Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
ORCID: https://orcid.org/0000-0003-1020-4341
Affiliation:
2Department of Medical, Surgical Sciences and Advanced Technologies G.F. Ingrassia, Catania 95123, Italy
ORCID: https://orcid.org/0000-0001-7136-9698
Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
Email: alexpoli345@gmail.com
ORCID: https://orcid.org/0000-0001-6717-8899
Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
ORCID: https://orcid.org/0000-0003-3059-4981
Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
ORCID: https://orcid.org/0000-0001-6086-2348
Affiliation:
1Department of General Surgery and Surgical-Medical Specialties, School of Dentistry, University of Catania, Catania 95124, Italy
Email: gaetano.isola@unict.it
ORCID: https://orcid.org/0000-0003-4267-6992
Explor Med. 2023;4:215–234 DOI: https://doi.org/10.37349/emed.2023.00135
Received: October 25, 2022 Accepted: December 12, 2022 Published: April 26, 2023
Academic Editor: Nermeen A. Elkasabgy, Cairo University Faculty of Pharmacy, Egypt
The article belongs to the special issue Biomaterials and Biomarkers in Dentistry: Up to Date
The periodontium is an appropriate target for regeneration, as it cannot restore its function following disease. Significantly, the periodontium’s limited regenerative capacity could be enhanced through the development of novel biomaterials and therapeutic approaches. Notably, the regenerative potential of the periodontium depends not only on its tissue-specific architecture and function but also on its ability to reconstruct distinct tissues and tissue interfaces, implying that the development of tissue engineering techniques can offer new perspectives for the organized reconstruction of soft and hard periodontal tissues. With their biocompatible structure and one-of-a-kind stimulus-responsive property, hydrogels have been utilized as an excellent drug delivery system for the treatment of several oral diseases. Furthermore, bioceramics and three-dimensional (3D) printed scaffolds are also appropriate scaffolding materials for the regeneration of periodontal tissue, bone, and cartilage. This work aims to examine and update material-based, biologically active cues and the deployment of breakthrough bio-fabrication technologies to regenerate the numerous tissues that comprise the periodontium for clinical and scientific applications.
Cementum periodontal ligament (PDL) and alveolar bone (AB) constitute the periodontium, an integrated and functional unit. The periodontal ligament is one of the three tissues that comprise the periodontium [1–3]. It is firmly attached to the AB and cementum, useful for mechanical stimulation while chewing, and provides a barrier against oral pathogens. The periodontium is a very complicated structure in which both tensile and compressive forces can regulate hard tissue remodeling through mechanical stress on soft tissue. Such cross-tissue communication is critical for maintaining microenvironmental homeostasis and regulating tissue remodeling [4–7].
In this regard, periodontitis is a multifactorial infectious disease characterized by inflammation of the supporting tissues of the tooth caused by periodontal bacteria organized in a complex biofilm [4–8], which, if not properly treated, can lead to the destruction of periodontal tissues and tooth loss in the long term. The onset and successive progression of periodontitis represent an unbalanced immune reaction of the host against an organized dysbiotic biofilm [1–4]. Nonetheless, periodontitis disrupts this mechanism and causes the degradation of both hard and soft tissues. This is accompanied by a cascade of cytokines and prostanoids that leads to the destruction of periodontal tissue. Even after the inflammation caused by periodontitis has been rebuilding the bone-PDL-cementum complex to a healthy level after it has been diminished remains a challenging endeavour [8, 9].
Because periodontitis accelerates the deterioration of tooth-supporting tissues and ultimately results in tooth loss, there have been a number of investigations into periodontal tissue regeneration. Some authors proposed that the kind of dominating cells on the root surface of periodontal wound tissue determined the style of tissue recovery. On the basis of this notion, new periodontal treatment strategies, such as directed guided tissue regeneration (GTR), have been presented [1–3]. The objective of GTR is the development of a new bone-PDL-cementum complex, the success of which has not yet been realized due to the limitations of regenerated bone, the absence of local vascularization, and the unpredictability of functional periodontium regeneration. The process of periodontal tissue repair is analogous to that of tissue healing during wound repair, which can be broken down into three phases: blood clot formation and inflammatory phase, proliferative phase, and remodeling phase [1, 10–12]. In this regard, other periodontal regeneration therapy techniques such as the use of growth factors (GFs) and amelogenin were developed. In periodontal regenerative medicine, combining cell regeneration together with biomaterials containing bioactive chemicals determined the development of a second- and third-periodontal regeneration technique [13, 14].
Therapies of the fourth and fifth generation for bioengineering periodontal tissue analogues, based on biomaterial and cell sheet constructs or genetically modified stem cells have evolved as a complement to existing therapeutic treatments. However, controlled clinical trials must be conducted to evaluate the danger and long-term efficacy of new bioimplants based on cell scaffolds. Embryonic stem cells (ESCs) and induced pluripotent stem (IPS) and [15–18] permit individualized cell therapy and periodontal regeneration can be enhanced by cell therapy, however, their usage is constrained by genetically modified cells. Moreover, there are areas of uncertainty in IPS biology, and it is proposed that their usage as bioimplants be implemented first in clinical trials on dental bone or periodontal abnormalities, where they can be conveniently monitored in long-term studies. It is proposed that recently acquired immortalized human periodontal cells replace embryonic constituents during periodontal tissue regeneration. Evolving tissue analogues made by three-dimensional (3D) cell printing methods aim for similarity to in vivo tissue properties and theragnostic applications, however, their functioning is still lacking [19, 20].
Based on the healing mechanism, the fundamental concept of periodontal regeneration is to enhance cell function and manage the local microenvironment at each phase. This article provides a summary of the characteristics of the microenvironment and the difficulties encountered during these crucial phases of periodontal regeneration. It also seeks to outline existing biomaterials and tactics for the modulation of critical biological events in each phase, which provides a theoretical foundation for in vivo research and clinical application of tissue-engineered periodontal regeneration.
In collaboration with the tissue-engineering technique, it is crucial to research two necessary substances: regulated drug delivery systems and the scaffold. The periodontal regeneration procedure employs two strategies, namely tissue engineering and the GTR. Clinical systems have made considerable use of GTR for periodontal therapy. It follows a regenerative surgical method that comprises the process of elevating, planning root surfaces, scaling, placing a barrier membrane beneath the gingiva for a period of time, and placing a mucous-gingival flap around the damaged teeth [21].
