study were under dispensary observation and outpatient treatment in the endocrinology department of “Ternopil University Hospital” and the polyclinic department of “City Communal Hospital No. 3”. Dentists examined them on an outpatient basis at the Department of Dental Therapy of “I. Horbachevsky Ternopil National Medical University”. Department doctors verified the leading systemic diseases based on valid national and international protocols (according to clinical and instrumental examination data and international classification of diseases).
The control group comprised 30 patients with clinically healthy periodontium without accompanying systemic pathology. In diagnosing periodontal diseases, basic periodontal diagnostic parameters were used: probing depths, bleeding on probing, clinical attachment levels, plaque index, and radiographs assessing alveolar bone level. Only patients aged 18–60 were included in the studied groups (main, control) to exclude age-related pathology.
The absence of a dentogingival junction was the principal criterion for diagnosing chronic generalized periodontitis (CGP). The stage of severity was determined based on the depth of periodontal pockets and the degree of destruction of bone tissue. Thus, a mild degree of CGP is characterized by periodontal pockets less than 3 mm deep and the X-ray pattern confirming signs of initial destruction of the inter-dental septa. The depth of periodontal pockets varied from 3 mm to 6 mm in patients with a moderate stage of CGP; I–II degree of pathologic tooth mobility (according to tooth mobility index by Miller scale in Fleszar modification) was frequently revealed during the examination. According to the data obtained by X-ray examination, the destruction of the interdental septa’s cortical plate and bone tissue was equal to 1/2–1/3 of the length of the tooth root.
Periodontal pockets more than 6 mm deep, II–III degree pathological tooth mobility, cortical plate, and bone tissue destruction by more than 1/3 of the tooth root length are typical for the severe stage of CGP. Cortical plate and bone tissue destruction was revealed by X-ray examination.
Smears from periodontal pockets to study the quantitative and qualitative composition of the microflora of this biotope were taken. Our team collected plaque samples with a sterile inoculation needle from periodontal pockets and immediately applied them to nutrient medium and glass plates.
Researchers took samples from the patient’s gingival sulcus in the morning hours on an empty patient’s stomach with a sterile standard ISO (International Organization for Standardization) size 30 paper endodontic pin (DiaDent), 1 cm long, which, after soaking, the tooth was placed in a sterile saline solution and washed thoroughly.
Sugar meat-peptone agar and blood agar were used to isolate aerobic, facultatively anaerobic, and microaerophilic microorganisms [growth mediums (dry), Farmaktiv LLC]. The identification of isolated pure cultures was carried out according to morphological, cultural, and biochemical characteristics following generally accepted methods and the identifier of bacteria by Bergey (1994) [15]. Bergey’s manual of systematic bacteriology is the primary resource for determining the identity of prokaryotic organisms, emphasizing bacterial species, using every characterizing aspect. An isolated pure culture was identified by a complex of morphological, cultural, and biochemical properties (sets MLT00014 STREPTOtest 16_P, MLT00012 STAPHYtest 16_R, Erba Lachema).
The presence of Fusobacterium, Veillonella, Prevotella, and Porphyromonas was determined according to morphological and cultural properties. Smears were taken from periodontal pocket content and stained using Gram and Romanowsky-Giemsa methods, followed by immersion microscopy. To inoculate bacteria, the method of sectoring on dense nutrient media was employed to determine the specific content of each group of microorganisms in 1 g of biomaterial. The number of colony-forming units (CFUs) expressed the degree of microbial colonization. After counting the CFU, the data was converted into decimal logarithms (lg CFU).
The Bioethics Committee of I. Horbachevsky Ternopil National Medical University Ministry of Health of Ukraine approved the study protocols (excerpt from protocol No. 73) on April 3, 2023. All the patient investigations conformed to the principles outlined in the Council of Europe Convention on Human Rights and Biomedicine (April 4, 1997), the World Health Association Helsinki Declaration on Ethical Principles for Scientific Research with Human Participation (1964–2000), and the Order of the Ministry of Health of Ukraine No. 281 of November 1, 2000; Declaration of Helsinki “World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects” (2001), Code of Scientist of Ukraine (2009). Doctors informed every patient about the study’s purposes. Patients signed their “consent of the patient”.
Statistical analysis and testing of hypotheses for the resulting findings were performed by means of parametric criterion Z-test [17, 18].
