Affiliation:
1Faculty of Medicine and Health Science, University of Science and Technology, Sana’a 15201, Yemen
Affiliation:
2Faculty of Medicine, Benha University, Benha 13511, Egypt
Email: manr170393@fmed.bu.edu.eg; manarahmedkamal37@gmail.com
ORCID: https://orcid.org/0000-0003-4339-1788
Affiliation:
3Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
ORCID: https://orcid.org/0009-0000-1888-2839
Affiliation:
3Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
ORCID: https://orcid.org/0000-0001-7531-1178
Affiliation:
1Faculty of Medicine and Health Science, University of Science and Technology, Sana’a 15201, Yemen
Affiliation:
4Department of Internal Medicine, University of Science and Technology Hospital, Sana’a 15201, Yemen
Affiliation:
5Department of Medicine, Brockton Neighborhood Medical Center, Massachusetts, Brockton, MA 02301, USA
Affiliation:
3Department of Medicine, Houston Methodist Hospital, Houston, TX 77030, USA
Email: AEsmail@houstonmethodist.org
ORCID: https://orcid.org/0000-0002-2337-8351
Explor Med. 2025;6:1001324 DOI: https://doi.org/10.37349/emed.2025.1001324
Received: February 08, 2025 Accepted: May 12, 2025 Published: May 25, 2025
Academic Editor: Alessandro Granito, University of Bologna, Italy
Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare and potentially lethal complication of blood transfusion. It occurs when transfused immune cells, such as lymphocytes, elicit an immune response against the recipient’s tissues, including the skin, bone marrow, and gastrointestinal tract. TA-GVHD leads to severe pancytopenia, resulting in increased vulnerability to severe, often untreatable infections. Although the pathogenesis of TA-GVHD is fairly well understood, management remains challenging given the paucity of successful outcomes. We present a case of an immunocompetent 40-year-old Yemeni male who developed TA-GVHD after receiving a single unit of irradiated blood from his brother. The transfusion was done prior to an upper endoscopy procedure that confirmed the diagnosis of a peptic ulcer disease after which the patient was treated appropriately. He presented later with severe dyspnea and productive cough, necessitating oxygen support via face mask. The patient rapidly deteriorated with unstable vitals and was subsequently intubated and mechanically ventilated. Unfortunately, the patient died later due to cardiopulmonary compromise despite appropriate resuscitative measures. In summary, TA-GVHD is a serious condition that attacks the recipient’s tissues and results in severe pancytopenia.
Graft-versus-host disease (GVHD) is a complication of hematopoietic stem cell transplantation (HSCT) which occurs when donor T-lymphocytes recognize the recipient’s tissues as foreign and create an immune response against them. GVHD manifests either acutely, which generally presents within 3 months of HSCT, or chronically, which typically presents more than 6 months following the transplant. Acute and chronic GVHD mainly differ in the underlying pathophysiology and the immune components that drive the immune response [1, 2]. GVHD has a wide range of clinical manifestations, predominantly involving skin, gastrointestinal tract, liver, and lungs [3]. Transfusion-associated graft-versus-host disease (TA-GVHD) is a rare and usually fatal complication of blood transfusion, in which lymphocytes from the transfused blood attack the recipient’s tissues, particularly the skin, bone marrow, and gastrointestinal tract [4, 5]. GVHD is associated with allogeneic HSCT [6], whereas in TA-GVHD, the lymphocytes from the transfused blood attack the recipient’s bone marrow, leading to bone marrow aplasia and profound pancytopenia, which is typically the cause of death [7, 8].
