Affiliation:
Iranian Centre for Endodontic Research, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran 1983963113, Iran
Email: saasgary@yahoo.com
ORCID: https://orcid.org/0000-0001-6691-0478
Affiliation:
Iranian Centre for Endodontic Research, Research Institute of Dental Sciences, Shahid Beheshti University of Medical Sciences, Tehran 1983963113, Iran
Explor Med. 2023;4:733–738 DOI: https://doi.org/10.37349/emed.2023.00173
Received: June 07, 2023 Accepted: June 30, 2023 Published: October 27, 2023
Academic Editor: Gaetano Isola, University of Catania, Italy
The article belongs to the special issue Biomaterials and Biomarkers in Dentistry: Up to Date
This case report demonstrates the successful induction of apexogenesis in an extensively carious lower right first molar with immature roots through the use of stepwise excavation and calcium-enriched mixture (CEM) cement as an indirect pulp capping material. The patient, an 8-year-old boy, presented with pain and carious pulp exposure. The initial treatment involved removing soft dentin and applying CEM cement as an indirect pulp cap. The patient experienced pain relief after 24 h, and subsequent follow-up appointments showed complete healing and maturation of the tooth. The case highlights the potential of indirect pulp treatment with CEM cement and emphasizes the importance of regenerative biomaterials in promoting healing and dentin bridge formation. Further clinical work and research are recommended to explore the efficacy of this treatment approach.
The dental practitioner is often familiar with the short roots and wide canals of immature teeth; it is also recognized that immature teeth have an excellent blood supply and therefore their ability to heal even when caries have reached the pulp can be extraordinary [1, 2]. Apexification of an immature root will evidently be unfavorable. Hence, the primary aim of treatment of a heavily carious vital immature molar is to remove the infection, induce apexogenesis, or continue root and tooth development of the immature tooth [3]. Teeth with deep caries are likely to have pulp exposures reported to be as high as about 75% [4]. A two-stage stepwise technique is popular for asymptomatic immature teeth, and it has shown success. Advantages include reduced risk of exposure and preservation of pulp vitality. However, the need for re-entry requires a further appointment and patient compliance.
The copious blood supply to the pulp can arguably allow even a heavily inflamed pulp to heal, highlighting the potential discrepancy between clinical symptoms and the histopathological status of the pulp [2]. Traditionally the likelihood of a heavily exposed pulp as well as painful immature molar sometimes shifted the treatment choice to pulpotomy procedures. A recent clinical trial showed that mature adult teeth responded better to stepwise excavation than to pulpal exposures and subsequent pulp capping even with traditional materials like calcium hydroxide (CH) [2]. This raises the question of whether indirect procedures like stepwise excavation can replace pulpotomy procedures shifting the treatment protocols to an extra-conservative approach.
Currently, more bio-regenerative materials have been used for pulp capping and pulpotomy procedures in immature teeth in the place of CH to avoid its common pitfalls such as poor adherence, micro-abscess, and lack of long-term seal [5]. Biomaterials such as mineral trioxide aggregate (MTA) [6] and calcium-enriched mixture (CEM) cement [2] have shown more favorable treatment outcomes [7] due to their hermetic seal [8], biocompatibility [9, 10] and dentinal bridge formation in humans [5, 11] and animal teeth [12]. Their ability to allow pulp healing has also been shown in indirect/direct pulp capping procedures [13, 14].
This case study shows an example of a stepwise technique of a deep carious lesion in an immature painful molar.
An 8-year-old boy presented with pain to a specialist endodontic clinic in Tehran. He complained of sharp pain on eating and drinking, and sometimes spontaneously lasting less than 1–2 min. Medical history taken from his mother was non-remarkable apart from taking retain for attention-deficit/hyperactivity disorder (ADHD).
On general examination, extensive caries was present in the lower right first molar. The patient was in the mixed dentition stage. Radiographs showed incomplete immature roots and caries reaching the pulp (Figure 1A). Emergency treatment was initiated after the explanation. Soft dentine was removed; however, somewhat harder affected dentine was left over the pulp horns with no evidence of clinical exposure. CEM cement dressing was placed and gently adapted over the affected/semi-soft carious pulpal floor, and the rest of the tooth was restored with glass ionomer (Figure 1B). The patient and his mother were informed of interim restoration and a recall appointment was made for 1 week and three months. At the one-week follow-up appointment, the young boy reported no pain and complete satisfaction with treatment.