The tissue engineering strategy employs bioactive chemicals to build a biomimetic system and progenitor/stem cells to trigger new tissue creation. This strategy for periodontal regeneration is characterized as scaffold-free or scaffold-based, depending on the type of biomaterials used. In a scaffold-free method, either cell aggregates or individual cells are transported to a wound site lacking a cell carrier. Numerous cell types, including adipose-derived stem cells (ADSCs), bone marrow-derived dental pulp stem cells (DPSCs), and bone marrow mesenchymal stem cells (BMSCs), as well as DPSCs, have been validated for dental regeneration. In contrast to the cell suspension in swine periodontal defect cells, sheet therapy induced a unique bone growth process [22, 23].
The Cell Sheet Protocol is only able to regenerate a tissue layer with the most basic configuration. If the regeneration of the AB complex and PDL cementum involves the complex structural design of periodontium involving soft tissue PDL and two hard tissues (AB and cementum), then the only viable option is a scaffold-based one. To simulate periodontal cells, scaffolds with different properties in each phase are necessary. Particularly, the architecture, cellular structure, and biochemical arrangement of each component must be tuned to achieve periodontal healing. On the basis of the number of GFs and medications, explanations of scaffolding design and biomaterials are provided. In a mono-drug delivery procedure, a GF or medicine is administered to achieve a specified goal. Bone morphogenetic protein-2 (BMP-2) was used to expedite mandibular bone defect healing and promote bone regeneration. Non-covalent methods, such as ionic complexation and physical entrapment, can be utilized to combine bioactive chemicals with biomaterials. However, these methods are incapable of managing the burst release, which is undesirable for drug delivery operations. Frequently, the bioactive compounds’ activity declines during the manufacturing procedure (Figure 1) [24].
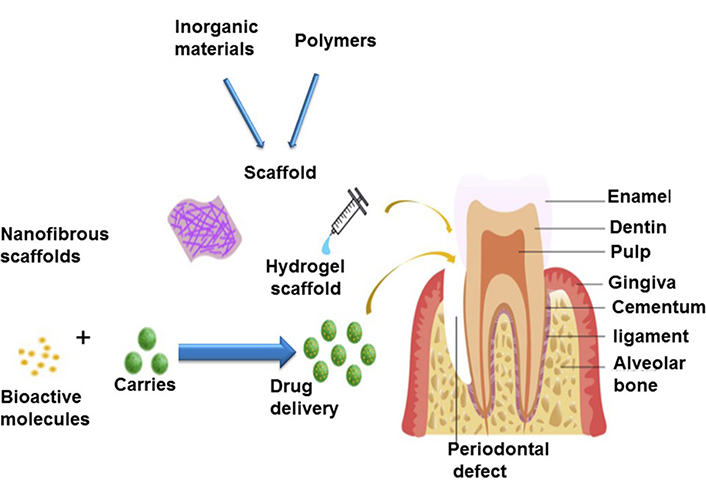
Scaffolds of a tissue-engineering approach, anatomy of periodontal tissues [24]
Note. Repinted from “Recent progress in materials development and biological properties of GTR membranes for periodontal regeneration,” by Ul Hassan S, Bilal B, Nazir MS, Naqvi SAR, Ali Z, Nadeem S, et al. Chem Biol Drug De. 2021;98:1007–24 (https://onlinelibrary.wiley.com/doi/10.1111/cbdd.13959). © 2021 John Wiley & Sons Ltd.
In the extracellular matrix (ECM), certain natural biomaterials, such as heparin sulfate and heparin glycosaminoglycan, include binding sites that produce strong interactions with bioactive chemicals. In this manner, the bioactive molecules are protected by this glycosaminoglycan (like GFs). Therefore, proteolytic degradation must be prevented, resulting in a longer and more sustainable release and denaturation. On the basis of this role for bone-tissue regeneration, a new technique, hierarchical nano-spheres encapsulating microspheres, has recently been created [20, 25, 26]. By using this technique, BMP-2 and, heparin was encapsulated in a gelatin nano-spheres heparin-conjugated system, which was then attached to microsphere nanofibers. This method makes it possible to include the control GF delivery system into a single injectable microsphere. BMP-2 has heparin-binding sites, which govern the sustained release of heparin and stabilize the protein. In addition, encapsulation in gelatin nano-spheres microspheres resembled the structure of collagen fibers and offered certain universal properties, such as a controlled disintegration rate, a larger surface area, and a reduced density value with high porosity. The nano-fibrous design inspired by the ECM enhanced the formation of new tissue, proliferation, adhesion, and differentiation of mesenchymal stem cells (MSCs) obtained from bone marrow. As demonstrated by an in vitro study, hierarchical microsphere nanofibers loaded with BMP-2 exhibited remarkable carrier capabilities and significantly enhanced bone regeneration. Combining regulated GF transport with an injectable biomimetic scaffold opens up new possibilities for cell-instructive scaffolds [22, 27].
Periodontal treatment is most effective when it combines the distribution of an osteogenic agent with the elimination of contaminants. The dual-drug delivery device successfully satisfies further GTR technical requirements [26, 28]. The dual-drug delivery procedure can sequentially release osteogenic and antibacterial medications, making it a viable method for periodontal therapy. In previously reported work, phase separation and a thermally-induced method were used to efficiently introduce a 3D dual-drug delivery system scaffold loaded with naringin (NAR) and metronidazole (MNA). The NAR and MNA were discharged in a sequentially structured fashion, with the MNA being discharged rapidly and the NAR following a more regular schedule. Numerous characteristics are created during the production of GTR electrospinning membrane: (a) broad adaptability and common methodologies; (b) with a varied structure, it can modulate fiber mats that improve cell attachment, achieve continuous and accurate local drug delivery, and boost drug loading. A unique core/shell configuration has been produced through the process of coaxial electrospinning, a novel type of spinning. By means of coaxial electrospinning, pharmaceuticals can be placed in the shell section and core portion simultaneously. Therefore, coaxial electrospinning is suitable for spinning multifunctional dual-drug delivery system membranes [13, 29–31].
The increased bioactivity, biological, and physiochemical features of dental membrane bioceramics have attracted a great deal of interest. Numerous studies have combined bioceramics such as bioactive glasses (BGs), hydroxyapatite (HA), and calcium phosphate (CP) in the field of dentistry due to their important biological properties, such as osteoinductivity and osteo-conductivity, and their capacity to reduce the natural inorganic bone constituent. Due to its favourable osteo-conductivity, bioactivity, and extreme in vivo resorb ability, beta-tricalcium phosphate (β-TCP) [32, 33] has been utilized as an exceptional bioceramic for bone healing/repair applications and dental membranes in a number of medical settings. Numerous types of membranes, including fibers, gels, foams, and mats, have been utilized. These membranes have been manufactured using a variety of techniques, including electrospinning, particle leaching, freeze-drying, self-assembly, solvent casting, and moulding. Nanofibers are promising for dental applications due to their ability to resemble ECM with a large porous structure, linked pores, and a high surface-to-volume ratio.