TA-GVHD carries a mortality rate of 90–100%. Although patients require immunosuppression and emergent transplantation, treatment is generally not helpful [9]. In most cases, death results from severe neutropenia, and septic shock, as seen in our patient, who rapidly developed multiorgan failure [9]. Therefore, preventive, such as the irradiation of blood components given to susceptible recipients, are essential to prevent TA-GVHD [9]. Currently, the prevention of TA-GVHD is routinely achieved by exposing blood products to γ-irradiation, which inhibits donor T-cell proliferation. In addition to the use of γ-irradiation, recent research on pathogen reduction protocols is emerging, which modify nucleic acids and interfere with their replication, significantly lowering the risk of TA-GVHD. Previous studies comparing γ-irradiation and pathogen reduction protocols in both in vitro and in vivo models have found that both protocols are equally effective in preventing T-lymphocyte proliferation and GVHD responses [10].
Despite the importance of timely identification and prevention of TA-GVHD, previous literature about TA-GVHD in Yemen is lacking. We report here a case of TA-GVHD in an immunocompetent 40-year-old Yemeni male with peptic ulcer disease, who received irradiated and pre-storage leukoreduced packed red blood cells (RBCs) from his brother, which was the only risk factor for TA-GVHD.
The case was presented according to the Consensus-based Clinical Case Reporting Guideline Development (CARE Guidelines). The study involved a human subject, and ethical approval was obtained from the Research Ethical Committee (REC) at the Faculty of Medicine and Health Science, University of Science and Technology, Sana’a, Yemen. Written informed consent was obtained from the patient for the publishing of this report.
A 40-year-old Yemeni man presented to the emergency department with a ten-day history of fever, chills, and diffuse rash. The patient also reported dyspnea, dry cough, and generalized arthralgia. Additionally, he had upper abdominal pain and anemia (hemoglobin: 8 g/dL), which had been documented 17 days prior. Although the patient had a hemoglobin level of 8 g/dL on admission, blood products were administered based on the treating physician’s judgment due to symptomatic anemia and pre-procedural optimization to prevent any possible complications. The patient received one unit of irradiated and pre-storage leukoreduced blood from his brother prior to undergoing upper gastrointestinal endoscopy, which confirmed peptic ulcer disease and then he was subsequently treated. His past medical history was unremarkable, with no evidence of chronic diseases, drug use, cancer, or chemotherapy.
The patient’s vital signs were documented during his emergency department visit, with a temperature of 38.5°C (101.3°F), pulse rate (PR) 126 beats per minute (BPM), respiratory rate (RR), 22 breaths per minute, and blood pressure (BP) 130/80 mmHg. The patient appeared ill but was alert, conscious, oriented to place, person, and time. No cyanosis, jaundice, or lower limb swelling was observed. Examination of the head, eyes, ears, nose, and throat (HEENT) revealed mucosal pallor along with bilateral eye redness and multiple oral ulcers. The skin examination showed a diffuse erythematous maculopapular rash over the entire body. Lung examination revealed decreased bilateral air entry with basal crepitations. The remainder of the systemic examination was unremarkable.
Initial laboratory investigations were ordered including a complete blood count (CBC) with differential, liver function tests, kidney function tests, c-reactive protein. Additional tests—such as dengue virus IgM, malaria test thick, and thin blood smear, hepatitis B virus (HBV) surface antigen, hepatitis C virus (HCV) antibody, human-immunodeficiency virus (HIV) 1 and 2, urinalysis, blood cultures, and peripheral blood smear were performed in the emergency room and no significant results were observed. The patient’s blood type was O-positive. Laboratory results revealed anemia, severe leukopenia, thrombocytopenia, elevated creatinine, and elevated liver enzymes including aminotransferase (ALT) and aspartate aminotransferase (AST). Tests for dengue virus, HBV, HCV, HIV 1 and 2, and malaria were all negative. The peripheral blood smear revealed severe pancytopenia. Biopsies were not obtained as the patient was admitted during a holiday season. Human leukocyte antigen (HLA) typing, which is essential for identifying shared antigens that may predispose patients to TA-GVHD following transfusions, was not performed due to limited resources. Consequently, the diagnosis of TA-GVHD was made clinically by a hematologist on the third day of hospitalization. Short tandem repeat-polymerase chain reaction (STR-PCR), which could have further supported the diagnosis, was also unavailable in the region. A chest x-ray revealed prominent lung vascularity and findings suggestive of pulmonary edema (Figure 1).