The researchers observed significant qualitative and quantitative changes in the character of microbiocenosis of periodontal pockets and gingival sulcus in patients with a concomitant gastrointestinal tract pathology compared to similar, practically healthy patients.
Microbiological studies have shown that various microorganisms inhabit the gingival sulcus and periodontal pockets in chronic catarrhal gingivitis and CGP in patients with primary hypothyroidism. In patients with hypothyroidism, 536 strains of microorganisms that were either aerobic, facultative aerobic, or anaerobic were identified.
The degree of general insemination of the studied microbial oral ecosystem of patients with primary hypothyroidism and periodontal disease was on average 4.4 lg CFU/mL ± 1.2 lg CFU/mL, ranging from 2.7 lg CFU/g ± 0.2 lg CFU/g (non-pathogenic Neisseria) to 6.8 lg CFU/g ± 0.2 lg CFU/g (oral streptococci, Table 2). In this study, the sample mean is denoted as M and the standard error as m.
Microbiome of periodontal pockets in patients with primary hypothyroidism in combination with periodontal disease
| Microorganism | Main group n = 83 | Control group n = 30 | |||
|---|---|---|---|---|---|
| Frequency of detection (%) | Colonization density (lg CFU/mL) (M ± m) | Frequency of detection (%) | Colonization density (lg CFU/mL) (M ± m) | ||
| Facultative anaerobic bacteria | |||||
| Gram-positive cocci | α-hemolytic streptococci | 93.30 | 6.80 ± 0.20 | 100.00 | 6.75 ± 0.11 |
| β-hemolytic streptococci | 51.70 | 5.20 ± 0.20 | 26.70 | 3.39 ± 0.17** | |
| Coagulase-positive staphylococci | 75.50 | 5.60 ± 0.10 | 33.30 | 3.94 ± 0.16** | |
| Coagulase-negative staphylococci | 78.30 | 4.40 ± 0.19 | 46.70 | 4.02 ± 0.18** | |
| Corynebacterium | 33.30 | 3.60 ± 0.10 | 13.30 | 1.44 ± 0.17** | |
| Gram-negative rods | Escherichia coli | 35.00 | 2.30 ± 0.10 | 10.00 | 0.13 ± 0.08** |
| Klebsiella | 15.00 | 1.22 ± 0.30 | 0.00 | - | |
| Non-pathogenic Neisseria | 55.00 | 2.73 ± 0.21 | 43.30 | 5.32 ± 0.11** | |
| Microaerophilic bacteria | |||||
| Lactobacilli | 68.30 | 3.41 ± 0.21 | 53.30 | 5.77 ± 0.18** | |
| Obligate anaerobic bacteria | |||||
| Fusobacteria* | 68.30 | - | 100.00 | - | |
| Veillonella* | 66.70 | - | 100.00 | - | |
| Prevotella | 43.30 | - | 0.00 | - | |
| Porphyromonas | 83.30 | - | 0.00 | - | |
| Spiral shaped bacteria | |||||
| Spirochaeta* | 75.00 | - | 100.00 | - | |
| Saccharomycetaceae fungus | |||||
| Candida fungi | 51.70 | 3.20 ± 0.10 | 6.70 | 1.23 ± 0.21** | |
*: microorganisms that are identified by the microscopic method; **: P < 0.05; -: not detected
Microorganisms’ colonization density of periodontal pockets has reached clinically significant indicators. Half of the patients have in periodontal pockets potentially pathogenic Gram-negative bacteria belonging to the following genera: Veillonella (in 66.7%), Fusobacterium (68.3%), Spirochaeta (75.0%); pathogenic β-hemolytic streptococci (51.7%); non-pathogenic Neisseria (55.0%). Gram-negative Enterobacteriaceae were in some patients, in particular strains of Escherichia coli and Klebsiella (35.0% and 15.0%, respectively). The density of their colonization of periodontal pockets was 2.3 lg CFU/mL ± 0.1 lg CFU/mL and 1.2 lg CFU/mL ± 0.3 lg CFU/mL, respectively.
The studies showed that the composition of the microflora of periodontal pockets in patients with thyroid hypofunction was poly-microbial and represented by various associations of microorganisms. Most of the associations consisted of 5 or more species of bacteria (in 78.3% of patients). In 15% of cases, associations with 3–5 types of microorganisms, only four patients (6.7%) have two-component associations.
Microbiomes of periodontal pockets are prevalent in species of anaerobic and facultative anaerobic microorganisms. Most patients with hypothyroidism have the leading “biomarker bacteria” and periodontopathogenic microorganisms: Prevotella, Porphyromonas, and Spirochaeta.
