Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar (Mohali), Punjab 160062, India
2M.M. College of Pharmacy, Maharishi Markandeshwar (Deemed to be University), Mullana-Ambala 133207, Haryana, India
ORCID: https://orcid.org/0000-0001-8816-3987
Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research (NIPER), S.A.S. Nagar (Mohali), Punjab 160062, India
Email: ptiwari@niper.ac.in
ORCID: https://orcid.org/0000-0003-0442-7880
Affiliation:
3Department of General Medicine, Government Medical College and Hospital (GMCH), Chandigarh 160030, India
ORCID: https://orcid.org/0000-0001-8831-8002
Affiliation:
4Department of Biochemistry, Government Medical College and Hospital (GMCH), Chandigarh 160030, India
ORCID: https://orcid.org/0000-0002-7202-7902
Explor Med. 2023;4:299–313 DOI: https://doi.org/10.37349/emed.2023.00141
Received: October 10, 2022 Accepted: February 22, 2023 Published: May 17, 2023
Academic Editor: Yingyong Zhao, Northwest University, China
The article belongs to the special issue Disease Diagnosis, Molecular Mechanism and Therapeutic Strategies in Kidney Injury and Fibrosis
Aim: In patients with cancer, ischemic heart disease, and peripheral vascular disease, the neutrophil-lymphocyte ratio (NLR), a measure of systemic inflammation, has been demonstrated to predict mortality. This study aimed to evaluate the inflammatory status, and also examine the impact of NLR on renal outcomes (mortality and composite endpoints) in non-dialysis chronic kidney disease (CKD) patients.
Methods: This prospective cohort was conducted at a tertiary care public teaching hospital. The NLR greater than 3.53 was taken as an indication of systemic inflammation. The outcome measures include composite endpoints (end-stage renal disease, dialysis commencement, doubling serum creatinine from the baseline), and mortality. Kaplan-Meier plots and a multivariate Cox proportional hazard model were employed to analyze the outcomes.
Results: A cohort of 360 patients aged 53.7 years ± 13.9 years had a median follow-up of 14 months ± 4.24 months and was evaluated for inflammatory status and renal outcomes. The proportion of inflammation was found to be 101 (28.7%). Higher NLR levels had shown an increased incidence of mortality (5.3%) and composite endpoints (12.3%). In reference to the NLR quartile (Q1), the highest quartile (Q4) had shown 3 times increased hazards for mortality and 95.0% increased risk of hazards for composite endpoints Q4 hazard ratio (HR) 3.09; 95% confidence interval (CI) 1.38–6.91 (P = 0.006), and Q4 HR 1.93; 95% CI 1.22–3.08 (P = 0.005), respectively. Higher NLR was positively associated with urea, creatinine, alkaline phosphatase, Pt-Global web tool©/Patient-Generated Subjective Global Assessment score and negatively correlated with estimated glomerular filtration rate, albumin, hemoglobin.
Conclusions: NLR is a potential predictor of mortality and composite endpoints in CKD patients even before they undergo dialysis. Additionally, inflammation should be regarded as a common comorbid condition in CKD patients due to its high prevalence.
Chronic kidney disease (CKD), although being a “silent epidemic” disease, is regarded as one of the major causes of mortality. It can cause deterioration in quality of life and has high costs for the health system [1]. People suffering from CKD have a lower life expectancy and a higher rate of cardiovascular complications including anemia, bone disease, infections, inflammation, and cancer, which compounds the patient’s poor prognosis [2].
The estimated global prevalence of CKD is 13.4% (11.7–15.1%), and the projected number of end-stage kidney disease (ESKD) patients who require renal replacement therapy varies from 4.902 million to 7.083 million [3]. According to Centers for Disease Control and Prevention (CDC), around 40% of people in the United States with significantly impaired kidney function (not on dialysis) are unaware of having CKD, which is considered as the biggest hurdle in kidney care. In 2019, treating Medicare beneficiaries with CKD cost $87.2 billion, and treating people with end-stage renal disease (ESRD) cost an additional $37.3 billion [4].
According to the Screening and Early Evaluation of Kidney Disease (SEEK)-India cohort, 17.2% of people in India have some level of CKD. The prevalence according to CKD stages are as follows: 1 (7%), 2 (4.3%), 3 (4.3%), 4 (0.8%), and 5 (0.8%), respectively. The age-adjusted incidence rate of ESRD is thought to be 229 per million people, and more than 100,000 new patients engage in renal replacement programs each year [5]. The most common infections including those related to dialysis catheters are tuberculosis. The global prevalence of hyperuricemia among CKD patients was reported as 43.6% ranging from 35.2% to 52.4% [6].
Chronic inflammation is thought to be involved in the onset and progression of diabetes mellitus, cardiovascular disease, and CKD [7]. In patients with ESRD, excessive mortality has been seen to be due, in part, to their heightened pro-inflammatory state [8]. In addition, patients with CKD tend to have elevated levels of inflammatory mediators including C-reactive protein (CRP), tumor necrosis factor-α (TNF-α), and interleukin-6 (IL-6) [9, 10]. These inflammatory mediators cause glomerular hypertension, leukocyte adhesion and infiltration of the vascular endothelium, tubulointerstitial fibrosis, and renal scarring by stimulating mesangial and endothelial glomerular cells. This stimulation of mesangial and endothelial glomerular cells leads to an increase in the production and decrease in degradation of extracellular matrix [11–13].