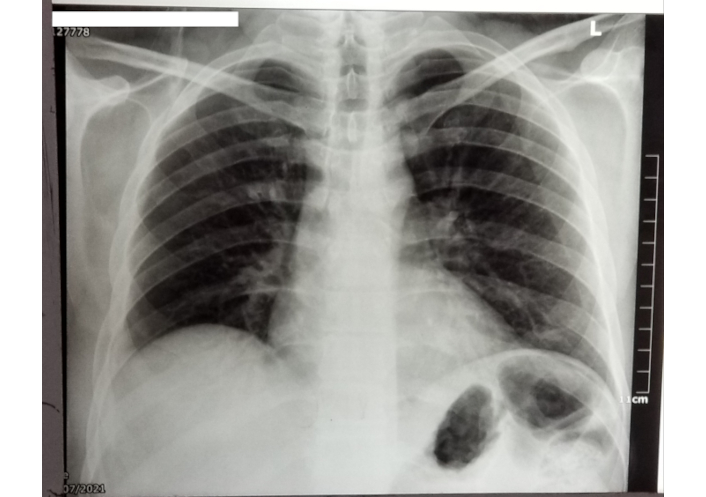
Chest X-ray of the patient revealed prominent lung vascularity suggestive of pulmonary edema
Initial treatment included intravenous (IV) infusion of 500 mL normal saline (0.9%) and broad-spectrum antibiotics: meropenem 1 g every eight hours (hrs) and levofloxacin 500 mg every 24 hrs. The patient also received hydrocortisone 100 mg, tramadol 100 mg, and a 1 g infusion of paracetamol infusion for fever (Table 1). Additionally, medication included gentamicin eye drops every 12 hrs, IV pantoprazole 40 mg every 24 hrs, IV tramadol 100 mg once again, and IV metoclopramide 10 mg (Table 1). The mean temperature and RR over the first five days were 37.5°C (99.5°F) and 21.86 breath per minute, respectively. The median HR, systolic, and diastolic BP were 122 BPM, 110 mmHg, and 63.91 mmHg, respectively (Table 2).
The medications administered to the patient throughout his admission
| Medication | Dose | Frequency |
|---|---|---|
| Meropenem | 1 g | Q8h (every 8 hours) |
| Levofloxacin | 500 mg | Q24h (every 24 hours) |
| Hydrocortisone | 100 mg | Q8h (every 8 hours) |
| Tramadol | 100 mg | PRN (given twice) |
| Paracetamol | 1 g | Q8h (every 8 hours) |
| Gentamicin eye drops | N/A | Q12h (every 12 hours) |
| IV pantoprazole | 40 mg | Q24h (every 24 hours) |
| IV metoclopramide | 10 mg | N/A |
| Amikacin infusion | 500 mg | Q12h (every 12 hours) |
| IV tranexamic acid | 500 mg | N/A |
| Salbutamol | N/A | N/A |
| Ipratropium | N/A | N/A |
| Filgrastim syringe | 300 mcg | N/A |
| IV furosemide | 40 mg | Q12h (every 12 hours) |
| Fluconazole infusion | 100 mg | Q12h (every 12 hours) |
| IV N-acetyl cysteine | 400 mg | Q8h (every 8 hours) |
IV: intravenous
Vital signs as documented during the patient’s hospitalization
| Variables | Total | Examination day | Units | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |||||||||
| Temperature mean (SD) | 37.5 | (0.90) | 38.5 | (0.5) | 37.167 | (1.8930) | 37.4 | (0.5657) | 37.875 | (0.6292) | 37.245 | (0.6563) | °C |
| Heart rate median (range) | 122 | (30–141) | 122 | (120–126) | 117 | (110–123) | 100.5 | (80–113) | 134.5 | (86–141) | 123 | (30–140) | BPM |
| Respiratory rate mean (SD) | 21.86 | (5.74) | 21 | (1) | 21.67 | (1.528) | 22.5 | (0.707) | 29.75 | (7.136) | 19.18 | (4.854) | Breaths per minute |
| Systolic blood pressure median (range) | 110 | (30–140) | 130 | (120–130) | 130 | (110–140) | 105 | (100–110) | 121 | (110–140) | 72 | (30–120) | mmHg |
| Diastolic blood pressure mean (SD) | 63.91 | (17.89) | 76.67 | (5.774) | 73.33 | (15.275) | 75 | (7.071) | 77.5 | (12.583) | 50.91 | (15.45) | mmHg |
SD: standard deviation; BPM: beats per minute
On the second day of hospitalization, vital sign revealed a persistent fever, tachycardia at 123 BPM, and BP of 110/70 despite receiving antipyretics and antibiotics. Laboratory investigations showed further reduction in the white blood cells (WBCs) and platelet count. Therefore, the patient remained on the same treatment regimen, which included a saline infusion 150 cc/hour, amikacin 500 mg IV every 12 hrs, tranexamic acid 500 mg IV, salbutamol, and ipratropium. The patient also received one unit of packed RBCs and filgrastim 300 mcg syringe (Table 1).