An α-hemolytic streptococci was isolated in almost all patients (93.3%). The density of their colonization of the periodontal ecological niche in average of 6.8 lg CFU/mL ± 0.2 lg CFU/mL is less than standard data. Only 68.3% of patients have lactobacilli cultures; their density was equal to 3.4 lg CFU/mL ± 0.2 lg CFU/mL.
It indicates the influence of systemic disease on the development of dysbiotic processes in the oral cavity. Porphyromonas strains, which belong to Gram-negative non-spore-forming anaerobes, were found in most patients with hypothyroidism (83.3% of patients) and coagulase-positive and coagulase-negative staphylococci in 75.0% and 78.3% of cases, respectively.
Analysis of the results of microbiological studies of patients with gastroduodenal pathology associated with Helicobacter pylori showed that the microflora of the pockets was poly-associative. Potentially pathogenic and pathogenic microorganisms were found in all patients. This microflora represents associations of various species of the Streptococcus genus and Staphylococcus genus, as well as lactic acid bacteria, fungi, and anaerobic microorganisms. On average, associations containing 3 to 6 types of microorganisms were inoculated from the sample. Patients with gastroduodenal pathology have aerobic-anaerobic associations in 40% of cases, aerobic-anaerobic-fungal in 48%, and anaerobic-fungal associations in 12% of observations. Streptococcus spp. represented the main part of the aerobic microflora. The dominant species was Staphylococcus haemolyticus (77.3%). Analysis of the species composition of the anaerobic microflora of the periodontal pockets showed significant inoculation with Gram-negative microorganisms (Bacteroides forsitus, Fusobacterium nucleatum, Prevotella intermedia, Porphyromonas gingivalis) (Figure 1). Peptostreptococcus anaerobius was detected in 35% cases in the whole spectrum of Gram-positive anaerobic microorganisms. The analysis of the quantitative composition of the microflora of the periodontal pockets showed that contamination by the previously mentioned types of bacteria in the main group averaged from 1 × 105 to 1 × 108 CFU/mL. Microscopic studies revealed significant microbial contamination of periodontal pockets of patients with gastric and duodenal ulcers. There was a shift in the microbial spectrum toward an increase in Gram-negative rods, associated with a decrease in Gram-positive rods and Gram-negative cocci. During bacteriological studies, staphylococci and streptococci were most often identified in diagnostically significant concentrations among facultative anaerobic microorganisms and among obligate anaerobes—Fusobacterium spp., Peptostreptococcus spp., Prevotella spp., Porphyromonas gingivalis. In high concentrations from 3 × 105 CFU/mL to 2 × 108 CFU/mL, Fusobacterium was isolated in 77.2% of patients. The dominance of these microorganisms in the microbial associations of periodontal pockets is characteristic. A high frequency of detection (67%) of Candida fungi with a colonization rate of 1 × 102–1 × 104 CFU/mL was determined.
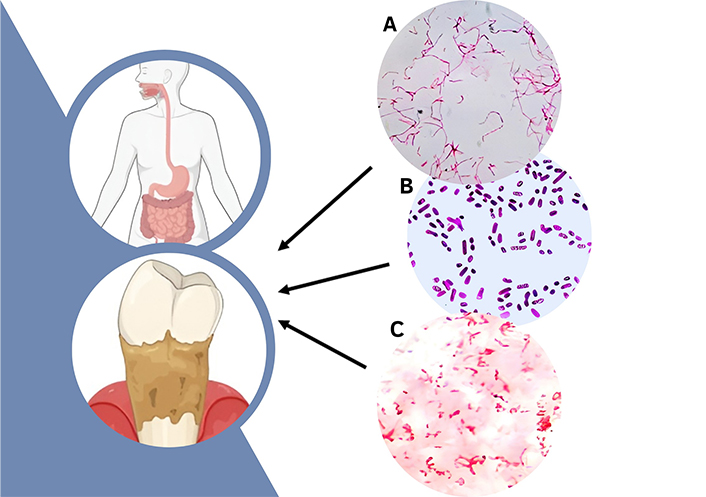
Microorganisms that prevail in patients with periodontal tissue pathology and gastroduodenal pathology. (А) Fusobacterium nucleatum (light microscopy, 1000× magnification, Gram stain); (В) Prevotella intermedia (light microscopy, 1000× magnification, Gram stain); (С) Porphyromonas gingivalis (light microscopy, 1000× magnification, Gram stain). The authors created the figure on the platform Canva (https://www.canva.com/) and with BioRender.com (https://app.biorender.com). Subpictures are original
The microflora of periodontal pockets of patients with colitis was dominated by cocci, which accounted for 49.8% of all cultured microorganisms. Gram-positive facultative anaerobic cocci belonging to the genera Staphylococcus and Streptococcus were dominant (Figure 2). In addition, the spectrum of microbiocenosis included anaerobic gram-negative rods, which made up 35.0% of the microbial community, Candida fungi (11.0%), and Spirochaeta (7.0%). The number of lactobacilli decreased (6.0%), which indicates dysbiosis of the periodontal pockets. Enterobacterium, such as Escherichia coli and Klebsiella spp., occupied the smallest segment in the microbiocenosis of the periodontal pockets (2.7%), which belonged to invasive microflora.