Inflammation is closely linked to the progression of renal disease. It involves complex interactions between parenchymal cells and immune cells, including macrophages and dendritic cells, which then recruit circulating monocytes, lymphocytes, and neutrophils.
A number of tests, including an estimation of CRP, IL-6, and ferritin levels, are being performed to evaluate inflammation among CKD patients. The role of neutrophil-lymphocyte ratio (NLR) as a potential marker for determining inflammation in cardiac and non-cardiac disorders is well established [14–16]. Furthermore, the existing literature also demonstrated associations between the NLR and other inflammatory markers such as IL-6 or high sensitivity-CRP, and TNF-α levels in non-dialysis and dialysis patients [17, 18].
Inflammatory markers such as CRP, procalcitonin (PCT), ferritin, TNF-α, and IL-6 are widely used in ESRD patients [19–21]. However, these traditional biomarkers require individual tests and have several limitations. In India, TNF-α tests typically cost between $30 to $40, while IL-6 tests typically cost between $12 to $14. For NLR to estimate, a complete blood counts (CBCs) test runs about $10. These individual tests are often inaccessible and exorbitantly expensive to measure.
The predictive value of CRP is rather nonspecific in that it is difficult to determine if a particular cause is present because multiple factors contribute to uremia inflammation [19]. PCT, a calcitonin precursor peptide, is a sensitive and specific indicator of infection [22]. Ferritin’s accuracy in evaluating inflammation in ESRD patients is low since patients with ESRD who have received intravenous iron also tend to have higher ferritin levels [20]. Therefore, a more efficient and convenient marker (NLR) has been employed in clinical practice, which allows us to calculate the marker from a patient’s full blood count without incurring additional costs.
There is growing evidence of the NLR as a predictor of mortality among CKD patients. In the case of solid organ malignancies [23], and peripheral vascular disease [24, 25], NLR has been identified as a marker of systemic inflammation and a predictive marker for mortality. However, a few studies have examined the relationship between NLR and non-dialysis CKD patients, prospectively. This study is an attempt to evaluate the inflammatory status and also examine the impact of NLR on renal outcomes (mortality and composite endpoints) among non-dialysis CKD patients.
In this prospective cohort study, we enrolled 510 patients in a renal clinic, department of general medicine, at a tertiary care public teaching hospital between August 2019 and April 2022. Patients with any malignancy, chronic inflammatory disease, prescription of immunosuppressants at enrollment, acute or chronic infections, acute exacerbation of CKD, lost to follow-up, and estimated glomerular filtration rate (eGFR) < 15 mL per min/1.73 m2 at baseline require dialysis or kidney transplantation were excluded from the study.
The concerned “Research Committee and Ethics Committee” of the hospital and mentor institute (IEC/43/2019 & RC-61-Aug-19) has approved the study protocol. The informed written consent was obtained from all participants or their legally authorized representatives.
All the enrolled patients were interviewed and clinically examined at the baseline and during the follow-up period. Their medical histories and outpatient records were evaluated in detail. A pre-designed case record form was employed to record the demographic details, biochemical parameters, follow-up investigations, nutritional status, etc.
Demographics include data related to age, gender, diagnosis, body mass index (BMI), comorbidities, socioeconomic status, smoking status, blood pressure, alcohol intake, and medications prescribed were recorded at the baseline.
For the biochemical parameters, blood samples were drawn from all the individuals using uniform techniques after an overnight fasting period. CBC and all biochemical analyses including serum creatinine, serum albumin, phosphorus, Hb1Ac, lipid profile, serum sodium, potassium, chloride, phosphate, calcium, serum uric acid, alkaline phosphatase (ALP), protein, and albumin were performed by automated procedures.
The NLR was calculated using the percentages of neutrophils and lymphocytes. NLR greater than 3.53 was considered to be an indication of inflammation [26]. All the laboratory investigations were in the laboratory of biochemistry department in accordance with the ethical principles expressed in the Declaration of Helsinki [27]. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) creatinine web application. Serum creatinine was measured by a modified kinetic Jaffe reaction via the phosphoenolpyruvate carboxylase method. Serum phosphate (normal range 2.5–4.5 mg/dL) was measured using an ammonium molybdate assay. Serum phosphate levels greater than 4.5 mg/dL were taken as an indication of hyperphosphatemia.
A digital tool “Pt-Global web tool©/Patient-Generated Subjective Global Assessment (PG-SGA)” was used to evaluate the nutritional status. Patients were categorized into normal nutritional status (A), moderately malnourished (B), and severely malnourished (C) groups based on Pt-Global web tool©/PG-SGA score. This digital tool is licensed, and a license was purchased to utilize this tool in this study. The evaluation process in Pt-Global web tool© is automatic and less time-consuming. This Pt-Global web tool© is now available at https://pt-global.org/pt-global-app/. Faith D. Ottery and the Hanze University of Applied Sciences have validated this tool in populations with chronic diseases and (frail) elderly people [28].
