Affiliation:
1Private Periodontal Implant Practice, 31100 Treviso, Italy
2Department of Prosthodontics and Implantology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600077, Tamil Nadu, India
ORCID: https://orcid.org/0000-0003-1449-8340
Affiliation:
2Department of Prosthodontics and Implantology, Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600077, Tamil Nadu, India
3Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, 00161 Rome, Italy
Email: rodolforeda17@gmail.com
ORCID: https://orcid.org/0000-0003-1532-6524
Affiliation:
3Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, 00161 Rome, Italy
ORCID: https://orcid.org/0000-0002-2062-8140
Affiliation:
4Department of Prosthetic Dentistry, Faculty of Dentistry, Medical University of Tirana, 1001 Tirana, Albania
ORCID: https://orcid.org/0000-0002-0843-5231
Affiliation:
5Centre of Molecular Medicine and Diagnostics (COMManD), Saveetha Dental College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Saveetha University, Chennai 600077, Tamil Nadu, India
6College of Dental Medicine, Roseman University of Health Sciences, South Jordan, UT 84095, USA
ORCID: https://orcid.org/0000-0001-7246-5497
Affiliation:
3Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, 00161 Rome, Italy
7Operative Research Unit of Dentistry, Fondazione Policlinico Universitario Campus Bio-Medico, 00128 Rome, Italy
ORCID: https://orcid.org/0000-0002-5054-0828
Affiliation:
3Department of Oral and Maxillofacial Sciences, Sapienza University of Rome, 00161 Rome, Italy
ORCID: https://orcid.org/0000-0003-3904-3000
Explor Med. 2024;5:243–256 DOI: https://doi.org/10.37349/emed.2024.00219
Received: October 02, 2023 Accepted: November 13, 2023 Published: April 22, 2024
Academic Editor: Gaetano Isola, University of Catania, Italy
The article belongs to the special issue Biomaterials and Biomarkers in Dentistry: Up to Date
Aim: The study was to evaluate the active matrix metalloproteinase-8 (aMMP-8) concentration in gingival crevicular fluid (GCF) and in peri-implant sulcular fluid (PISF) in healthy and diseased conditions, before and after non-surgical treatment, and to compare it with the various clinical parameters used to estimate the gingival and peri-implant inflammation.
Methods: Plaque index/modified PI (PI/mPI), gingival index/simplified GI (GI/sGI), probing depth (PD), bleeding on probing index/modified BOPI (BOPI/mBOPI), radiographic bone loss/radiographic marginal bone loss (rBL/rMBL), and GCF/PISF samples were evaluated, before and 3 months after non-surgical treatment, GCF/PISF samples were analyzed by a chair-side mouth-rinse test (ImplantSafe®) in combination with a digital reader (ORALyzer®).
Results: In all groups, aMMP-8 median levels were statistically higher in the PISF than in GCF and they did not change after treatment. Moreover, it was statistically higher in Group 3 (periodontitis/peri-implantitis) compared to the other groups. A positive correlation of the GCF/PISF and aMMP-8 median concentration was seen with increasing PD and BOPI/mBOPI values. A higher covariation of aMMP-8 mean levels in GCF with PD was found when compared to PISF levels. aMMP-8 mean levels in PISF expressed a higher covariation with increasing grades of sGI, rMBL, and BOPI while aMMP-8 GCF concentration established a better covariation with PD and PI.
Conclusions: PISF of sites with peri-implant mucositis and peri-implantitis showed higher levels of aMMP-8 compared to sites with gingivitis and periodontitis. Compared to clinical indices, aMMP-8 concentration in GCF/PISF can be a beneficial adjunctive diagnostic tool for early identification and screening of the risk of peri-implant diseases. After non-surgical therapy, PISF aMMP-8 concentration remained mostly unchanged, while the GCF concentration of aMMP-8 significantly decreased.