As an amorphous biomaterial capable of biodegradation, bioactive glass is mostly composed of sulphur dioxide (SO2) as its main component. Bioactive glass is capable of discharging calcium (Ca) and silicon (Si) ions, thereby enhancing the osteoblast’s activity and forming a bond with the bone. The deposition of minerals on the surface of osteoblast cells is promoted by BGs encased in biodegradable membranes, it has been discovered. Maximum bioceramic remains unsuitable for use in dental regeneration as a result of its high brittleness and poor flexibility as a result of manufacturing processes involving elevated pressure and temperature [34].
In gene therapy, the regeneration of the periodontium ex vivo and its dissemination in vivo involves two major approaches: ex vivo and in vivo. Even though in vivo distribution is a one-step process, its therapeutic efficacy is low and it elicits a strong immunological response from the host. Ex vivo, on the other hand, is a two-step process in which tissues and cells of interest can be extracted, and the improved in vitro location, strength, and efficacy of the transduced cells can be costly and labor-intensive.
Even though gene therapy in dental regeneration is in its infancy and requires further suggestions for clinical trials, the treatment efficacy of gene therapy in the dental regeneration process can generate promising therapeutic opportunities and increase the likelihood of well-designed, integrated periodontium regeneration.
Carbon nanotubes (CNTs) [25, 35–37] not only facilitate the regeneration process but also function as a stable mechanical component. Due to their specific chemical, physical, and mechanical properties, CNTs have demonstrated considerable potential in the biomedical field. In vivo studies have demonstrated the bioactivity of multi-walled CNTs (MWNTs) in a variety of cells, especially osteoblast cells. Numerous researches have discovered that HA improves the biological properties of polylactic acid (PLA) and that nano-sized HA crystallites are optimally arranged on the surface of MWNTs [38].
Hydrogel is a cross-linked macromolecular polymer with hydrophilic strength and adequate absorption characteristics. As a hydrogel, several types of biomolecules can be created. The advantages of a hydrogel structure consist of bioactivity, a higher water content, and a flexible construction process. Numerous types of hydrogel have been used to regenerate the periodontium. When biphasic CP (BCP) and a collagen membrane were coupled with hydroxypropylmethyl cellulose (HPMC), the membrane produced exceptional outcomes, including the prevention of soft tissue attack into periodontium defects and considerable bone repair [39].
The lack of mechanical strength is a significant limitation of hydrogel in tissue engineering. Numerous surveys used a chemical modification to circumvent the obstacle to a certain extent.
Wool keratin and polylactic-co-glycolic acid (PLGA) are two excellent biological substances. These two biomaterials have applications in the field of GTR technology. However, due to the lack of inherent strength of molecular recognition sites on the PLGA side, they can bind the PLGA application in the medical field to a specific place and block specific cell adhesion [38]. Therefore, materials with greater cell affinity, such as HA and collagen, are predominantly reformatted with PLGA to improve biocompatibility. Wool keratin is an organic biomaterial. It is capable of causing the biodegradation of harmless compounds in vivo and in vitro without eliciting an immunological or inflammatory response. In addition, its treatment performance is superior, and it may be manipulated into many forms. However, keratin biomaterials often have limited mechanical strength, as the membranes of pure wool keratin exhibit fragility [40, 41]. Consequently, wool keratin is mixed with PLGA to produce composite GTR membranes. However, wool keratin can reduce the rate of PLGA-induced sterile infection and boost PLGA’s bioactivity and cell affinity. By enhancing the mechanical properties of wool keratin with PLGA, an ideal GTR membrane may be manufactured in accordance with the specifications. Using solvent casting and electrospinning, a bilayer-graded membrane with a bottom layer of non-porous PLGA and wool keratin membrane and a top layer of porosity PLGA/wool keratin membrane was created. The porous layer encourages regeneration, but the thick layer can inhibit gingival epithelium and connective tissue from interfacing with the root surface: remodel periodontal ligament, cementum, and AB; make a new link; and ultimately accomplish periodontal tissue regeneration. The resultant membranes were then utilized in a GTR technique on beagle dogs. The research will create the groundwork for a deeper comprehension of bilayer wool keratin/PLGA membranes for the treatment of dental problems and the use of GTR [42].
Biodegradable polymer (BP) has also been used in the dental regenerative technique as powders or scaffolds for tissue engineering, and microparticles to stimulate local tissue healing or solutions. Previous studies indicated that an inorganic covering can promote implant surface differentiation, mineralization, cell proliferation, and early cell adhesion [41, 43]. In order to retain alveolar ridge volume after extraction, resveratrol is placed onto electro-spun PLA and polycaprolactone (PCL) membranes. The two membranes were able to release resveratrol in a continuous manner adjustable, and kinetically diverse manner simultaneously on two fronts as promoting new bone formation and inhibiting bone resorption [9, 26, 35, 43–46].
Scaffolds primarily serve as a platform for cell adhesion, tissue ingrowth, and early structural support in tissue engineering. In GTR, non-degradable or degradable membranes limit epithelium growth by contact, allowing for a rather gradual (4–6 weeks) repair of periodontal connective tissue and PDL. However, a protracted duration of periodontitis may diminish the efficacy of GTR by diminishing the healing capacity of PDL cells, impeding the immunological response of the host, or denaturalizing cellular matrix (CM) to an extreme degree [27, 47].
Biomaterials should ideally be distinguished by their biocompatibility, ease of surgical site adaption and placement, maintenance of space, clot stability, tissue integration, cell invasion/guidance, and encouragement of cellular proliferation. On the basis of their origin, scaffolds are categorized as allogenic, xenogeneic, alloplastic, or living structures (when they include cells) [40–44].
Scaffolds used for the regeneration of periodontal tissues can provide contact guidance that enhances the migration of cells into periodontal defects in a timely manner, leading to increased regeneration. Various bioactive cues, like GFs and cytokines, serve as stimuli and have also been supplied with scaffolds to enhance cell migration and tissue ingrowth. To the best of our knowledge, however, there is no experimental evidence that scaffold-mediated periodontal repair is as rapid as GTR. Still, insufficient evidence suggests that scaffold-mediated periodontal repair is equivalent to GTR [48–50].
The majority of prior investigations on biodegradable scaffolds [4, 7, 30, 31]. Previous authors created a PCL, collagen type I (COL-I), and recombinant human cementum protein 1 (rhCEMP1)/amorphous CP (ACP) electrospun multiphasic scaffold that promoted the formation of a CM-like structure when implanted in the calvarium of rats carrying periodontal ligament stem cells (PDLSCs) [16, 51].