The follow-up was commenced at the date of enrollment and continues up until the death or other censoring events including loss of follow-up, kidney transplantation, or the last date of available follow-up data. The patients were followed up for the period of more than 12 months. The study outcomes were prevalence of inflammation at the baseline and the renal outcomes (primary and secondary) after the pre-specified follow-up. The primary outcomes or composite endpoints (commencement of dialysis, doubling serum creatinine from the baseline, progression to ESRD) and secondary outcomes (mortality) were recorded by the face-to-face encounter at the OPD or via phone call.
Descriptive statistics were employed at the baseline. The study results were presented as mean ± standard deviation (SD) or median with interquartile range (IQR) for continuous data, based on their distribution and frequency with percentages (%) for categorical variables. Association of the baseline characteristics and patient categories (inflammatory and non-inflammatory group) were examined using the Chi-square test for categorical variables and analysis of variance (ANOVA) test for continuous variables. The Pearson product-moment correlation was performed to assess the correlation of NLR and different covariates.
Renal outcomes were assessed by using survival analysis. Patient survival curves for mortality based on NLR quartiles were generated according to the Kaplan-Meier method. Statistical significance was assessed using Breslow test or log-rank test. Subsequently, the association between NLR quartiles and renal outcomes was evaluated by using Cox proportional hazards models. The results were described in the hazard ratio (HR) and 95% confidence interval (CI). The relationship between NLR and each outcome (primary and secondary) was evaluated in two ways: with inflammatory status based on NLR values and with NLR quartiles.
The product-limit approach, often known as the Kaplan-Meier method, is a nonparametric technique used to calculate the probability of surviving past specific time points (i.e., it calculates a survival distribution) [29]. Additionally, the survival distributions of two or more groups of a between-subjects factor can be compared for equality. Before moving forward with the Kaplan-Meier analysis, a number of conditions must be satisfied, including that the event status should consist of two mutually exclusive and collectively exhaustive states; that the time until an event or censorship (known as the “survival time”) should be precisely defined and measured; that left-censoring should, whenever possible, be minimized or avoided; and that there should be a similar level and pattern of censorship for each group.
The log-rank test is used to determine whether or not there is a statistically significant difference in the survival times between two groups, but it does not allow for testing the effects of the other independent variables. The log-rank test can only evaluate one variable’s impact on prognosis at a time [30].
The Cox proportional hazards model is a frequently used approach that allows the investigator to study relationships between the time to event outcome (renal outcomes) and a set of explanatory variables (NLR quartiles). Cox proportion hazard makes it easier to quantify differences in survival distribution between two groups than the log-rank test (and other non-parametric models). To accomplish this, we estimate an HR. The HR is the ratio of the event rate in one group (such as the treatment group) over the other at any given time (e.g., control group) [30].
In this study, Cox regression analysis was examined by employing two distinct models (unadjusted and adjusted model) to draw conclusions. The unadjusted Cox-model (A) employed NLR quartiles as the predictive variable, follow-up period as the time variable, and mortality and composite endpoints as the outcome variables. However, in order to provide better agreement with the results, the adjusted Cox-model (B) utilized the distribution of confounders or covariates for the entire study population in addition to NLR quartiles as the predicting variable, follow-up period as the time variable, and mortality & composite endpoint as the outcome variable.
NLR was categorized into four different quartiles [Q1 (n = 94, 26.1%; NLR ≤ 2.10), Q2 (n = 88, 24.4%; NLR = 2.11–2.72), Q3 (n = 91, 25.3%; NLR = 2.73–3.75), and Q4 (n = 87, 24.2%; NLR ≥ 3.76)]. Quartile (Q1) was taken as reference group. To strengthen the robustness of the results, patients were again divided into two groups: inflammatory (QA; NLR > 3.53) and non-inflammatory (QB; NLR ≤ 3.53) on the basis of NLR ratio. All data were recorded and analyzed by using Microsoft Excel and SPSS software (Version 21; IBM, Armonk, New York). The P values < 0.05 were considered to be statistically significant.
Based upon the exclusion criteria, 150 patients were left out due to incomplete data, lost to follow-up, and declined participation. A cohort of 360 patients were eligible for quantitative analysis.
The average age of the patients was found to be 53.7 years ± 13.9 years. This cohort contains more men (58.3%), adults (62.2%), malnourished patients (61.4%), patients with hyperuricemia (63.9%), and 45.8% of patients with diabetes (Tables 1 and 2).