Several clinical indices based on the characteristics of gingival tissues have been proposed to categorize the inflammatory state of periodontal tissues [1–2]. In addition to the plaque index (PI) which gives an estimate of the prime factor causing the resulting inflammation, the most used are the gingival index (GI) and the sulcular bleeding on probing index (BOPI). These indices, together with the measurement of probing depth (PD) and radiographic examination, have also been proposed to categorize peri-implant pathologies [3]. Although easily applicable, these clinical indices lack the capability to objectively assess the onset and progression of periodontal and peri-implant destructive changes [4–7]. Moreover, they also have a weak sensitivity and specificity to evaluate the effect of the therapeutic intervention [4–7]. The World Workshop on periodontal and peri-implant disease classifications [8], highlighting the need for different diagnostic methods to supplement clinical ones, emphasized the utilization of validated biomarkers in the case definition systems [9]. The gingival crevicular fluid (GCF) and the peri-implant sulcular fluid (PISF) are tissue fluids that seep through the crevicular and junctional epithelium of periodontal and peri-implant tissues. They have a similar production mechanism due to an increase in the permeability of the vessels underlying the junctional and sulcular epithelium, which might occur due to inflammation, trauma, mechanical stimulation, or presence of highly osmotic substances (bacterial products, plaque) [10, 11]. GCF/PISF analysis for inflammation-associated molecules, tissue destruction markers, enzymes, and other proinflammatory mediators has been proposed as an adjunctive diagnostic tool for the early identification of periodontal/peri-implant diseases [10, 11]. One of the most investigated biomarkers is represented by the active matrix metalloproteinase-8 (aMMP-8) which is easily detectable in GCF and PISF. aMMP-8 is an enzyme that has several proteolytic properties, including the capacity of splitting tri-helical collagen type I, II, III, and is regarded as primarily responsible for the irreversible destruction of periodontal and peri‐implant tissues [12–14]. A significant positive correlation between aMMP-8 concentration in GCF and periodontal disease has been demonstrated, indicating that the GCF levels of this biomarker would be able to effectively differentiate clinically healthy sites and gingivitis from chronic periodontitis and to monitor and effectively treat patients with chronic periodontitis [15]. It is believed that the measurement of the concentration of aMMP-8 in PISF may be very helpful also in assessing the degree of inflammation within peri-implant tissues [16–19]. Several studies assessing PISF aMMP-8 levels in different peri-implantitis lesions reported a positive correlation between their concentration and clinical inflammatory conditions around dental implants [20–22]. Moreover, the determination of aMMP-8 levels in PISF has been shown to be useful for detecting peri-implant tissue health or/and inflammatory status before clinical and radiographic measurements indicate pathologic changes, screening susceptible sites and patients, evaluating the progression of bone loss in peri-implantitis, and to monitor the effectiveness of treatments [18–25]. There is a limited number of studies on the comparative assessment between the aMMP-8 concentration in GCF and PISF and the clinical indices commonly used to estimate gingival and peri-implant inflammation [26]. Moreover, insufficient knowledge exists concerning the effects of non-surgical therapy on clinical and immunological parameters specifically characterizing peri-implant diseases [27]. The determination of aMMP-8 levels in PISF has been shown to be useful for detecting peri-implant tissues health or/and inflammatory status before clinical and radiographic measurements indicate pathologic changes and to monitor the effectiveness of treatments. Insufficient knowledge exists concerning the relationship between the aMMP-8 concentration and the clinical indices commonly used to estimate the peri-implant inflammation and effects of non-surgical therapy. Therefore, the aim of the current study was to quantify aMMP-8 GCF and PISF levels and comparatively assess their relationship with inflammatory clinical indices, before and after a non-surgical treatment.
The sample population included 45 patients (23 women and 22 men), mean age of 41 years standard deviation (SD, 11.2). The demographic data of the study participants is reported in Table 1.
Demographic data of the study population
| Demographic variable | Health | Gingivitis/peri-implant mucositis | Periodontitis/peri-implantitis |
|---|---|---|---|
| Number of patients | 15 | 15 | 15 |
| Sex (males/females) | 7/8 | 8/8 | 7/7 |
| Mean ageRange (years) | 4018–62 | 4218–64 | 3119–61 |
All patients read and signed an appropriate consent document prior to participation and agreed to attend all scheduled follow-up appointments, as required by the ethical committee. This study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Università Campus Bio-Medico di Roma [protocol code: PAR 30.21 (OSS) ComEt CBM-30/03/2021].