Several prior works also utilized tri-phasic scaffolds to facilitate the regeneration of CM, PDL, and AB [48–51]. The tri-phasic scaffolds with spatiotemporal delivery of three separate bioactive signals and dental stem/progenitor cells efficiently stimulated the creation of a periodontium-like multi-tissue construct when implanted on the dorsum of immunodeficient mice for six weeks. Furthermore, some authors created tri-phase, 3D printed scaffolds with regionally distinct micro-architectures that have the ability to promote the integrated healing of multi-tissue periodontium by PDLSCs [52].
In this context, acellular dermal matrix (ADM) is a soft tissue transplant derived from decellularized human skin. Devoid of epithelium and cellular components, the ECM functions as a scaffold to enable cellular migration and revascularization from the surrounding host tissues [52–55]. A potential explanation may be that the ADM is incapable of promoting keratinization of the overlaying epithelia, which appears to be a favorable predictor of gingival margin stability. In the existence of a specific amount of keratinized tissue width at baseline, it is possible to achieve comparable root coverage outcomes with ADM as with connective tissues, according to studies [52–56].
The ADM was first developed for the treatment of burn wounds but has since been extensively utilized for a variety of additional purposes, including face augmentation, dural replacement, breast reconstruction, and aesthetic plastic surgery. In the field of dentistry, ADM was initially tested for the enhancement of connected and/or keratinized gingiva. However, the clinical results of the ADM are inferior to those of the free gingival graft (FGG). Specifically, the ADM appears to be more susceptible to shrinking, which may potentially account for the decreased tissue thickness seen. Compared to FGG-treated sites, ADM-treated sites exhibit a “scar”-like tissue appearance, while a superior aesthetic and color match with the surrounding tissue has been noted.
As discussed previously, different scaffold methods have demonstrated considerable potential for periodontal regeneration. Recent technical advancements in micro-precise regional control in the construction of scaffolds have established a significant milestone in the direction of integrated regeneration of multi-tissue periodontium.
Bacteria (such as Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) are the bacteria linked with periodontitis, and related host immune responses [10, 53–57]. Even though the current periodontal treatment involves the removal of dental plaque and calculus, the sources and niche of bacteria causing pathological symptoms, the recurrence rate of periodontitis after the initial periodontal treatment is strongly associated with maintenance to prevent secondary infection. In the case of GTR, reducing dental plaque deposition around the surgical site for 4–6 weeks post-treatment is crucial for a successful surgical outcome. Not only can GTR membranes hinder epithelial and connective tissues ingrowth, but they also obstruct blood flow gingival soft tissue commonly recedes, which exposes the membrane to the oral cavity and increases the risk of re-infection. e-Polytetrafluoroethylene (e-PTFE) membranes are more susceptible to such membrane exposure [58]. According to a number of prior research, such bacterial re-infection will surely affect the clinical outcome of periodontal tissue recovery. Unfortunately, the adoption of biodegradable membranes in place of non-biodegradable membranes does not eliminate the possibility of re-infection. Once exposed to the oral cavity, degradable membranes are susceptible to bacterial re-infection, which can result in rapid structural failure.
Current periodontal therapies pose a danger of re-infection with germs. As a basic and straightforward strategy, antimicrobial material components have been incorporated to scaffold materials. Chitosan, a natural polymer generated from seashells, has antibacterial, antifungal, bioadhesive, and hemostatic properties. Several prior research demonstrated the positive benefits of chitosan on periodontitis-causing bacteria. Some authors observed that chitosan particles at a concentration of 5 mg/mL suppressed periodontal infections such as Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. Similarly, it was observed that chitosan membrane containing grafted epigallocatechin-3-gallate and lovastatin was bactericidal against periodontopathic bacteria such as Aggregatibacter actinomycetemcomitans, Prevotella nigrescens, and Porphyromonas gingivalis [59].
Chitosan has shown antimicrobial properties when applied to scaffolds. Magnesium (Mg) and silver are more examples of anti-microbial components. It was observed that electrospun nanofibers containing silver nanoparticles exhibited antibacterial activity over 32 days [60]. Furthermore, some others observed that Mg oxide (MgO)-conjugated nanofibrous membrane exhibited dose-dependent antibacterial activity against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus) and showed promise in vivo efficacy in periodontal regeneration in rats [61].
The antibacterial activity of chitosan has been proven in vivo and in vitro against a variety of microorganisms, including Gram-positive and Gram-negative bacteria, yeast, fungus, and algae, making it a promising option as an antimicrobial agent in solution, film, and composite [58–63]. This antibacterial characteristic of chitosan has been linked to its cationic composition. However, its antibacterial action may also depend on other intrinsic and extrinsic variables (such as the kind of chitosan or the degree of chitosan polymerization and deacetylation, and the live host, the microorganisms, and the environmental conditions). Chitosan’s antibacterial action has not been completely understood till now [61–63].
Moreover, chitosan has also been widely exploited for periodontal regeneration because of its biocompatibility, biodegradability, non-immunogenicity, and anti-microbial properties against bacteria and fungus. Given its disadvantages as a standalone scaffold material, such as poor solubility and particle aggregation, chitosan is typically coupled with another scaffold material to provide antibacterial activity. Chitosan nanoparticles coupled with PLA nanofibers exhibited better mechanical characteristics and hydrophilicity than PLA alone. When coupled with HA and amelogenin scaffolds, chitosan displayed improved in vitro and in vivo antibacterial activity [62].
In addition to these material components, scaffolds have been treated with antimicrobial drugs such as tetracycline, doxycycline, and MNA. It was developed as a continuous release of tetracycline targeting long-lasting antibacterial properties in periodontal therapy. Similarly, coaxial electrospun PLGA fibers loaded with MNA and NAR were created and demonstrated the ability to suppress anaerobic bacteria [63].
In general, natural biomaterials offer superior cell biocompatibility. They are far less toxic and seldom produce inflammatory or immune responses. Thus, natural biomaterials have been extensively exploited as scaffolds for the regeneration of periodontal tissues [64]. Collagen and chitosan are two of the most studied natural biomaterials for the regeneration of periodontal tissue. For instance, a 3D collagen scaffold with aligned pores of 50–100 μm induced dynamic and rapid PDL migration with encouraging results. In research, collagen hydrogel absorbed with BMP-2 produced a substantial quantity of new PDL and increased periodontal attachment. Similarly, in a rat model, fibroblast GF-2 (FGF-2)-containing collagen sheet inhibited epithelial down growth into a periodontal lesion and enhanced periodontal regeneration [65].