Baseline demographic characteristics of study participants based on inflammatory status
| Variable | All n = 360 (%) | Inflammatory status | P value | ||
|---|---|---|---|---|---|
| No (QB)NLR (≤ 3.53)n = 259 (71.9%) | Yes (QA)NLR (> 3.53)n = 101 (28.1%) | ||||
| Age groups (years) | Adult (< 60) | 224 (62.2) | 164 (63.3) | 60 (59.4) | 0.491 |
| Elderly (≥ 60) | 136 (37.8) | 95 (36.7) | 41 (40.6) | ||
| Gender | Male | 210 (58.3) | 147 (56.8) | 63 (62.4) | 0.331 |
| Female | 150 (41.7) | 112 (43.2) | 38 (37.6) | ||
| BMI | Underweight | 33 (9.2) | 21 (8.1) | 12 (11.9) | 0.715 |
| Normal | 156 (43.3) | 114 (44.0) | 42 (41.6) | ||
| Overweight | 143 (39.7) | 103 (39.8) | 40 (39.6) | ||
| Obese | 28 (7.8) | 21 (8.1) | 7 (6.9) | ||
| Socio-economic status | Lower | 159 (44.2) | 112 (43.2) | 47 (46.5) | 0.495 |
| Middle | 198 (55.0) | 144 (55.6) | 54 (53.5) | ||
| Higher | 3 (0.8) | 3 (1.2) | 0 (0) | ||
| Residence | Rural | 188 (52.2) | 136 (52.5) | 52 (51.5) | 0.861 |
| Urban | 172 (47.8) | 123 (47.5) | 49 (45.5) | ||
| Smoking | Yes | 65 (18.1) | 46 (17.8) | 19 (18.8) | 0.576 |
| No | 295 (81.9) | 213 (82.2) | 82 (81.2) | ||
| Alcohol | Yes | 152 (42.2) | 107 (41.3) | 45 (44.6) | 0.476 |
| No | 208 (57.8) | 152 (58.7) | 56 (55.4) | ||
| Diabetic status | Yes | 165 (45.8) | 113 (43.6) | 52 (51.5) | 0.179 |
| No | 195 (54.2) | 146 (56.4) | 49 (48.5) | ||
| CKD stages | CKD stage 2 | 27 (7.5) | 22 (8.5) | 5 (5.0) | 0.249 |
| CKD stage 3a | 34 (9.4) | 28 (10.8) | 6 (5.9) | ||
| CKD stage 3b | 112 (31.1) | 81 (31.3) | 31 (30.7) | ||
| CKD stage 4 | 187 (51.9) | 128 (49.4) | 59 (58.4) | ||
| Nutritional status | Normal | 139 (38.6) | 111 (42.9) | 28 (27.7) | 0.008 |
| Malnourished (severe/moderate) | 221 (61.4) | 148 (57.2) | 73 (72.3) | ||
| Hyperuricemia | No | 130 (36.1) | 93 (35.9) | 37 (36.6) | 0.897 |
| Yes | 230 (63.9) | 166 (64.1) | 64 (63.4) | ||
| Hyperphosphatemia | No | 223 (61.9) | 168 (64.9) | 55 (54.5) | 0.050 |
| Yes | 137 (38.1) | 91 (35.1) | 46 (45.5) | ||
Data presented as number and percentage. Percentages within the sub-groups are calculated on the basis of inflammatory status. P value < 0.05 taken as significant. eGFR in all patients were estimated by the CKD-EPI formula
Baseline biochemical characteristics of study participants based on inflammatory status
| Variables | Overall mean ± SDn = 360 (108.2 ± 44.6) | Inflammatory status | P value | |
|---|---|---|---|---|
| No (QB)NLR (≤ 3.53)n = 259 (71.9%) | Yes (QA)NLR (> 3.53)n = 101 (28.1%) | |||
| Age (years) | 53.7 ± 13.9 | 53.4 ± 14.2 | 54.4 ± 13.2 | 0.549 |
| Biochemical parameters | ||||
| Sodium (mg/dL) | 139.6 ± 5.5 | 140.0 ± 5.5 | 138.5 ± 5.4 | 0.020 |
| Potassium (mEq/L) | 4.79 ± 0.7 | 4.76 ± 0.6 | 4.88 ± 0.8 | 0.162 |
| Urea (mg/dL) | 77.8 ± 42.0 | 73.4 ± 36.3 | 89.2 ± 52.4 | 0.001 |
| Creatinine (mg/dL) | 2.43 ± 2.7 | 2.23 ± 0.7 | 2.93 ± 5.0 | 0.029 |
| Calcium (mg/dL) | 9.20 ± 1.16 | 9.29 ± 1.17 | 8.97 ± 1.14 | 0.021 |
| Phosphorus (mg/dL) | 4.44 ± 1.40 | 4.32 ± 1.18 | 4.75 ± 1.82 | 0.008 |
| Uric acid (mg/dL) | 7.49 ± 2.10 | 7.49 ± 2.07 | 7.48 ± 2.19 | 0.973 |
| ALP (mg/dL) | 108.2 ± 44.6 | 105.3 ± 37.3 | 115.6 ± 59.0 | 0.049 |
| eGFR (mL per min/1.73 m2) | 33.8 ± 15.3 | 33.9 ± 16.1 | 30.2 ± 12.9 | 0.040 |
| S albumin (g/dL) | 3.99 ± 0.75 | 3.75 ± 0.77 | 4.06 ± 0.73 | ≤ 0.005 |
| Hb1Ac (%) | 6.93 ± 1.85 | 6.93 ± 1.81 | 6.95 ± 1.96 | 0.912 |
| Hemoglobin (g/L) | 10.73 ± 2.31 | 10.92 ± 2.22 | 10.23 ± 2.48 | 0.011 |
| TC (g/L) | 179.9 ± 56.6 | 180.4 ± 52.5 | 178.7 ± 66.1 | 0.800 |
| TG (g/L) | 159.1 ± 72.9 | 163.9 ± 78.3 | 146.8 ± 55.0 | 0.046 |
| HDL-c (g/L) | 45.8 ± 15.3 | 45.0 ± 13.2 | 48.1 ± 19.6 | 0.080 |
| LDL-c (g/L) | 100.9 ± 43.2 | 101.2 ± 40.3 | 100.2 ± 50.0 | 0.848 |
| VLDL (g/L) | 34.8 ± 28.2 | 36.6 ± 32.3 | 29.9 ± 11.5 | 0.043 |
| Anthropometry | ||||
| BMI (kg/m2) | 24.0 ± 4.3 | 24.2 ± 4.31 | 23.6 ± 4.35 | 0.281 |
| SBP (mmHg) | 149.3 ± 24.8 | 147.6 ± 25.0 | 153.6 ± 23.8 | 0.038 |
| DBP (mmHg) | 88.3 ± 14.0 | 88.1 ± 14.5 | 88.8 ± 12.6 | 0.706 |