Inclusion criteria: age > 18 years, no systemic diseases, presence of one or more dental implants placed in the native bone, with prosthetic crowns loaded for at least 1 year.
In all groups, all eligible implants present were included until 15 implants per group (according to our sample size calculation) were sampled. The procedure for patient and implant selection and conduction of the study is presented in Figure 1. For the assessment of the dental clinical status, PD (6 sites per tooth), PI, GI, and BOPI were employed. The clinical status of peri-implant tissues was evaluated by assessing the PD (6 sites per implant) and corresponding indices for implants, including a modified PI (mPI) [28], simplified GI (sGI) [29], and a modified BOPI (mBOPI) [28].
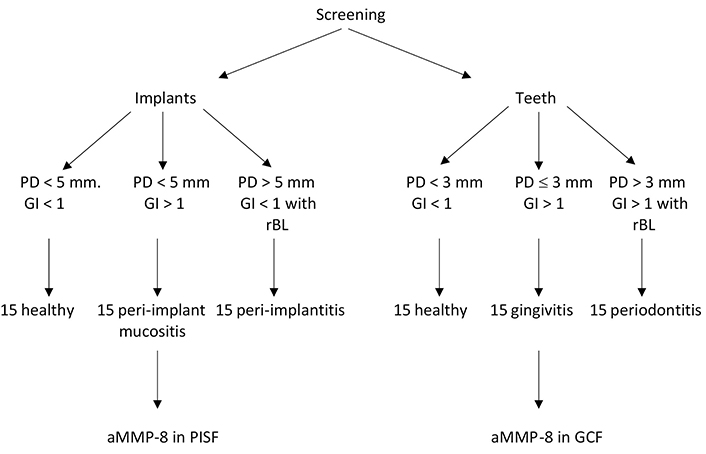
Schematic diagram for the pattern followed during the study. rBL: radiographic bone loss
The rBL around teeth was calculated as the distance from the cementoenamel junction to the bone crest area most in contact with the tooth root. When the cementoenamel junction was not evident (due to decay, fillings, crowns, or overlapping images), its location was approximated by considering the adjacent or contralateral teeth. The peri-implant radiographic marginal bone loss (rMBL) was calculated as the distance from the most coronal portion of the intra-osseous part of the implant to the bone crest area most in contact with the implant. A computer-assisted measurement automatically provided by a software program (Gendex VixWin Platinum, DEXIS, United States) was used for radiographic measurements of digital radiographs taken with the same radiologic device. After clinical recordings, patients were divided into 3 groups. The patients not meeting the specific criteria for inclusion in each group were excluded. Control Group (CG) included periodontally healthy patients exhibiting healthy peri-implant tissues. Group 1 included patients with gingivitis and peri-implant mucositis. Finally, Group 2 included patients with periodontitis and peri-implantitis. rMBL ≥ 3 mm apical from the most coronal portion of the intra-osseous part of the implant together with PD ≥ 5 mm and BOPI has been considered when diagnosing peri-implantitis. To avoid the risk of aMMP-8 fluctuation due to mechanical irritation, the clinical examination was performed a week before PISF and GCF sampling. Clinical and biochemical evaluations were assessed at baseline (T0) and 3 months (T3) after therapy.
After preparing the site according to the manufacturer’s instructions, the aMMP-8 intrasulcular levels were obtained by means of PerioSafe® test (composed of highly specific monoclonal antibodies MoAB 8706 and MoAB 8708 conjugated to latex particles), in combination a digital reader (ORALyzer®, dentognostics, Germany). The software is able to provide a numerical value referring to the concentration of the different inflammation factors in the sterile paper point that is used for GCF/PISF sampling. The test has been independently validated in Europe, United States, and Africa, to determine the aMMP-8 PISF/GCF concentration [16–18].
All patients received instructions in oral hygiene, and their teeth and implants were polished professionally with rubber cups and paste.