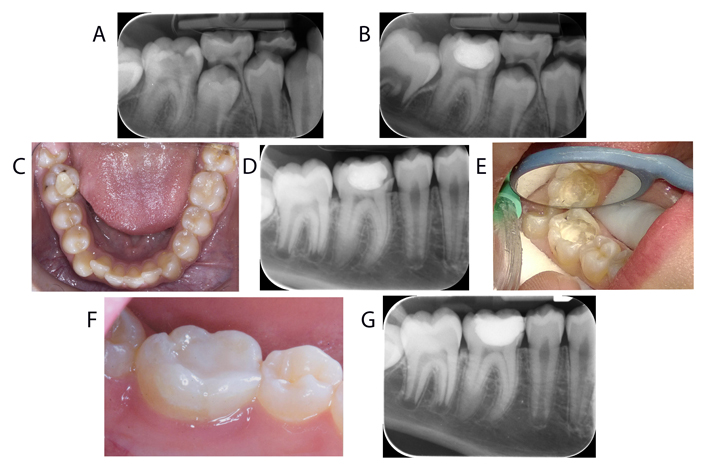
Clinical and radiographic presentation of the case. (A) Radiograph showing extensive caries in the lower right first molar with incomplete immature roots and caries reaching pulp; (B) immediate post-operative radiograph showing the CEM cement dressing placed over the affected dentine; (C) intraoral photograph showing the fractured mesiolingual wall of the lower right first molar at the follow-up appointment three years later; (D) radiograph demonstrating complete apexogenesis and maturation of the lower right first molar with no signs of periradicular or apical lesions; (E) removal of the old restoration revealing calcified pulp horns; (F) intraoral photograph after restoration with lingual cuspal coverage composite; (G) post-operative radiograph taken after restoration
The patient failed to attend the 3-month follow-up appointment, where the tooth was due to be assessed and permanently restored. A telephone conversation revealed that the tooth was functional and no signs or symptoms were reported, though the mother promised to make an appointment, they did not attend until three years later.
At this appointment patient was 12 and was due to have orthodontic treatment. He attended with his mother and was concerned that the filling had become “sharp” recently. On examination, the mesiolingual wall of the lower right first molar had fractured (Figure 1C). There were no signs or symptoms present. Radiographs showed no signs of periradicular, or apical lesion or resorption and complete apexogenesis and maturation of the treated tooth (Figure 1D). The healthy vital tooth was sensitive to electric and ethyl chloride pulp testing. Removal of the old restoration showed calcified pulp horns (Figure 1E).
At this appointment, the tooth was restored with lingual cuspal coverage composite (Figure 1F), and a post-operative radiograph was taken (Figure 1G). The patient was then referred back to the orthodontist for treatment.
Calcification of pulp horns and hard tissue formation beneath indirect pulp capping material and excavated caries is a clear indication of clinical and histological success [15, 16]. Moreover, in immature teeth continued apexogenesis after pulp capping procedures will guarantee vitality and long-term prognosis of the immature permanent tooth with reduced risk of fracture [6]. Our case showed calcification of pulp horns and complete absence of caries beneath the CEM cement and glass ionomer restoration on re-entry as well as complete apexogenesis/maturation of the lower first molar evident on the periapical radiograph. The patient experienced pain alleviation after 24 h and no pain at 7 days; previous trials showed similar results with their stepwise excavation group and direct complete excavation group [4].
Recent research has shown that even in extensive caries, indirect pulp capping with a suitable biomaterial may induce the same success as direct pulp capping and avoids the risks of exposing pulp and removing excessive coronal tissue as in full coronal pulpotomy [4, 17]. Mejare and Cvek [1] advocated partial pulpotomy in pulp-exposed carious immature molars or even stepwise indirect technique (group 1) with some success. His early trial used CH as a capping material; however, higher success rates resulting in apexogenesis have been reported with materials like MTA and CEM cement, as materials like CH may leak or disintegrate through time [16, 18]. In this case, the hard tissue formation beneath the CEM cement and the obvious calcification of the pulp horns on re-entry demonstrates complete success. A recent clinical trial with CEM cement and MTA carried out a complete coronal pulpotomy of cariously exposed immature molars +/– pain showed a radiographic success rate of 78.9% and 81.5%, respectively. The extent of caries in our case was similar to those of the clinical trial, however in an indirect approach like this case study, further tooth tissue is preserved, reducing the likelihood of fracture [6].