| SGA score | 10.4 ± 6.5 | 9.7 ± 6.4 | 12.3 ± 6.5 | 0.001 |
Data presented as mean with SD. eGFR in all patients were estimated by the CKD-EPI formula. P value < 0.05 taken as significant. DBP: diastolic blood pressure; HDL-c: high-density lipoprotein cholesterol; LDL-c: low-density lipoprotein cholesterol; S albumin: serum albumin; SBP: systolic blood pressure; SGA: subjective global assessment; TC: total cholesterol; TG: triglyceride; VLDL: very low-density lipoprotein
A significant mean difference was observed in the mean of inflammatory and non-inflammatory groups for nutritional status (P = 0.008), hyperuricemia (P = 0.050), sodium (P = 0.020), urea (P = 0.001), creatinine (P = 0.029), calcium (P = 0.021), phosphorous (P = 0.008), serum ALP (P = 0.049), eGFR (P = 0.040), albumin (P ≤ 0.005), hemoglobin (P = 0.011), TG (P = 0.046), VLDL (P = 0.043), SBP (P = 0.038), and Pt-Global web tool©/PG-SGA score (P = 0.001) (Tables 1 and 2).
According to NLR, the prevalence of inflammation among CKD patients was found to be 101 (28.1%). Male patients 63 (17.5%) were having a higher prevalence rate than female patients 38 (10.6%). The prevalence of inflammation was found to increased [CKD stage 2 (1.4%), CKD stage 3a–b 37 (10.3%), CKD stage 4 64 (17.8%)] with decrease in glomerular filtration rate. Inflammatory group (NLR > 3.53) was more likely to be adults 60 (16.7%) and malnourished 73 (20.3%) (Table 1).
The Pearson product-moment correlation provided a significant input on correlation of NLR and different covariates. The findings demonstrated that NLR tends to increase continuously as eGFR, albumin, and hemoglobin levels decrease (r = –0.138**, P = 0.009; r = –0.217**, P < 0.01; r = –0.131*, P = 0.013 respectively), whereas NLR was positively correlated with urea (r = 0.258**, P < 0.01), creatinine (r = 0.258*, P < 0.05), ALP (r = 0.158**, P = 0.003), Pt-Global web tool©/PG-SGA score (r = –0.158**, P = 0.003). **Correlation is significant at the 0.01 level (2-tailed). *Correlation is significant at the 0.05 level (2-tailed).
This cohort has a median follow-up of 14 months ± 4.5 months. The renal outcomes both primary or composite endpoints (advancing to ESRD, dialysis initiation, double serum creatine from baseline) and secondary outcomes (mortality) were assessed based on NLR quartiles (Table 3).
Incidence of renal outcomes according to NLR quartiles
| Outcomes | Overall n = 360 (%) | NLR quartiles | |||
|---|---|---|---|---|---|
| Q1n = 94 (26.1%) | Q2n = 88 (24.4%) | Q3n = 91 (25.3%) | Q4n = 87 (24.2%) | ||
| Death | 50 (13.9) | 9 (2.5) | 12 (3.3) | 10 (2.8) | 19 (5.3) |
| Composite endpoints* | 135 (37.6) | 32 (8.9) | 28 (7.8) | 31 (8.3) | 44 (12.3) |
| Commencement of dialysis | 79 (21.9) | 15 (4.2) | 18 (5.0) | 19 (5.3) | 27 (7.5) |
| Doubling of serum creatinine | 126 (35.6) | 29 (8.1) | 26 (7.2) | 31 (8.6) | 40 (11.1) |
| Advance to ESRD | 106 (29.4) | 24 (6.7) | 21 (5.8) | 25 (6.9) | 36 (10.0) |
Incidence of renal outcomes according to NLR quartiles. * Renal outcomes include death and composite endpoints (ESRD, dialysis initiation, double serum creatine from baseline). Q1 (NLR ≤ 2.10), Q2 (NLR = 2.11–2.72), Q3 (NLR = 2.73–3.75), and Q4 (NLR ≥ 3.76). Composite endpoints are further evaluated as commencement of dialysis, doubling of serum creatinine, and advance to ESRD
For the highest NLR quartile (Q4), the incidence rates for composite endpoints (commencement of dialysis, doubling of serum creatinine from the baseline, patient advancement to ESRD) and death were found to be 12.3% (7.5%, 11.1%, 10.0%), and 5.3%, respectively. When composite endpoints were further examined, the results showed that 126 patients (35.6%) had their serum creatinine double from the baseline, 106 patients (29.4%) had progressed to ESRD, and 79 patients (21.9%) had started receiving dialysis. Comparatively to the other NLR quartiles, the NLR quartile 4 showed a larger proportion of composite endpoints (Q1, Q2, Q3) (Table 3).