On the third day of hospitalization, the patient developed severe dyspnea and a productive cough. Vital signs revealed a temperature of 38°C (100.4°F), HR 113 BPM, and RR of 23 breaths per minute, and BP 100/80 mmHg. Laboratory results showed a further reduction of WBCs count to 0.2 × 103/uL, a markedly elevated procalcitonin level of 28.6 ng/mL, and a negative dengue virus IgM. Serum creatinine was 1.66 mg/dL. The patient was placed on oxygen via face mask, continued on the same treatment regimen, and was referred to the intensive care unit (ICU).
On the fourth day of hospitalization, the patient’s condition continued to deteriorate. He remained febrile with a temperature of 38.5°C (101.3°F) despite antipyretic and antibiotics, an HR 136 BPM, and an RR 38 breaths per minute. His BP was 112/80 mmHg, laboratory results showed WBCs of 0.2 × 103/uL. The patient’s condition was managed with IV fluids, which were reduced to 100 mL/hour, along with IV furosemide 40 mg and fluconazole infusion 100 mg every 12 hrs. IV N-acetyl cysteine 400 mg was administered every 8 hrs.
On the fifth day of hospitalization, the patient experienced a significant clinical decline, presenting with an altered level of consciousness, and hypotension (BP 73/44 mmHg), bradypnea (12 breaths per minute), tachycardia (HR 140 BPM) and a temperature of 37°C (98.6°F). His oxygen saturation dropped to 40%, necessitating the initiation of inotropes and subsequent intubation with mechanically ventilation. Unfortunately, the patient passed away later that day due to cardiorespiratory failure.
We present the first reported case of TA-GVHD in Yemen, highlighting its rapid and fatal clinical course. The patient, who received irradiated blood from his brother, exhibited clinical presentations consistent with TA-GVHD, including fever, diffuse maculopapular rash, dyspnea, cough, painful bilateral red eyes, tachycardia, bilateral basal lung rales, elevated liver transaminases, pancytopenia with severe neutropenia, and high levels of inflammatory markers. Despite aggressive management, the patient died due to cardiopulmonary compromise. This case underlines the importance of maintaining a high index of suspicion for TA-GVHD even when blood products are irradiated, as partial HLA matching may still pose a significant risk.
This finding aligns with a case reported by Malladi et al. [11], for an immunocompetent woman who developed fatal TA-GVHD after receiving unirradiated blood from her son following surgery. She developed symptoms within 10 days post-transfusion, including fever, rash, oral ulcers, jaundice, and pancytopenia. Despite treatment, she died within days. Together, the cases highlighted the risk of TA-GVHD in transfusion involving first-degree relatives, emphasizing the need for cautious screening and more stringent transfusion protocols [11].