Despite encouraging advances, the aforementioned strategies for incorporating anti-microbial material components or pharmacological compounds have been restricted to in vitro or in vivo periodontal healing modes without clinically relevant re-infection conditions.
Periodontitis is a multifactorial inflammatory illness marked by the gradual deterioration of the periodontal tissue. Despite the fact that bacteria appear to be the primary cause of periodontitis, the excessive host immune response and/or the inadequate resolution of inflammation are major contributiors to the pathogenesis of periodontitis. Consequently, they control inflammation with pharmacological treatments, such as nonsteroidal anti-inflammatory medications drugs (NSAIDs) [66]. However, they are only utilized as adjunctive treatments because the effects of interventions that rely solely on proinflammatory pathways and/or signaling without addressing causal variables are limited.
Clinical periodontal treatment begins with the removal of both the causal elements (such as tooth plaque and calculus) and the granulomatous inflammatory tissue. Since biomaterials, either as a scaffold or a contact inhibitory membrane, are administered after full clearance of irritants and inflammatory granulation tissues, inflammation management during periodontal treatment may not be a significant concern. Nevertheless, inflammation caused by a possible foreign body reaction may demand the use of a biomaterial-based scaffold or membrane. As the majority of wound healing processes involve an inflammatory phase, the therapy in question is susceptible to mild inflammatory conditions. However, the inflammation caused by incorrect surgical care and poor systemic health of patients may cause periodontal tissue destruction [67].
In prior investigations, chitosan-based scaffolds containing the prolonged release of meloxicam (NSAID) or aspirin were shown to reduce post-treatment inflammation. In a separate investigation, PCL scaffolds were administered ibuprofen, which demonstrated anti-inflammatory properties. A 3D PCL scaffold coupled with tannic acid, an anti-oxidant with anti-inflammatory properties, inhibited lipopolysaccharides-induced inflammation. The majority of earlier anti-inflammatory biomaterials were created for GTR membrane function rather than included in the scaffold architecture utilized in periodontal regeneration [68].
In periodontium, materials derived from bioceramics, including HA, β-tricalcium phosphate (β-TCP), and BG, have been widely used to enhance the healing of AB. Typically, bioceramic scaffolds have good mechanical stability and biodegradability, making them suitable for periodontal regeneration. Compared with other natural and manmade materials, bioceramic scaffolds have superior osteoconductive and osteoinductive capabilities. In addition, bioceramics can be injected into periodontal defects in granule, paste, and injectable formats. Conversely, the slow breakdown rate of ceramics can be detrimental to periodontal regeneration because residual ceramic particles can cause mechanical irritation or inflammation [69, 70].
Biodegradable bioceramics, such as HA and β-TCP, have been extensively utilized as scaffold materials for bone tissue regeneration. In a clinical experiment with a six-month follow-up, β-TCP comprising BMP-2 and platelet derived GF (PDGF) given with a collagen membrane demonstrated a high level of clinical attachment and radiographic bone gain. Another clinical study employing β-TCP with 0.1% to 0.4% FGF-2 as a scaffold implanted in vertical bone lesions of patients revealed that FGF-2-combined β-TCP resulted in better clinical attachment than control β-TCP alone. In rat and dog models, nano β-TCP scaffolds coupled with FGF-2 demonstrated increased cell infiltration and periodontal hard tissue regeneration compared to collagen scaffolds. The combination of HA and vascular endothelial GF (VEGF) exhibited osteoconductive characteristics with PDLSCs and VEGF production in vitro. CP cement (CPC) coupled with the anti-diabetic medication metformin has shown promising osteogenic differentiation activity in PDLSCs [71].
Bioactive glass, an additional well-known bioceramic with exceptional osteogenic properties, is also commonly utilized for periodontal tissue regeneration. The disintegration rate of bioactive glass can be adjusted and it can be mixed with both soft and hard scaffold materials. A clinical trial utilizing bioactive glass putty on 15 patients with grade II furcation abnormalities demonstrated a good improvement in vertical probing depth decrease at 9 months compared to platelet rich fibrin (PRF) treatment.
In addition, inorganic polyphosphates are polymers of orthophosphate connected by phosphoanhydride bonds that are rich in energy to form polymeric chains. Calcium-polyphosphate (CPP) is an excellent bone replacement because it can be manufactured with mechanical qualities similar to trabecular bone, regulated degradability, and excellent integration to host bone when implanted in vivo. CPP has been utilized in several configurations, including sintered porous blocks, particulates, and nanoparticles [68–73].
The mechanical qualities of bioceramic-based materials are a significant restriction. They are frequently too brittle to construct a 3D structure with the necessary shape and dimensions. Recent technological advancements have enabled the 3D printing of bioceramic scaffolds with patient-specific anatomical shapes and sizes. However, the limited elasticity and excessive brittleness of most bioceramics prevent their application as scaffolds for periodontal tissue regeneration in areas that require strong mechanical stability for an extended period of time (Figure 2) [72–74].
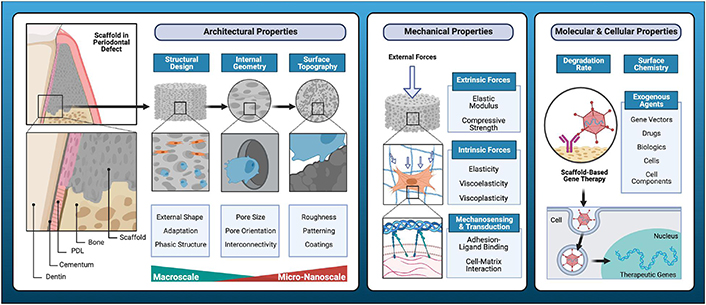
Key determinants of cell-scaffold interactions [74]
Note. Reprinted from “Regenerative medicine technologies to treat dental, oral, and craniofacial defects,” by Latimer JM, Maekawa S, Yao Y, Wu DT, Chen M, Giannobile WV. Front Bioeng Biotechnol. 2021;9:704048 (https://doi.org/10.3389/fbioe.2021.704048). CC BY.
As a replacement for the non-resorbable polytetrafluoroethylene (PTFE) membrane, the second-generation degradable membrane is primarily composed of synthetic polymers. These polymers have also been utilized as scaffolding components. For periodontal scaffold materials, polyester-based polymers such as PLA, polyglycolic acid (PGA), PLGA, and PCL have been utilized frequently. Due to the negligible quantity of leftover particles that are produced at a very slow rate, the degradation byproduct of polyester has been deemed safe. Synthetic polymers have a number of distinct advantages, including highly modifiable physiochemical properties, a biodegradation rate that can be controlled, and a quick and straightforward fabrication technique that enables mass production [72, 73].