Further analysis of the incidence of mortality and composite outcomes was done using NLR quartiles stratified by CKD stages (Figures 1 and 2). When compared to patients with other CKD stages, those with stage 4 had the largest number of deaths (Q1 = 7, Q2 = 7, Q3 = 7, Q4 = 13) and composite endpoints (Q1 = 23, Q2 = 25, Q3 = 20, Q4 = 30). This was used to explain how patient survival declines as ESRD advances.
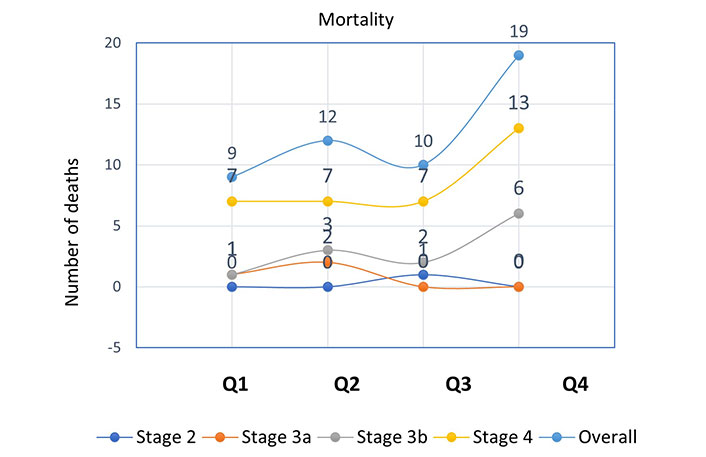
Incidence of mortality according to NLR quartiles stratified by CKD stages. NLR quartile 1, n = 94 (≤ 2.10); quartile 2, n = 88 (2.11–2.72); quartile 3, n = 91 (2.73–3.75); quartile 4, n = 87 (≥ 3.76)
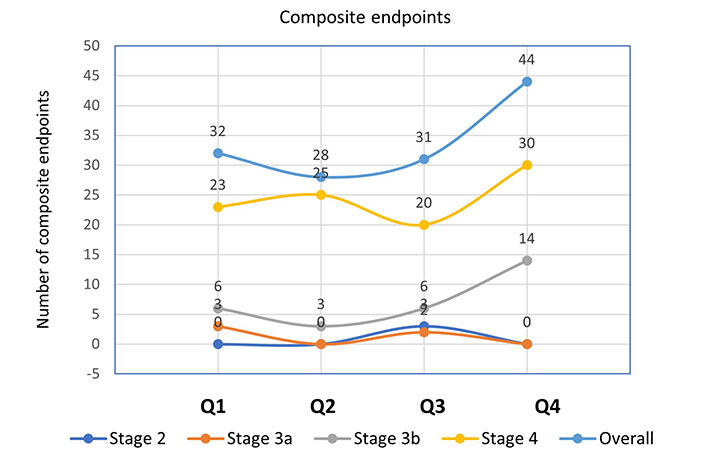
Incidence of composite endpoints according to NLR quartiles stratified by CKD stages. NLR quartile 1, n = 94 (≤ 2.10); quartile 2, n = 88 (2.11–2.72); quartile 3, n = 91 (2.73–3.75); quartile 4, n = 87 (≥ 3.76)
Cox regression hazard model was used to assess the relationship between NLR and renal outcomes. In an unadjusted model, a comparison was made between NLR quartiles (Q4, Q3, & Q2) with the reference quartile (Q1).
In reference to NLR quartile (Q1), NLR quartile (Q4) has shown three times increased hazards for mortality [Q4 HR 3.09 (95% CI) 1.38–6.91], while a graded increase in hazards for composite endpoints was observed [Q4 HR 1.93 (95% CI) 1.22–3.08]. Also, a statistically significant association was obtained between NLR quartile Q4 and NLR quartile Q1 for mortality (P = 0.006) and composite endpoints (P = 0.005) (Table 4).