TA-GVHD is a rare complication of blood transfusion that occurs when viable donor lymphocytes elicit an immune response against the recipient’s lymphoid tissue. In typical situations, donor lymphocytes are recognized as foreign and are destroyed by the recipient’s immune system. However, if the recipient is immunocompromised or shares the same HLA type with the donor [9], such as in transfusion from first-degree relatives, the recipient’s immune system cannot recognize donor lymphocytes as foreign. As a result, these viable donor lymphocytes able to engraft and stimulate destructive inflammatory injury to bone marrow, skin, liver, and bowel, which are the main targeted organs [12]. Thus, HLA typing is therefore essential to identify individuals with shared antigens which increase the risk for TA-GVHD.
The diagnosis of TA-GVHD is challenging due to its non-specific symptoms. Histopathological examination of biopsy specimens from the skin, rectum, liver, or bone marrow biopsy remains the gold standard for establishing a diagnosis [13–20]. Another test for diagnosing TA-GVHD is a demonstration of WBC chimerism using STR-PCR [13–15, 17–20]. Fluorescent in situ hybridization (FISH) is an alternative cytogenetic test that can be used to confirm TA-GVHD. Additionally, cytometric analysis of the peripheral blood can detect the presence of donor T cells in the recipient’s circulation, which may lead to TA-GVHD [7, 13–15, 18]. Notably, the FISH test is rapid and more sensitive when the donor and recipient are of different sexes [13, 15, 19, 20]. In this case, the lack of HLA typing and STR-PCR due to limited resources in Yemen posed significant diagnostic challenges, reflecting broader issues in resource-poor settings. Future strategies, such as centralized testing facilities, could improve diagnostic accuracy in such contexts.
TA-GVHD shares immune-mediated mechanisms with other severe transfusion-related complications, such as transfusion-related acute lung injury (TRALI) and transfusion-associated immunomodulation (TRIM). TRALI results from donor antibodies activating recipient neutrophils, causing pulmonary edema, which was observed in our patient’s chest X-ray but attributed to TA-GVHD. Bux highlights TRALI as a leading cause of transfusion-related mortality, driven by immune-mediated neutrophil activation [21].
TRIM, as described by Kumar et al. [22], involves transfusion-induced immune suppression, potentially activating autoreactive B and T cells via autoantigen presentation to antigen-presenting cells, which may exacerbate immune dysfunction and infection risk. Similarly, in HSCT, GVHD involves donor T-cell-mediated tissue damage, with chronic forms potentially driven by autoreactive lymphocytes, as noted by Hill et al. [2]. These mechanisms underscore the critical role of immune dysregulation in transfusion complications, particularly when HLA similarity, as likely in our case due to familial donation, allows donor lymphocytes to evade recipient immune clearance.
Preventive measures are critical, given the high mortality rate of TA-GVHD (90–100%). Foukaneli et al. [17] emphasize the importance of irradiating blood components for susceptible recipients, though this case suggests that irradiation alone may not suffice when HLA similarity exists. Avoiding transfusions from first-degree relatives and implementing stricter donor selection protocols could further reduce risk.
The cornerstone of TA-GVHD treatment is immunosuppressive therapy, which aims to suppress the donor’s immune response against the recipient’s tissues. New guidelines hold the hope of using monoclonal antibodies as part of immunosuppression in such cases. Emergency allogeneic HSCT remains the most effective treatment option for severe TA-GVHD [16, 18–20].
In a systematic review [12] of TA-GVHD case reports published from inception through September 2013, which was published in 2015, most of the 348 patients involved presented within a median of 11 days after cessation of transfusion. The most clinic laboratory findings were rash in 80.2%, fever in 67.5%, elevated liver enzymes in 66.4%, pancytopenia in 65.2%, diarrhea in 43%, bone marrow aplasia in 22.7% or bone marrow hypocellularity in 17.2% and hepatomegaly in 13.5%. Only one case exhibited all seven manifestations of TA-GVHD, while four had an average number of these features. The diagnosis was made using the National Healthcare Safety Network (NHSN) criteria for definitive diagnosis, and 49 patients (14.1%) had a probable diagnosis of TA-GVHD [7, 16–20].