Various polymeric scaffolds for periodontal regeneration have been studied. Electrospun PLGA/PCL composite scaffolds containing FGF-2 and bone marrow MSCs enhanced periodontal tissue repair within 6 weeks in vivo in a rat model. After one month without root resorption, the PLGA scaffold conjugated with plasmid DNA expressing FGF-2 enhanced the proliferation of human PDL cells and the formation of PDL-like tissue. In vitro evaluation of 3D printed PCL/PLGA composite scaffolds for potential use with human PDL cells indicated significantly enhanced adhesion, proliferation, and osteogenic activity [75, 76]. In a rat fenestration defect model, a multi-phase composite scaffold composed of micro-patterned PCL/PLGA for PDL and amorphous PCL for bone, with PDGF and BMP-7 for spatial delivery from the scaffold, enhanced bone-periodontal ligament interface regeneration (Figure 3) [37, 49, 52, 77–79].
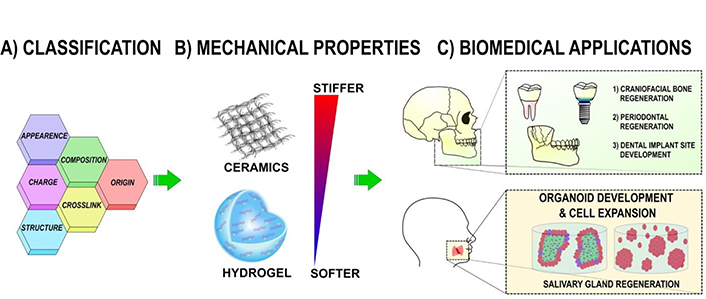
Polymeric scaffolds for dental, oral, and craniofacial regeneration [79]
Note. Reprinted from “Polymeric scaffolds for dental, oral, and craniofacial regenerative medicine,” by Wu DT, Munguia-Lopez JG, Cho YW, Ma X, Song V, Zhu Z, et al. Molecules. 2021;26:7043 (https://doi.org/10.3390/molecules26227043). CC BY.
Hydrogel is a network of cross-linked macromolecular polymers with absorption and hydrophilic characteristics. Several types of biomaterials can be generated as the hydrogel. Hydrogels are advantageous of because their high water content, biocompatibility, structural design, and formation flexibility. Hydrogels have been utilized to repair periodontal tissue in various forms. In a canine model compared to collagen membrane with BCP, HPMC hydrogel membrane with BCP revealed improved results, including suppression of soft tissue invasion into the periodontal defect and substantial bone repair [80].
In a canine model with class II furcation deficits, a collagen hydrogel scaffold injected with FGF-2 produced CM-like tissue and PDL-like Sharpey’s fibers devoid of ankyloses and root resorption. HydroMatrix, an injectable peptide nanofiber hydrogel, increased in vitro PDLSC adhesion, migration, and proliferation [81]. In a rat model, periodontal regeneration was facilitated by polyethylene glycol (PEG) hydrogel and rhCEMP1. Separate studies co-polymerized PEG and PLGA in order to develop thermosensitive PLGA-PEG-PLGA hydrogel. This hydrogel, when injected with PDGF-BB, demonstrated the potential to direct periodontal regeneration in a rat model by promoting angiogenesis, osteogenic differentiation of PDLSCs, and bone repair. Other hydrogels, such as transglutaminase crosslinked gelatins (TG-gels) and self-assembling peptides (P11-4), have demonstrated promising cell motility, adhesion, and metabolic activity when examined with PDL cells [25, 26, 82, 83].
As a developing technology, 3D printing enables greater control over the macro- and micro-structure of tissue engineering scaffolds. The periodontium is a complicated structure composed of many types of tissue since both soft and hard tissues are interconnected [40]. Recently, the 3D printing approach has been used to construct scaffolds with regionally distinct internal microstructures that are suited for CM, PDL, and/or AB in order to mimic such multi-phase tissue compositions. In addition, various growth agents can be coupled with 3D printed scaffolds to aid in the regeneration of periodontal tissues. In addition to the internal microstructure [84], 3D printing with layer-by-layer deposition enables the creation of a custom-designed scaffold in a shape and size that corresponds to the anatomic shape of each periodontal lesion [85]. Despite the limited number of publications to date, the use of 3D printing for periodontal regeneration looks to be on the rise.
As a new technique for therapeutic applications, complex biomaterials are currently intended to contain biologically active components such as BMP, enamel matrix derivatives (EMD), and GFs. In vivo, both the controlled release and protection of biomolecules throughout the repair process are crucial. The simplest method of production is compound attachment to the surface of the biomatrix. This approach is effective when rapid biomolecule release is sought, resulting in direct cell contact and activation [86]. The inclusion of biomolecules into matrices appears to offer even more advantages, however, the efficiency of the process depends on the conditioning type. Combining micro/nanoparticles containing biomolecules with polymeric biomatrices has been established as a universal technique for incorporating bioactive chemicals. Thus, alginate microspheres encapsulating BMP-6 were incorporated into porous chitosan matrices, allowing for the regulated release of the biologically active substance and the promotion of stem cell osteogenesis in periodontal regeneration. Regeneration of tissues is a complex process involving the activity of several GFs and cytokines. Thus, the inclusion of several biofactors into biomatrices and their distribution in concentrations greater than physiological ones was achieved for the creation of effective bone regeneration therapies [87]. The administration of two distinct components necessitates their separate integration into micro/nanoparticles for simultaneous release. Thus, biomaterials comprising microspheres expressing BMP-2 and insulinlike GF-1 (IGF-1) were produced to promote the independent release of GFs over time. Alternately, micro/nanocarriers with varying release kinetics are utilized for each biofactor of interest, based on the transporters’ variable biodegradability, size, porosity, and permeability. The introduction of two types of nanocapsules, poly (betahydroxybutyratecobetahydroxyvalerate) and PLGA, was found to result in the regulated release of BMP-2, followed by BMP-7, which positively promoted bone regeneration [88]. The coupling of a nanotransporter for one GF with a biomatrix carrying another growth factor was also investigated in an effort to rapidly release one factor while sustaining the release of the other. This technique may be applied well in periodontal regeneration for the first dispersion of anti-inflammatory mediators, the next molecules involved in regeneration or angiogenesis, and finally osteogenic mediators.