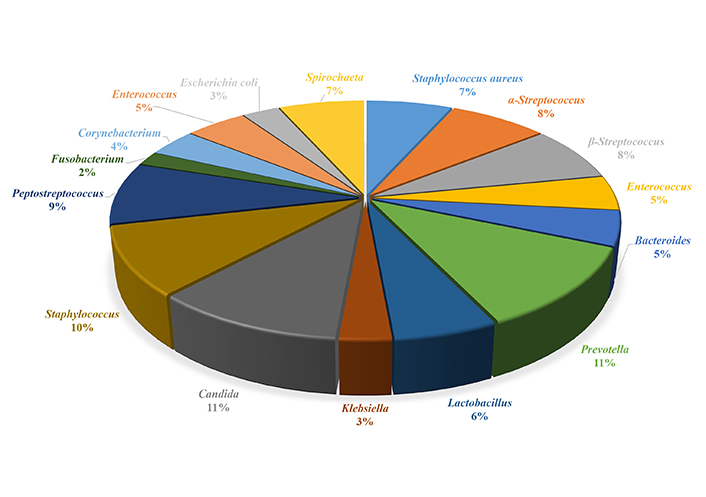
Qualitative composition of the microbiocenosis of the periodontal pocket of patients with chronic colitis
The degree of total microbial colonization of the gingival sulcus of patients with catarrhal gingivitis was 5.62 lg CFU/mL ± 2.32 lg CFU/mL. The periodontal pocket microorganism colonization density was 6.17 lg CFU/mL ± 2.56 lg CFU/mL, which is a few times higher than in patients with chronic gingivitis (Figure 3).
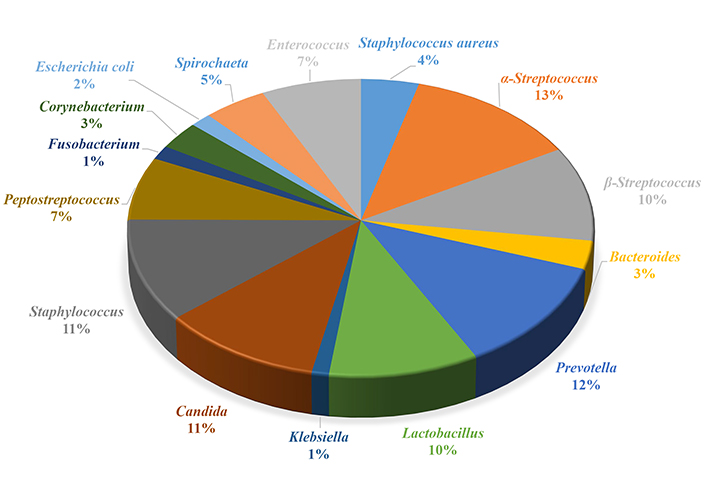
Qualitative composition of the microbiocenosis of the gingival sulcus of patients with chronic colitis
The microbiological studies of samples from patients with chronic pancreatitis showed that various microorganisms inhabit periodontal pockets and gingival sulcus (Table 3). The degree of its total colonization ranged between 7.23 lg CFU/mL ± 0.09 lg CFU/mL in the case of chronic catarrhal gingivitis and 7.37 lg CFU/mL ± 0.15 lg CFU/mL in the case of CGP. Microorganisms in periodontal pathology were inoculated from gingival and periodontal pockets with approximately the same inoculation density. Most microorganisms were isolated within the same order, except for Escherichia coli. Their number was a few times higher in patients with CGP than in those with chronic catarrhal gingivitis (2.19 lg CFU/mL ± 0.13 lg CFU/mL vs. 1.83 lg CFU/mL ± 0.10 lg CFU/mL, respectively). α-hemolytic streptococci, Spirochaeta, Fusobacterium, and Veillonella, which formed the main normal microflora of gingival and periodontal pockets, dominated this biotope. Staphylococci (78.6%) and lactobacilli (73.8%) were patients’ second most frequently detected bacteria. In patients with CGP, the frequency of detection of coagulase-positive staphylococci was approximately the same (77.8%), while the frequency of coagulase-negative staphylococci was slightly lower (66.7%). Lactobacilli, which also belong to the normal microflora of the oral cavity and gum pockets, were cultured in periodontitis with a frequency almost 20% lower than in gingivitis. Saprophytic Neisseria and Corynebacterium were also detected less often in the second case. The frequency of detection of β-hemolytic streptococci, which, like staphylococci, are considered pyogenic cocci and play a significant role in the development of the inflammatory process, has increased significantly (from 26.2% in chronic catarrhal gingivitis to 72.2 % in CGP). At the same time, Escherichia coli, Klebsiella, and Candida fungi were cultured more frequently from the contents of gingival sulcus and periodontal pockets. Microbiological