Group 1 patients also received an adjunctive therapy which is recognized as effective in treating peri-implant mucositis lesion [30] with supra-subgingival debridement in all 4 quadrants with tips A and P (Swiss Instruments PM, EMS, Switzerland) of ultrasound (PIEZON®, EMS, Switzerland) and curettes (Standard Gracey SG3/4, 11/12, 13/14, Hu-Friedy, United States). Implants were instrumented with special plastic curettes.
Group 2 patients were treated with an adjunctive single non-surgical therapy by means of the AIRFLOW Master Piezon® (Electro Medical Systems, EMS, Switzerland), applied all around the implant.
Five patients were examined before the study for calibration. Clinical data were recorded twice by a single examiner (RG) and another blinded examiner carried out the GCF/PISF sample analysis.
Considering an average Cohen’s effect size of 0.9 and a power of 80%, 13 teeth and implants were investigated, to detect a mean of 10 ng/ mL aMMP-8 difference between the groups before and after treatment. A t-test for independent samples was used with a significance level of 0.05. The Sidak test was used for multiple comparisons within the groups. The Kruskal-Wallis and Mann-Whitney U tests were used for nonparametric data analysis. The relationship between PISF and GCF values and the different clinical parameters was analyzed with the Pearson correlation coefficient. A P value < 0.05 was considered to be statistically significant. IBM SPSS Statistics (version 23.0 for Windows, IBM, Australia) and GraphPad Prism (version 7.02 for Windows, GraphPad Software, United States) were used for data analyses.
The clinical parameters of tooth and implant sites, along with the mean aMMP-8 values in CGF/PISF for the different groups before and after treatment, were reported in Table 2 and Table 3, respectively (mean ± SD). The r correlation values can be read in Table 4. In all groups, before and after therapy, the concentration of aMMP-8 was higher in the PISF than in the GCF, albeit without statistical significance (P > 0.05).
aMMP-8 GCF mean values and clinical parameters of tooth for the different groups, before and after treatment
| Variable | Time-point | Healthy (H) | Gingivitis (G) | Periodontitis (P) | Multiple comparisons |
|---|---|---|---|---|---|
| aMMP-8 (ng/mL) | T0T3 | 9.62 ± 4.308.99 ± 5.10 | 13.98 ± 4.7011.08 ± 3.90 | 21.82 ± 5.8015.03 ± 4.10 | H vs. G*H vs. P*G vs. P* |
| GI | T0T3 | 0.48 ± 0.030.42 ± 0.05 | 2.44 ± 0.421.12 ± 0.35(T0 vs. T3*) | 2.83 ± 0.892.04 ± 0.67(T0 vs. T3*) | H vs. G*H vs. P*G vs. P |
| PI | T0T3 | 0.65 ± 0.390.32 ± 0.23 | 1.82 ± 0.821.13 ± 0.76(T0 vs. T3*) | 2.66 ± 0.711.83 ± 0.45(T0 vs. T3*) | H vs. G*H vs. P*G vs. P* |
| BOPI | T0T3 | 0.000.00 | 1.94 ± 0.870.93 ± 0.36(T0 vs. T3*) | 2.57 ± 0.581.13 ± 0.68 | H vs. G*H vs. P*G vs. P |
| PD (mm) | T0T3 | 1.55 ± 0.331.52 ± 0.35 | 2.93 ± 0.631.91 ± 0.78(T0 vs. T3*) | 4.58 ± 0.742.89 ± 0.86 | H vs. G*H vs. P*G vs. P* |
| rBL (mm) | T0T3 | 2.30 ± 0.512.20 ± 0.33 | 3.00 ± 0.422.80 ± 0.28 | 6.40 ± 0.195.10 ± 0.55 | H vs. GH vs. P*G vs. P* |
Values are presented as mean ± SD. *: P ≤ 0.05