To rebuild bone abnormalities, autogenic biomatrices consisting of bone autografts augmented with plateletrich plasma (PRP) and PRF [89–92]. PRP is characterized as “a modest volume of autologous plasma with a high platelet concentration” and is a significant source of GFs. In dental clinics, PRP is utilized alone or in conjunction with bone replacements for the restoration of mandibular bone, the treatment of periodontal abnormalities, and the regeneration of dental alveoli after tooth extraction [89]. The outcomes of continuity defects, sinus lift augmentation grafts, horizontal and vertical ridge augmentations, and ridge preservation grafts were improved. PRP injections were also utilized to treat the musculoskeletal system, increase muscle recovery of acute muscle injuries and knee osteoarthritis, allow early implant in osteoporotic bone, and boost soft tissue, mucosal, and skin healing. Nevertheless, clinical investigations are necessary to confirm the effectiveness of these applications.
When coupled with an anticoagulant, PRP is an inexpensive, widely available material that is stable for eight hours. Due to its low mechanical strength, it is challenging to handle during implantation. The fast release of GFs may inhibit the stimulation of bone repair. There is various research on the favorable effect of PRP in periodontal regeneration as well as reports on its limited efficacy; however, the latter might be attributed to the quality of the PRP. It is believed that GFs, such as IGF-1, PDGF, transforming growth factor, FGF, and blood proteins acting as osteoconductive adhesion molecules are responsible for PRP’s beneficial effect on bone regeneration [90].
PRF was recently discovered and is regarded as the second generation of platelet concentrate, helpful for stimulating tissue healing. It is a fibrin-enriched platelet biomaterial comprising, in addition to the GFs from PRP, tumor necrosis factor, and cytokines such as interleukin 1, interleukin 6, and interleukin 4. It is readily available, simple to procure and apply, inexpensive, and requires no anticoagulation. Due to its flexibility and strength, PRF can be utilized as a clot or membrane, and it is simple to manipulate. After employing PRF and an allograft of demineralized bone matrix (DBM) or PRF in combination with bioactive glass for root cysts, bone regeneration was found. It has also been demonstrated that PRF stimulates the proliferation of osteoblasts, periodontal ligament cells, and gingival fibroblasts, showing its periodontal regeneration capability. Positive results were observed in the treatment of intrabony deficiencies when a dense matrix constructed of PRF was employed as a membrane in GTR to shield and support the bone graft at the implant and pulp site [91].
DBM was evaluated for osteoconductivity, osteoinductivity, and ability to produce bone tissue in preclinical and clinical trials. Currently, DBM is extensively employed for bone regeneration in periodontal therapies. In periodontal regeneration, commercial DBM in conjunction with EMD, BMP-2, PRP, and PRF was successfully evaluated [92]. It has been demonstrated that similarly demineralized dentin stimulates the proliferation and differentiation of periodontal ligament cells due to the presence of GFs. Combining demineralized dentin with exogenous GFs such as BMP-2 stimulated cement production and periodontal regeneration [93].
In tissue engineering, the creation of cell monolayers as an alternative to cell injection is an extremely promising approach. Monolayers of cells are grown on the temperature-responsive surface of culture plates, allowing for their noninvasive takeover in combination with synthetic ECM or by magnetic force or polyelectrolyte processes [94]. Cell monolayers were coupled with biomaterials or integrated into multilayered structures because of their fragile nature and challenging periodontal implantation. Thus, a biomatrix composed of TCP and several cell sheets of periodontal ligament cells demonstrated osteoblastic/cementoblastic development in vitro and good clinical results of periodontal regeneration in a 3-wall intrabony lesion in the dog [95]. A trilayered composite composed of a bone component enriched with CP and a periodontal component consisting of electrospun PCL provided stability to multiple cell sheets of osteoblasts, which adhered, filled the pores of the scaffold, and secreted mineralized matrix after 3 weeks of cultivation in osteogenic induction medium. In vitro, the composite displayed notable osteoinductivity, while in vivo, the combination of cell sheet technology improved bone mineralization, vascularization, and periodontal adhesion to dentin [96]. On animal and human periodontal abnormalities, stratified cellular biomatrices were evaluated for their safety and efficacy in preclinical investigations.
The evolution of rapid prototyping permits the production of 3D printed matrices tailored to each osseous defect. The image of the periodontal defect generated by a computed tomography scan is translated to a digital format utilizing computer-aided design. Using computerassisted manufacturing technologies, the scaffold’s architecture, including precise exterior shape and features such as porosity and pore size, can be modified to enhance the implant’s influence on new tissue creation [97]. Multiple techniques-based printers overlay polymeric biomaterials of varying viscosities to construct 3D structures. PCL is primarily employed for printing periodontal tissue regeneration structures. Compared to random porous scaffolds, these 3D printed matrices with controlled physico-chemical properties shown encouraging outcomes in space preservation, regulation of tissue infiltration, and cell homing.
Currently, the most challenging aspect of periodontal regeneration is the formation of multiple interfaces for AB growth, periodontal ligament orientation, and tissue fusion. Thus, using 3D printing, multiphasic matrices based on PCL and HA with 100 μ microchannel compartments for dentin cement, 600 μ microchannel compartments for a periodontal ligament, and 300 μ microchannel compartments for AB were created. In combination with encapsulated bioactive signals and DPSCs, its four-week ectopic implantation in mice resulted in the creation of integrated tissues (Figure 4) [47, 98, 99].
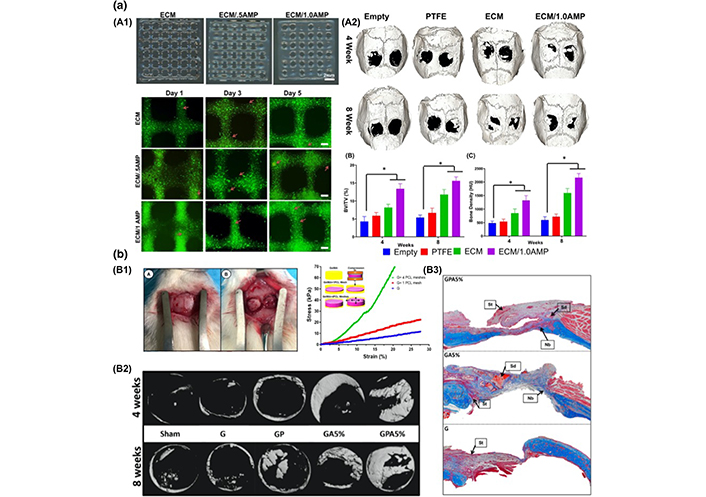
Scaffolds of a tissue-engineering approach, anatomy of periodontal tissues [99]
Note. Reprinted from “Advanced biomaterials for periodontal tissue regeneration,” by Daghrery A, Bottino MC. Genesis. 2022;60:e23501 (https://onlinelibrary.wiley.com/doi/10.1002/dvg.23501). CC BY-NC.