Among the 348 cases involved in the systematic review, the criteria for blood component irradiation were met in 121 subjects with either congenital or acquired cellular immunosuppression. Of those, 50 patients received blood from related donors. On the other hand, the remaining 227 patients had no underlying indications that met the criteria for irradiation of blood products. This highlights that immune status is not sufficient to indicate increased risk of TA-GVHD. The primary underlying pathophysiology is HLA-sharing antigens between the donor and recipient, which was confirmed by HLA typing in 84 of 348 cases showing that HLA-sharing antigens were present in 60 patients. Most of these are related to donor transfusion and immunocompetence and are not indicated for blood component irradiation [12].
A previously published systematic review [12] reported 312 fatalities and only 29 cases survived. Treatment administered consisted mainly of immunosuppressants, immunomodulatory drugs, and stem cell transplantation. Among 175 patients who received immunosuppressive or immunomodulatory drugs, 157 were treated with corticosteroids, and only 15 of those cases survived. Cyclosporine was given to 34 patients, of whom only 3 cases survived. Four out of 19 patients who received IV immune globulin (IVIg) survived. No patients survived among the 33 patients who received antithyroglobulin and 3 out of 8 patients who underwent bone marrow transplantation survived.
A study by Elliot et al. [23] analyzed 10 years’ data from the UK which revealed 956 incidents where patients did not receive irradiated blood products. These patients mainly were treated with purine analogues, alemtuzumab, or had a history of Hodgkin lymphoma. However, no cases of TA-GVHD were reported. This suggests that leukodepletion may reduce the risk of TA-GVHD but it also questioned the recommendation for indefinite irradiation for all stages of Hodgkin lymphoma due to lack of strong evidence.
In summary, TA-GVHD occurred in our patient following the transfusion of a single fresh unit of blood from the patient’s brother, prior undergoing an upper gastro-endoscopy that confirmed peptic ulcer disease. He subsequently began appropriate medical treatment. The patient later developed severe dyspnea and a productive cough, requiring oxygen therapy via a face mask. Unfortunately, the patient’s condition worsened, with an altered level of consciousness and unstable vital signs, BP, RR, HR, temperature, and oxygen saturation. He received inotropic support, was intubated, and placed on mechanical ventilation, but ultimately succumbed to cardiorespiratory compromise.
ALT: aminotransferase
AST: aspartate aminotransferase
BP: blood pressure
BPM: beats per minute
GVHD: graft-versus-host disease
HBV: hepatitis B virus
HCV: hepatitis C virus
HEENT: head, eyes, ears, nose, and throat
HIV: human immunodeficiency virus
HLA: human leukocyte antigen
IV: intravenous
IVIg: intravenous immune globulin
RBCs: red blood cells
TA-GVHD: transfusion-associated graft-versus-host disease
TRIM: transfusion-associated immunomodulation
WBCs: white blood cells
AA, HAD, and DM: Conceptualization, Data curation, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. MAK, EAN, BK, and DB: Data curation, Resources, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. AE: Conceptualization, Data curation, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflict of interest.
This observational study on a human subject was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and received ethical approval from the Research Ethical Committee (REC) of the Faculty of Medicine and Health Sciences, University of Science and Technology, Sana’a, Yemen (Approval No. UST-IRB-2024-014). The authors are accountable for all aspects of the work, ensuring that any questions regarding the accuracy or integrity of the study are appropriately investigated and resolved.
Informed consent to participate in the study was obtained from the patient.
Written informed consent was obtained from the patient to publish this case report and any accompanying images. A copy of the written consent is available for review by the journal’s Editor-in-Chief.
The datasets used and/or analyzed during the current study are available from the corresponding author on a reasonable request.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4936
Download: 38
Times Cited: 0