studies of the microbiocenosis of periodontal pockets of patients with CGP and gastrointestinal tract pathology revealed that the frequency of anaerobic microorganisms’ detection was higher than in patients with generalized periodontitis without concomitant systemic pathology, i.e., Helicobacter pylori (7.0 times), Bacteroides forsythias (4.2 times), Fusobacterium nucleatum (5.2 times), Prevotella intermedia, and Porphyromonas gingivalis (5.0 times).
Colonization density and frequency of detection of microorganisms in the gingival sulcus and periodontal pockets
| Microorganism | Main group n = 102 | Control group n = 30 | ||
|---|---|---|---|---|
Frequency of detection (%) | Colonization density (lg CFU/mL) (M ± m) | Frequency of detection (%) | Colonization density (lg CFU/mL) (M ± m) | |
| Coagulase-positive staphylococci | 77.8 | 4.44 ± 0.11 | 33.3 | 3.94 ± 0.16** |
| Coagulase-negative staphylococci | 66.7 | 4.50 ± 0.14 | 46.7 | 4.02 ± 0.18** |
| α-hemolytic streptococci | 100.0 | 6.82 ± 0.11 | 100.0 | 6.75 ± 0.11** |
| β-hemolytic streptococci | 40.0 | 5.39 ± 0.19 | 26.7 | 3.39 ± 0.17** |
| Neisseria | 55.0 | 6.54 ± 0.16 | 43.3 | 5.32 ± 0.11** |
| Lactobacilli | 73.8 | 3.95 ± 0.12 | 53.3 | 5.77 ± 0.18** |
| Klebsiella | 15.0 | 1.80 ± 0.21 | 0.0 | - |
| Escherichia coli | 41.7 | 2.08 ± 0.11 | 10.0 | 0.13 ± 0.08** |
| Candida fungi | 60.0 | 3.21 ± 0.11 | 6.7 | 1.23 ± 0.21** |
| Corynebacterium | 33.3 | 3.49 ± 0.09 | 13.3 | 1.44 ± 0.17** |
| Spirochaeta* | 100.0 | - | 100.0 | - |
| Fusobacterium* | 100.0 | - | 100.0 | - |
| Veillonella* | 100.0 | - | 100.0 | - |
*: microorganisms that are identified by the microscopic method; **: P < 0.05; -: not detected
Systemic disorders can significantly influence a person’s quality of life and may require long-term management and care. Research has shown a bidirectional relationship between CGP and certain systemic disorders. CGP is characterized by chronic inflammation in the gums. It is a kind of infectious disease initiated by subgingival periodontal pathogens, which destroy ligament and alveolar bone supporting the teeth. Ultimately, it results in the loss of the affected teeth, compromising patients’ well-being. The systemic inflammatory response triggered by periodontal infection can contribute to developing or exacerbating other systemic inflammatory conditions, such as cardiovascular disease, diabetes, and rheumatoid arthritis. Oral bacteria from periodontal infections can enter the bloodstream and potentially spread to other body parts. It can lead to infections in distant organs and tissues.