Using fused deposition modeling, biphasic solid porous biomaterials were created. In order to promote the simultaneous transport of two cell types (stem cells and osteoblasts) for the repair of the AB-periodontal ligament complex, they were fabricated with TCP as the bone component and PCL microfibrous membrane as the periodontal component [100–103].
According to in vitro investigations, the bone compartment promoted the proliferation of osteoblasts and mineralization of the ECM, whereas the periodontal compartment was populated with numerous layers of periodontal ligament cells. Combining a 3D printed PCL structure with an osteoconductive CP membrane and cell sheet technology. Implantation of the biphasic scaffold in an ectopic rat model stimulated AB development and periodontal attachment.
The spatial orientation of the periodontal ligament has a significant influence on masticatory forces [104, 105]. The 3D printing technology has enabled the fabrication of a biomaterial that resembles the structure of the periodontal ligament, allowing dental roots to attach to the AB and successfully controlling the perpendicular and oblique orientation of connective tissue regenerated fibers. Its insertion resulted in the formation of bone tissue and the guidance of periodontal ligaments, cementogenesis on the root surface, and functional regeneration of the periodontal complex [16, 56, 62, 80, 82, 88, 106–108]. In order to facilitate in vivo functional orientation of the periodontal ligament, scaffolds with perpendicular microchannels were fabricated using an additive manufacturing process with PCL-PGA copolymer-based synthetic copolymers [38, 63].
Periodontitis is a chronic infectious condition that necessitates efficient treatments for biological applications. GTR is a beneficial procedure for stimulating the challenging regeneration of tooth-supporting tissues. Present commercial GTR membranes are unable to achieve the desired therapeutic effects and exhibit a number of limitations, such as limited mechanical strength, a diminished capacity to stimulate the hierarchical periodontal regeneration process, and an unsuitable degradation rate, among others. To achieve therapeutic properties, novel GTR membranes must satisfy the following three requirements: (a) appropriate degradation, biocompatibility, and mechanical strength; (b) organized PDL regeneration activity and optimal AB; and (c) antibacterial activity. To ensure their exceptional biocompatibility, the proper additives, and biopolymers should be identified. Moreover, chemicals and biopolymers in the correct proportions make it possible to manage the membrane’s deterioration rate.
Synthetic polymers with high mechanical strength and natural polymers with biomedical uses and electrospinning capability can be combined to maximize their mutual advantages. An important characteristic required for GTR membranes is the capacity to stimulate periodontal regeneration. Due to their inherent potential to induce endogenous ECM, electrospun nanofibers are remarkably effective at inducing osteodifferentiation. MWNTs derived from carbon and inorganic ceramics are expected to facilitate tissue regeneration while simultaneously enhancing mechanical applicability. In addition, effective biomaterials, such as GFs and proteins, are utilized to induce degenerative pathways. In clinical employment, inflammation is the leading cause of GTR-related adverse effects. Immediate anti-inflammatory conditions and periodontal regeneration are promoted by the use of numerous medications. To date, several medicines and functional proteins such as antimicrobial peptides (AMPs), NSAIDs, metal nanoparticles such as silver nanoparticles (AgNPs), and oxide components such as zinc oxide (ZnO) have been incorporated into electro-spun nanofibers to generate an optimal anti-inflammatory environment and suppress bacterial growth. To maintain the control of drug release and bioactivity, polymer-based factors involving component weight and polymer weight, as well as drug-based factors including drug loadings, the crystallinity of pharmaceuticals, and molecular weight, must be considered. Due to the ever-increasing use of antibiotics in clinical settings, bacterial resistance has become a formidable obstacle in microbial prevention. Hence, it is vital to seek out more types of medications to effectively control periodontal inflammation and reduce bacterial resistance. The fast depletion of GFs in vivo drastically lowers their therapeutic effectiveness. The release of macromolecules is unnecessary when electrospun nanofibers are utilized. Electrospinning techniques can be combined with other procedures to create effective means for the convenient release of GFs and to detect the temporally unique discharge throughout the regeneration procedure.
Stem cells, such as PDLCs, play a vital role in periodontal tissue engineering. Dental cells cannot regenerate spontaneously due to a lack of viable stem cells. Using a tissue-engineering approach, it is possible to cultivate stem cells on a scaffold in vitro and then insert them into damaged sites to provide the necessary precursors for periodontal regeneration. However, the basis of stem cells, the in vitro culture state, the clinical impacts, and the viability of stem cells are thought-provoking in terms of their therapeutic applications. The subsequent step is to choose acceptable stem cells and GFs, identify adequate quantities in scaffolds and improve the viability of these stem cells for optimal periodontal regeneration.
Before human clinical trials, additional research is required to optimize the content and structure of new biomatrices for dental applications and to customize them to the specific needs of the patient population. For periodontal tissue engineering, the developed composite materials are beneficial for the production of bioactive implants incorporating dental cells. Using in vivo experimental models, it will be necessary to conduct additional research on osteoblast activity, differentiation, and the creation of ECM components in order to evaluate the efficacy of bioactive implants to induce bone defect repair. In addition, more biotechnological study on the production of bioactive implants and their inflammatory response after implantation is required. All of these innovative processes and techniques augur the creation of future therapeutic programs.
3D: three-dimensional
AB: alveolar bone
ADM: acellular dermal matrix
BCP: biphasic calcium phosphate
BGs: bioactive glasses
BMP-2: bone morphogenetic protein-2
CM: cellular matrix
CP: calcium phosphate
DBM: demineralized bone matrix
DPSCs: dental pulp stem cells
ECM: extracellular matrix
FGF-2: fibroblast growth factor-2
GFs: growth factors
GTR: guided tissue regeneration
HA: hydroxyapatite
MNA: metronidazole
MWNTs: multi-walled carbon nanotubes
NAR: naringin
NSAIDs: nonsteroidal anti-inflammatory medications drugs
PCL: polycaprolactone
PDGF: platelet derived growth factor
PDL: periodontal ligament
PDLSCs: periodontal ligament stem cells
PEG: polyethylene glycol
PLA: polylactic acid
PLGA: polylactic-co-glycolic acid
PRP: plateletrich plasma
SS: Conceptualization. SF: Methodology. AP: Validation, Formal analysis. VR: Validation. GR: Formal analysis. ALG: Writing—original draft. GI: Conceptualization, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Data from the present manuscript is available from the corresponding author upon reasonable request (gaetano.isola@unict.it).
The present research was funded by departmental funding only by the Department of Surgical Medical Specialties, University of Catania, Catania, Italy. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Saeed Asgary, Laleh Alim Marvasti
Elio Minetti ... Francesco Inchingolo
Renzo Guarnieri ... Luca Testarelli
Domenico Baldi ... Jacopo Colombo
Salwa Mekled ... Geraldine Weinstein