A recent Cochrane review has recommended indirect pulp capping as opposed to direct in primary teeth even when it entails leaving carious dentine over the pulp without re-entry [19]. Stepwise excavation has been recommended in permanent teeth affected by deep caries that may/may not expose the pulp, as compared to complete excavation, which increases the risk of exposure by approximately 11%. Removing the source of inflammation even when the superficial pulp may be somewhat inflamed can result in complete healing and regeneration, especially when a biomaterial like MTA [6] or CEM cement [2] is used. A review on a stepwise excavation in adult permanent teeth also reached the same conclusion, however, these studies did not use a biomaterial like CEM cement [4]. Materials like CEM cement have been shown to be biocompatible with pulp tissue [20], contrary to CH which is more likely to cause inflammation when adjacent to the pulp, gaps in the dentinal bridge as well as long-term failure. However, CH has shown better success rates in primary (92%) than permanent teeth (74%) in stepwise indirect pulp capping procedures [4, 19]. CEM cement on the other hand, like MTA, has been able to achieve a success/healing rate of 100% with pulpotomy procedures in immature teeth [2]. The success rate of CEM cement direct pulp capping in immature/mature teeth is 96% [18].
Indirect pulp capping has not been frequently assessed in deep carious lesions. Whether the tooth is suffering from irreversible or reversible pulpitis may be irrelevant as clinically a definitive diagnosis cannot be backed [15]; irreversible pulpitis may be regarded as a misnomer. Clinical trials of pulp-involved carious mature permanent molars have shown success rates with pulpotomy treatment [11]. A recent systematic review showed that vital pulp therapy is a good treatment option even for definitive mature teeth with symptomatic irreversible pulpitis [21]. It is likely that nano-exposures not visible to the naked eye allow regenerative biomaterial to induce healing and hard tissue formation in the indirect approach. In addition, the removal of the bulk of bacteria and toxins and infected dentine and the sealing off of the cavity will help to reduce inflammation and trigger hard tissue barrier formation even with the presence of some semi-soft dentine [1, 17, 22]. CEM cement has repeatedly been shown to produce hard tissue barrier formation like MTA [23] in permanent mature and immature teeth [16] superior to that of CaOH and Dycal® due to their long-term seal, biocompatibility [9, 11], non-toxicity [24], resistance to bacterial penetration and dentine bridge formation [16, 21]. Additionally, CEM cement has been claimed to have easier handling characteristics [11]. Clinical trials would be beneficial to assess whether immature cariously exposed teeth that would otherwise undergo pulpotomy can be successfully treated like this case study.
In conclusion, this case study demonstrates the successful apexogenesis of a symptomatic immature molar that had radiographic evidence of carious pulp exposure with CEM cement indirect pulp capping. It also shows that leaving a biomaterial over affected/semi-soft dentine will result in caries arrest, calcification of the pulp horns, continued root formation, and successful clinical outcome.
CEM: calcium-enriched mixture
CH: calcium hydroxide
MTA: mineral trioxide aggregate
SA: Conceptualization, Investigation, Data curation, Supervision, Writing—review & editing. LAM: Investigation, Data curation, Methodology, Writing—original draft. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study conforms to the Declaration of Helsinki ethical principles for medical research and is approved by Research Institute for Dental Sciences of Shahid Beheshti University of Medical Sciences.
Informed consent to participate was obtained from the patient’s guardian.
Informed consent to publication was obtained from the patient’s guardian.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Simona Santonocito ... Gaetano Isola
Elio Minetti ... Francesco Inchingolo
Renzo Guarnieri ... Luca Testarelli
Domenico Baldi ... Jacopo Colombo
Salwa Mekled ... Geraldine Weinstein