aMMP-8 PISF mean values and clinical parameters of implant sites for the different groups, before and after treatment
| Variable | Time-point | Healthy (H) | Peri-implant mucositis (PIM) | Peri-implantitis (P) | Multiple comparisons |
|---|---|---|---|---|---|
| aMMP-8(ng/mL) | T0T3 | 11.44 ± 6.609.83 ± 3.10 | 13.69 ± 4.7011.98 ± 4.90 | 24.60 ± 3.2020.85 ± 5.40 | H vs. PIMH vs. P*PIM vs. P* |
| sGI | T0T3 | 0.54 ± 0.050.42 ± 0.05 | 2.18 ± 0.271.53 ± 0.35(T0 vs. T3*) | 2.82 ± 0.502.11 ± 0.62 | H vs. PIM*H vs. P*PIM vs. P* |
| mPI | T0T3 | 1.20 ± 0.050.32 ± 0.23(T0 vs. T3*) | 1.88 ± 0.611.33 ± 0.76(T0 vs. T3*) | 2.29 ± 0.541.93 ± 0.21(T0 vs. T3*) | H vs. PIM*H vs. P*PIM vs. P |
| mBOPI | T0T3 | 0.11 ± 0.320.08 ± 0.09 | 1.74 ± 0.401.17 ± 0.28(T0 vs. T3*) | 2.22 ± 0.271.53 ± 0.68 | H vs. PIM*H vs. P*PIM vs. P |
| PD (mm) | T0T3 | 1.93 ± 0.271.88 ± 0.35 | 2.70 ± 0.462.47 ± 0.78 | 5.06 ± 1.124.78 ± 0.74 | H vs. PIMH vs. P*PIM vs. P* |
| rMBL (mm) | T0T3 | 2.20 ± 0.402.10 ± 0.70 | 3.30 ± 0.503.10 ± 0.40 | 6.40 ± 1.206.10 ± 0.90 | H vs. PIMH vs. P*PIM vs. P* |
Values are presented as mean ± SD. *: P ≤ 0.05
Pearson correlation between clinical parameters and corresponding aMMP-8 PISF and aMMP-8 GCF concentration
| Variable | aMMP-8 GCF | aMMP-8 PISF |
|---|---|---|
| PI/mPI | r = 0.803 | r = 0.737 |
| GI/sGI | r = 0.649 | r = 0.684 |
| PD | r = 0.873 | r = 0.789 |
| BOPI/mBOPI | r = 0.672 | r = 0.734 |
| rBL/rMBL | r = 0.888 | r = 0.898 |
At the baseline (T0) the aMMP-8 PISF mean values were significantly higher at sites with peri-implant mucositis (13.69 ± 4.70 ng/mL) and peri-implantitis (24.60 ± 3.20 ng/mL) compared to clinically healthy sites (11.44 ± 6.60 ng/mL, P < 0.05). This finding was similar for the aMMP-8 GCF concentration, whose mean value for the healthy teeth was 9.62 ± 4.30 ng/mL, for gingivitis was 13.98 ± 4.70 ng/mL, and for periodontitis was 21.82 ± 5.80 ng/mL, with a statistically significant difference between the groups (P < 0.05). Three months after therapy (T3) the aMMP-8 mean value in GCF showed a statistically significant decrease (gingivitis = 11.08 ± 3.90 ng/mL; periodontitis = 15.03 ± 4.10 ng/mL), whereas in PISF the aMMP-8 decrease did not show statistical significance (peri-implant mucositis = 11.98 ± 4.90 ng/mL; peri-implantitis = 20.85 ± 5.40 ng/mL). When compared to aMMP-8 PISF concentration, aMMP-8 GCF concentration demonstrated a better correlation with increasing PI (r = 0.803 vs. r = 0.737) and increasing GI (r = 0.649 vs. r = 0.684, Figure 2 and 3).
When the aMMP-8 GCF and PISF concentrations were compared with PD, both showed a positive correlation (Figure 4). However, aMMP-8 GCF concentration demonstrated a better correlation with increasing PDs (r = 0.873) when compared to aMMP-8 PISF (r = 0.789). Compared to aMMP-8 GCF concentration, the aMMP-8 PISF concentration expressed a higher correlation with increasing grades of BOPI, with r, respectively, 0.672 and 0.734 (Figure 5). When the aMMP-8 GCF and PISF concentrations were compared with rBL/rMBL, both showed a positive correlation (Figure 6). However, aMMP-8 PISF level demonstrated a better correlation with increasing bone loss (r = 0.898) when compared to aMMP-8 GCF level (r = 0.888).