Association between NLR quartiles, inflammatory status with renal outcomes
| Variables | Death | P value | All-cause mortality (composite endpoints) | P value |
|---|---|---|---|---|
| Categorical variables (NLR quartiles) | ||||
| Q4 vs. Q1 | 3.09 (1.38–6.91) | 0.006 | 1.93 (1.22–3.08) | 0.005 |
| Q3 vs. Q1 | 1.09 (0.44–2.70) | 0.837 | 1.00 (0.61–1.65) | 0.979 |
| Q2 vs. Q1 | 1.76 (0.73–4.20) | 0.204 | 1.15 (0.69–1.92) | 0.589 |
| Inflammatory status | ||||
| QB vs. QA | 2.06 (1.17–3.61) | 0.011 | 1.50 (1.06–2.14) | 0.022 |
Association between NLR quartiles, inflammatory status with renal outcomes. NLR quartile 1, n = 94 (≤ 2.10); quartile 2, n = 88 (2.11–2.72); quartile 3, n = 91 (2.73–3.75); quartile 4, n = 87 (≥ 3.76). Renal outcomes include death and composite endpoints (ESRD, dialysis initiation, double serum creatine from baseline). QA: inflammatory group; QB: non-inflammatory group
In order to increase the strength of the findings, Cox regression analysis was also extended to assess the renal outcomes based on inflammatory status. Patients having inflammation (NLR > 3.53) were found to have two times increased hazards for death as compared to non-inflammatory group [QB HR 2.06 (95% CI) 1.17–3.61]. In addition, there has been increased risk for composite endpoints for the patients having inflammation in reference to non-inflammatory group [Q4 HR 1.50 (95% CI) 1.06–2.14]. Furthermore, the relationship between inflammatory group (QA) and non-inflammatory group (QB) for death (P = 0.011) and composite endpoints (P = 0.022) was found to be statistically significant (Table 4).
The model adjusted for covariates (sodium, urea, creatinine, calcium, phosphorous, ALP, albumin, hemoglobin, TG, VLDLs, SBP, Pt-Global web tool©/PG-SGA score) revealed that the hazards for mortality and composite endpoints in case of NLR quartile Q4 contrasted to NLR quartile Q1 was found to be adjusted HR 1.566 (95% CI) 0.653–3.755 and adjusted HR 1.144 (95% CI) 0.691–1.893, respectively. These results confirm that renal outcomes (mortality and composite endpoints) are predicted by a higher NLR quartiles. However, in an adjusted model it was determined that neither mortality (P = 0.302) nor composite endpoints (P = 0.601) showed a statistically significant association between the NLR quartiles Q4 and Q1. More quantifiable data are needed to corroborate the findings.
The hazards for mortality for different covariates were determined to be as follows: sodium [HR 0.994 (0.948–1.042)], urea [HR 1.003 (0.996–1.009)], creatinine [HR 1.040 (0.881–1.228)], calcium [HR 0.886 (0.698–1.124)], phosphorous [HR 0.961 (0.803–1.151)], ALP [HR 1.006 (1.001–1.011)], albumin [HR 0.564 (0.358–0.890)], hemoglobin [HR 0.917 (0.789–1.065)], TG [HR 1.001 (0.996–1.006)], VLDL [HR 0.998 (0.978–1.019)], SBP [HR 1.008 (0.997–1.019)], SGA score [HR 1.022 (0.968–1.080)].
ALP was included as a covariate because previous research has connected serum ALP concentrations to the outcomes of various adverse events like impaired lung function, inflammation, endothelial dysfunction, and solidification [31]. The existing data also highlight that the relationship between serum ALP concentration and CVD should be interpreted in terms of inflammation because atherosclerosis is associated with inflammatory processes [32], and high expression of ALP is observed in atherosclerotic plaque [33], whereas vascular inflammation promotes the initiation and progression of atherosclerosis [34]. Thus, in the presence of vascular inflammation, inflammatory activities may contribute to an increase in serum ALP levels.
The survival curves, depicting the time to renal outcome, demonstrated that the renal survival rates were degraded with increased NLR levels (log-rank P = 0.007) (Figure 3). This KM plot depicts the cumulative survival of CKD patients past specific time points in months, as per the NLR quartiles. It also identifies the patient who is at risk after a particular period of time. This investigation, which used log-rank (Mental-Cox), came to the conclusion that NLR can be a credible predictor of mortality among CKD patients. The Figure 3 also depicts the number of participants at risk during the during the study time (in months).
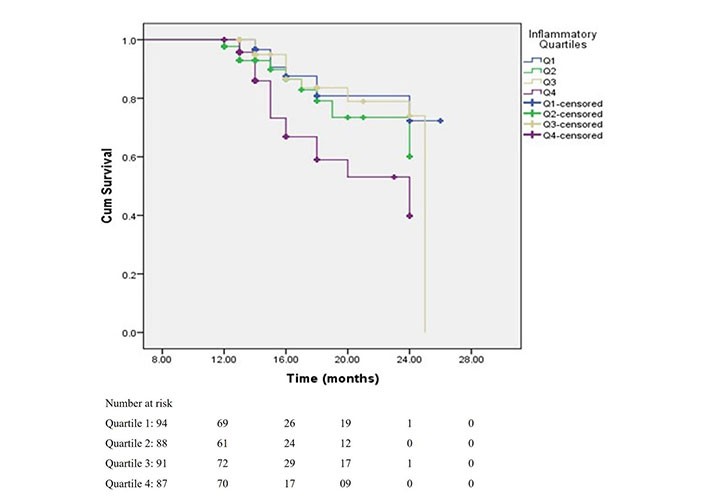
Survival rates of patients according to NLR quartiles. This shows survival rates of patients according to NLR quartiles with log-rank (Mental-Cox) P = 0.007, and Breslow (Generalized Wilcoxon) P = 0.004
The survival analysis was also extended to patients categorized on the basis of inflammatory status. The results demonstrated that renal survival rates were degraded for inflammatory group as compared to non-inflammatory group [HR 2.06 (1.17–3.61); log-rank P = 0.008] (Figure S1). Furthermore, the survival curves plotted for inflammatory status and renal outcomes stratified with malnutrition and hyperphosphatemia, showed that renal survival rates were attenuated for malnourished patients [HR 1.694 (0.957–2.998), P < 0.05; log-rank P = 0.050] and the patients with hyperphosphatemia [HR 1.984 (1.130–3.484), P = 0.01; log-rank P = 0.012] (Figures S2 and S3).