However, maintaining good oral hygiene, seeking regular dental care, and managing risk factors (i.e., smoking, alcohol intake, drug intake, etc. [19]) can help reduce the risk of gum disease and associated systemic health problems. Additionally, individuals with systemic disorders should be aware of the potential impact of their condition on oral health and consult with healthcare professionals as needed.
It is vital to comprehend the histology of clinically healthy tissues and inflamed gingival/periodontal tissues to understand periodontal pathogenesis better. A gingival crevice is the place where the pathological inflammatory process in the periodontium occurs and then progresses. This low-grade inflammation occurs in response to the continued presence of bacteria and their by-products in the gingival sulcus. A continuous exudation of fluid from the gingival tissues enters the crevice and seeps out as gingival crevicular fluid [20]. Toxins, by-products of microorganisms and enzymes, are inactivated by cellular and plasma components of the blood, which, in response to the action of pathogenic stimuli, penetrate from the capillaries through the tissue base into the sulcus. Therefore, even in normal conditions, there is always a particular microbial attack and the reaction of protective complexes to it in the gingival crevice, which belongs to the inflammatory category [21]. An explicit confirmation of this is the inevitable presence in the gingival sulcus of at least a minimal amount of gingival fluid, which is blood exudate from the capillaries network, as well as single leukocytes, exfoliated epithelial cells, a set of enzymes and proteins [22].
In all patients, there is a violation of the qualitative and quantitative composition of the microflora of the ecological niche of the periodontium. Dysbiosis was detected in all patients, as evidenced by a decrease in the frequency of culture of obligate oral microorganisms (i.e., streptococci, lactobacilli), an increase in the number of staphylococci, and the detection of invasive species of bacteria (i.e., Klebsiella and Escherichia coli).
Pathological disorders occur when the intensity of the microbial attack increases sharply. It happens in several cases: firstly, when the number of clusters of microorganisms increases; secondly, when, in addition to the usual saprophytic bacteria, pathogenic microorganisms appear in their composition [23]. As a rule, these are Spirochaeta, mobile forms of cocci, but they are mainly anaerobes. The peculiarity of these pathogenic bacteria is as follows: if saprophytic bacteria release exotoxins, to which tissue structures are sufficiently tolerant, then anaerobes release endotoxins, actively damaging cells, connective tissue formations, and the main substance. These bacteria appear with massive accumulations of microbial plaque, as they actively reproduce only in deep, oxygen-deprived layers. The reduction of specific and non-specific local and general protection mechanisms is usually accompanied by a sharp activation of constantly present microbial clusters. The consequence of this is the development of a clinically pronounced inflammatory reaction, the purpose of which is to neutralize tissue-destroying enzymes and toxins released by microbes, as well as to destroy the microbial cells.
Anaerobic microorganisms play the leading periodontopathogenic role among microorganisms [24, 25]: Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis, Prevotella intermedia, etc. It was established that the qualitative and quantitative composition of the microflora of the periodontal and gingival pockets is influenced by the presence of concomitant chronic pathology of the body’s digestive system. It is impossible to unequivocally talk about which microorganisms are the main ones in the pathogenesis of periodontal diseases due to the variability of the microbial composition of dental plaque. When studying the relative ratio of microbes in the subgingival areas of healthy and affected periodontium, it was found that cocci, straight rods, filaments, and fusiform bacteria predominate in healthy areas. In sites with affected periodontium, the number of motile rods and spirochetes increases proportionally to the growth of the plaque index, periodontal index, and pocket depth. Recently, great importance has been given to the synergism of microbes in the pathogenesis of periodontal diseases [26, 27]. Bacterial antagonism also plays a significant role in microbial colonization of the oral cavity. For example, hemolytic Streptococcus produces a high-molecular peptide and Streptococcus mitis—a substance of a non-protein nature, which in vitro inhibits black-pigmented Bacteroides. On the other hand, haematin produced by Bacteroides nigra inhibits the growth of Streptococcus mutans and a protein-like compound from Bacteroides nigra (melanin) inhibits, Bacteroides oralis, S. mutans, Streptococcus salivarius, and some species of Actinomyces. The pathogenic potential of bacteria is determined by the direct harmful effect of their by-products and by triggering immune response mechanisms and inflammatory reactions [28, 29]. Many microorganisms found in periodontitis produce powerful necrotizing exotoxins and enzymes, such as collagenase, elastase, fibrinolysin, hyaluronidase, etc., which cause the destructive effect of periodontal tissues. Bacteroides release volatile sulfur compounds that increase the permeability of the mucous membrane of the oral cavity [30, 31]. Many Bacteroides can produce enzymes that inactivate antibiotics—β lactamase, cephalosporinase, and penicillinase. Thus, the occurrence, severity, and intensity of the development of inflammatory and destructive periodontal diseases directly depend on the qualitative and quantitative composition of the microflora of the oral cavity.