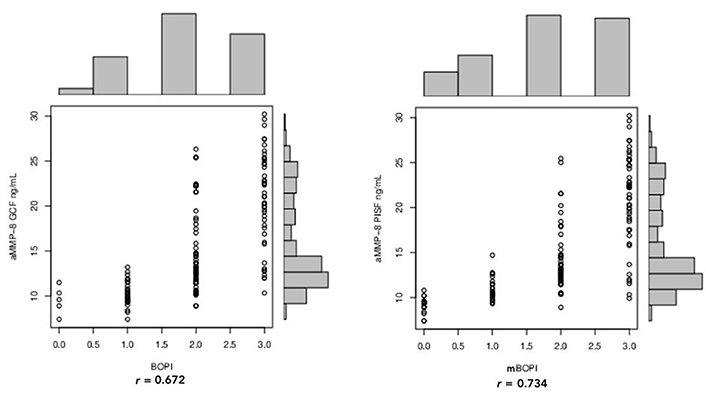
Pearson correlation between BOPI/mBOPI and aMMP-8 GCF and aMMP-8 PISF concentration
Still today periodontal and peri‐implant disease diagnosis is based on clinical and radiological examinations. However, the clinical indices conventionally used lack the capability to monitor disease activity, progression, and treatment effects [4–7]. To overcome this limitation the GCF and PISF have been tested for the presence of diverse host-derived immunological biomarkers associated with periodontal and peri‐implant tissue destruction [7, 10–13]. Among these, proteolytic enzymes capable of degrading the connective tissue have proven to be increasingly useful in the rapid diagnosis of periodontitis and peri-implantitis. Metalloproteinasis-8 (MMP‐8) is considered one of the major host‐derived collagenolytic proteinases responsible for the irreversible destruction of periodontal and peri‐implant tissues [12–14]. MMP‐8 is stored in specific granules and is released from neutrophils as latent procollagenase under the action of various stimuli, among which interleukin 1 and 8, tumor necrosis factor α, and granulocyte-macrophage colony-stimulating factor [31]. It is activated by reactive oxygen species, tissue and plasma proteinases, or opportunistic microbial proteinases (alone or in concert) [31]. Once activated, aMMP‐8 acts as a potential initiator of interstitial collagenolysis at inflammatory sites. It is the pathologically elevated concentration of aMMP‐8 and not the total or latent form, which has been demonstrated to distinguish healthy tissue from gingivitis, periodontitis, peri‐implant mucositis, and peri‐implantitis [14, 18, 32]. In the current study, the aMMP-8 PISF/GCF concentration has been determined in healthy and diseased conditions, before and after non-surgical treatment, and values have been compared with the various clinical parameters used to estimate the gingival and peri-implant inflammation. In all groups of patients, the concentration of aMMP-8 was higher in the PISF than in the GCF. These results agree with previously published data highlighted that experimental peri-implant mucositis sites present significantly higher levels of MMP-8 than experimental gingivitis sites [33], and that peri-implantitis PISF contained higher aMMP-8 levels than GCF from similar deep periodontitis sites [34, 35]. While several studies showed the effectiveness of nonsurgical therapy on decreasing aMMP-8 levels at sites affected by gingivitis and periodontitis [36–38], as far as the authors are aware, few studies have evaluated the influence of non-surgical therapy on PISF aMMP-8 levels [39–42]. In the current study, the non-surgical therapeutic procedures allow to lower the concentration of aMMP-8 in GCF with statistical evidence, while they had little effect on PISF aMMP-8 level. Our data are aligned with what is reported by Renvert et al. [39] and Hentenaar et al. [40], who neither found statistically significant differences in aMMP-8 PISF concentration before and after non-surgical therapy. A significant difference in aMMP-8 PISF concentration was found after 12 months by Schmidt et al. [41] in a group of patients undergoing non-surgical therapy. However, each patient was involved in a supportive therapy program with a high-frequency recall (every 3 to 6 months), used as preventive implant care, and none of the implants showed the baseline signs of peri-implant disease. Lower levels of MMP-8 at 3 months after non-surgical therapy were also found by Bassetti et al. [42], who used additional delivery of local minocycline microspheres to the mechanical debridement with titanium curettes and glycine powder air polishing.
As for the PI, results indicated that, although higher in the peri-implantitis group, it was not significantly statistically different from that of the peri-implant mucositis group. Otherwise, compared to sites with gingivitis, in periodontitis sites, the PI increase presented a statistical correlation. When compared to aMMP-8 PISF levels, aMMP-8 GCF levels demonstrated a better correlation with increasing PI (r = 0.803 vs. r = 0.737). These data suggest that contrary to what is recorded around the natural teeth, an increase in the severity of inflammation around implants may not necessarily require an increase in plaque accumulation. GI and sGI have been used for their easy applicability around teeth and implants for mucosal inflammation assessment. Findings of the current study showed a similar manifestation of inflammation in peri-implant mucosa and gingiva, with the GI and sGI revealing only limited statistically significant differences. However, aMMP-8 PISF expressed a higher covariation with increasing grades of sGI (r = 0.684), while a lower correlation of r = 0.649 was established for aMMP-8 GCF concentration with GI. It is known that the tissue texture and color of the peri-implant mucosa may be influenced by the appearance of the recipient tissues before implant placement, their keratinization status (with nonkeratinized tissues appearing redder than keratinized tissues), and the material characteristics of the implant surface [43, 44]. These factors that have not been recorded in the present study, could therefore also have influenced the results. As of today, it is not yet possible to define a range of PD compatible with an implant health status [8]. PD around implants may vary according to the condition of the overlying mucosa, amount of keratinized tissue, the probing pressure, implant design and implant-abutment connection (i.e., standard versus switched platform and one- versus two-piece implants), apico-coronal implant position, and restoration design (emergence profile) [45–47]. In the present study, according to indications of the 2017 World Workshop [8], a PD > 5 mm with the presence of clinical signs of inflammation was used to define an implant pathologic status around implants. When compared to aMMP-8 GCF levels, aMMP-8 PISF levels demonstrated the worst correlation with PD (r = 0.789 vs. r = 0.873). These data suggest that, compared to natural teeth, an increase in PD around implants is not necessarily connected to increased immunologic inflammatory status.
Generally, PD measurements are deeper around implants compared with natural dentition [48]. This has been linked to differences in the physiologic characteristics between peri-implant soft tissues relative to natural teeth which result also in different expectations for bleeding on probing (BOP) tendency [49]. When teeth and implants in the same patients were compared in the absence of disease, BOP was significantly higher at implants compared with teeth [50]. A systematic review with meta-analysis [51, 52] testing the reliability of modified BOP (mBOP) as clinic index for the diagnosis of peri-implantitis, reported a low prevalence of peri-implantitis for BOP-positive implants (24.1%) and for patients with implants displaying BOP (33.8%). Although the presence of BOP is not predictive of peri-implant disease status [51, 52], some literature data indicated that it could have a higher diagnostic accuracy around implants than around teeth [53]. This was confirmed in the current study, where periodontitis sites failed to show some significant rise in the BOPI over gingivitis, while all three implant subgroups displayed an important rise in BOPI/mBOPI. These data indicate that peri-implant mucosa gives a bleeding response more readily than periodontal sites. Moreover, the higher correlation detected between BOPI/mBOPI and the aMMP-8 PISF concentration, compared to aMMP-8 GCF concentration, suggests that this index could better express the immunologic inflammatory state around implants rather than around natural teeth.
MBL is considered the main factor in peri-implantitis diagnosis, since it is the unique differential factor between mucositis and peri-implantitis, as BOP and deep PD can be present in both entities. Compared to sites with gingivitis and peri-implant mucositis, both periodontitis and peri-implantitis sites showed significantly increased bone loss. Moreover, sites affected by peri-implantitis had a statistically higher concentration of aMMP-8 than those with periodontitis (24.60 ± 3.20 ng/mL vs. 21.82 ± 5.80 ng/mL).