The microflora of periodontal pockets is diverse and depends on the nature of the disease [32]. There is no doubt that populations of microorganisms that colonize the oral cavity are involved in pathological processes. In this regard, studying the microflora of periodontal pockets is essential for assessing the quality of treatment strategies [33, 34].
This study’s limitations imply that microorganisms are extracted from their environment and then studied microscopically or in culture. There is clear evidence that not all organisms in the periodontal pockets are subsequently cultivated, as revealed by microscopy of direct smears and correlative light and electron microscopy. The diversity of microbial communities is usually based on complex environmental, nutritional, and communicative interactions between species. By necessity, taking a sample underestimates the number and variety of species present in the original ecological niche. The sampling method, transport conditions, storage, culture (dilution, handling, media, incubation, and atmosphere), and laboratory sub-culture all have the potential to bias the resulting types and numbers of species.
Based on research results within the framework of evidence-based medicine, doctors can optimize treatment strategies and provide individual approaches in selecting pharmacotherapy for patients with combined pathologies. Moreover, treatment regimens will be developed for patients with periodontal diseases in the case of comorbidity.
Cytokine study can facilitate a more thorough correlation analysis and will be a crucial contribution to current clinical research in the future. It is also suggested to use “omics” technology for future research, which allows massively parallel analysis of molecules, including genes, transcripts, and proteins. This technology can quickly generate a massive amount of data in a single experiment, so it features a unique high output.
According to the research results, we can assume that a significant prevalence of periodontal diseases and lesions of the hard tissues of teeth in patients with associated systemic pathology indicates the direct influence of these concomitant diseases on the state of the patient’s dental health.
The degree of colonization by microorganisms in these pathologies differed slightly. At the same time, the frequency of detection of specific populations of opportunistic bacteria (i.e., β-hemolytic streptococci, Escherichia coli, Klebsiella, Candida fungi) increased in CGP compared to chronic catarrhal gingivitis.
The microbiological study of periodontal and gingival pockets of patients with periodontal pathology accompanied by systemic diseases indicates the need for a differentiated approach to antibacterial therapy.
It is important to emphasize that systemic diseases, disorders, or conditions do not cause periodontitis but instead predispose, accelerate, or otherwise increase disease progression.
CFU: colony-forming unit
CGP: chronic generalized periodontitis
NGS: next generation sequencing
rRNA: ribosomal RNA
The authors would like to thank professor of Lviv Polytechnic National University, Orest Kochan, for his intellectual assistance, advice and support at all stages of preparation of this paper. The work is a fragment of inter-department scientific research work “Comorbid conditions in the clinic of internal medicine and the practice of a family doctor: predictors of development, early diagnosis, prevention, and treatment” (state registration number 0133U4001244).
OD: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. SC: Conceptualization, Investigation, Writing—review & editing. SB and KL: Investigation, Data curation, Formal Analysis, Writing—review & editing. NM: Conceptualization, Investigation, Writing—original draft. NC and ML: Validation, Writing—review & editing, Supervision. NT: Investigation, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The Bioethics Committee of I. Horbachevsky Ternopil National Medical University Ministry of Health of Ukraine approved the study protocols (excerpt from protocol No. 73) on April 3, 2023. All the patient investigations conformed to the principles outlined in the Council of Europe Convention on Human Rights and Biomedicine (April 4, 1997), the World Health Association Helsinki Declaration on Ethical Principles for Scientific Research with Human Participation (1964–2000), and the Order of the Ministry of Health of Ukraine No. 281 of November 1, 2000; Declaration of Helsinki “World Medical Association Declaration of Helsinki Ethical Principles for Medical Research Involving Human Subjects” (2001), Code of Scientist of Ukraine (2009). All patients’ examinations were done with informed consent.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets for this study can be found in the corresponding author upon reasonable request.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Weinan Du ... Juxiu Li
Alejandra Vargas ... David A. Johnson
Shin Takasawa ... Maiko Takeda
Zeneng Wang ... Robert Koeth