About that, it is important to highlight that the activity of aMMP-8 is blocked by tissue inhibitors of metalloproteinases (TIMPs) [54]. Interestingly, evidence indicated that fibroblasts from peri-implantitis granulation tissue showed up-regulation of mRNA MMP-1 and reduced gene expression for TIMP-1 when compared to cells collected from chronic periodontitis granulation tissue [55]. Results of recent studies suggest that, both in the presence of the same gingival good clinical tissue health conditions and in the presence of the same inflammatory condition, soft tissues around implants are characterized by a higher pro-inflammatory state compared to soft tissues around teeth [56–58]. The different molecular responses of gingival tissues around teeth and implants are likely related to different tissue anatomy and physiology. Soft tissue around teeth develops during tooth eruption, while the peri-implant mucosa forms after the creation of a wound in oral soft and hard tissues. Since wound healing occurs in the presence of a biomaterial (i.e., a foreign body), interference of wound-healing events with this biomaterial and adaptation of the soft tissue to this biomaterial has been taken into consideration to explain the anatomic and physiologic differences [56–58]. Little is known about structural and chemical surface properties that may influence biological responses [59, 60]. By means of expression profiling by DNA microarray, it has been documented that titanium implant surface is able to modulate the expression of some genes that cover a broad range of functional activities, such as signaling transduction, translation, cell cycle regulation, enzyme and cytoskeleton development, and apoptosis [61]. Therefore, it is possible to speculate that the differences in aMMP-8 concentration observed between the groups of the current study could be also associated with the presence of biomaterial (i.e., titanium implant). However, further studies are needed to confirm this hypothesis.
Limitations of this study include the influence of gingival phenotype on aMMP-8 GCF/PISF concentration, which has been considered. Another confounding factor could be linked to the standardization of PISF sampling due to the atypical morphology of the implant prosthesis. Furthermore, it must be emphasized that due to the cyclic progression of peri-implant diseases, the biomarkers of the immune-inflammatory event responsible for tissue breakdown may not always be detected in cross-sectional studies with a single moment of fluid collection. A minor drawback of the study might be the difference in therapies applied in groups 1 and 2 of patients. However, considering the limited effect of non-surgical peri-implantitis interventions, the influence of therapy difference on aMMP-8 GCF/PISF concentration was considered rather low. Future studies employing a larger sample size should be conducted to confirm our results; in addition, further research should be carried out investigating other biomarkers to determine the peri-implant tissue inflammatory status after non-surgical therapy.
PISF of sites with peri-implant mucositis and peri-implantitis showed higher levels of aMMP-8 compared to sites with gingivitis and periodontitis. After non-surgical therapy, the PISF aMMP-8 concentration remained mostly unchanged, while the GCF concentration of aMMP-8 significantly decreased.
aMMP-8: active matrix metalloproteinase-8
BOP: bleeding on probing
BOPI: bleeding on probing index
GCF: gingival crevicular fluid
GI: gingival index
mBOPI: modified bleeding on probing index
MMP‐8: matrix metalloproteinase-8
mPI: modified plaque index
PD: probing depth
PI: plaque index
PISF: peri-implant sulcular fluid
rBL: radiographic bone loss
rMBL: radiographic marginal bone loss
SD: standard deviation
sGI: simplified gingival index
RG: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. RR: Conceptualization, Data curation, Investigation, Writing—original draft. AZ: Supervision, Methodology, Formal analysis. EX: Conceptualization, Writing—review & editing. SP: Visualization, Formal analysis, Writing—review & editing. DDN: Validation, Investigation, Writing—review & editing. LT: Supervision, Project administration. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Ethics Committee of Università Campus Bio-Medico di Roma [protocol code: PAR 30.21 (OSS) ComEt CBM-30/03/2021].
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The datasets for this manuscript are not publicly available because they expose sensitive data from included patients. Requests for accessing the datasets should be directed to Dr. Guarnieri and Dr. Reda.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Simona Santonocito ... Gaetano Isola
Saeed Asgary, Laleh Alim Marvasti
Elio Minetti ... Francesco Inchingolo
Domenico Baldi ... Jacopo Colombo
Salwa Mekled ... Geraldine Weinstein