This study provides a comprehensive assessment of the inflammatory status and predictive utility of NLR in patients with CKD before they undergo dialysis. The results of this study showed that non-dialysis CKD patients had a higher percentage of systemic inflammation (28.1%). The prevalence of inflammation was also found to be increased [CKD stage 2: 5 (1.4%), CKD stage 3a: 5 (1.6%), CKD stage 3b: 31 (8.6%), CKD stage 4: 59 (16.4%)] with decrease in renal function. This evidence was supported by a study reported by Gupta et al. [35] who also documented an increased prevalence of inflammation with a decline in renal function.
The results of this study showed that inflammation was more prevalent in patients with poor nutritional status [mild/moderate and severe (20.3%)] than in well-nourished patients (7.8%), but the numbers were smaller as compared to the results reported by Prakash et al. [36] (63.0%) in a similar population. The study’s findings also showed that the prevalence of inflammation was higher in patients with hyperuricemia (17.8%), and male gender (17.5%).
The preponderance of immune cells in the human body, neutrophils serve as the body’s first line of defense against infection and tissue damage. It is noteworthy that neutrophils, through a variety of biochemical processes, mediate the inflammatory response in kidney injury, causing further tissue damage. These mechanisms include the release of myeloperoxidase, reactive oxygen species, and proteolytic enzymes which can affect kidney function [37].
The lymphocyte is a type of cell line with an immune recognition function. It is mostly found in the lymph fluid that circulates through lymphatic veins and is a crucial cell in the body’s immune response process. Hypo-lymphocytosis may even occur when there is a significant amount of inflammation. A decrease in immune regulation results from neurohumoral stimulation, while lymphocyte death, lymphocyte differentiation, and downregulated proliferation are caused by inflammatory response [38]. Additionally, lymphocyte counts have been used to gauge nutritional status because it raises the risk of all-cause mortality [39, 40]. Due to its widespread availability and low cost, the NLR, which can be determined by regular blood testing, has garnered attention and can serve as an important predictor of renal outcomes among CKD patient.
The current study investigated that higher NLR was positively associated with urea, serum creatinine, serum ALP, and Pt-Global web tool©/PG-SGA score, which indicates that the two variables operate in unison; one variable rises or falls, the other does the same. Additionally, it was observed that NLR had a negative correlation with albumin, hemoglobin, and eGFR levels. Rise in serum creatinine, urea, serum ALP, and Pt-Global web tool©/PS-SGA score and decline in albumin, hemoglobin, and eGFR levels contributes to the development of CKD. This relationship between these variables and NLR implies that NLR may have some role in the development of CKD.
In the literature, the prognostic value of the NLR in patients with cardiovascular disease, myocardial infarction, and malignant tumors has been well elucidated. Our results in this cohort of non-dialysis patients also showed that the mortality rate was higher (5.3%) in quartile (Q4) as compared to other quartiles. The patients in quartile (Q4) were found to have a higher incidence of composite endpoints (12.3%). When composite endpoints were further evaluated, the results showed that patients in quartile (Q4) reported a higher incidence of commencing dialysis (7.5%), having doubled the serum creatinine from baseline (11.1%) and advancing to ESRD (10.0%). In this study, overall mortality was found to be 50 (13.8%) which is quite a small percentage compared to the results of 54 (34.0%) reported by Neuen et al. [41] from Australia.
The role of NLR in dialysis patients has been very well explained in the literature. A study by Johnson et al. [42] found that inflammation is involved in the initiation and progression of atherosclerosis, which can lead to atherosclerotic cardiovascular events being one of the most common causes of mortality for dialysis patients. In another study carried out by An et al. [43], NLR > 3 was found to be associated with an increase in the risk of cardiovascular and all-cause mortality in peritoneal dialysis patients.
In this study, researchers investigated the relationship between NLR and renal outcomes (mortality and composite endpoints) in non-dialysis patients. After a median follow-up of 14 months, the results demonstrated that NLR quartile (Q4; NLR ≥ 3.76) have shown three times increased hazards for mortality in reference to NLR quartile (Q1; NLR ≤ 2.10). Also, an increased risk of hazards for composite endpoints was observed for NLR quartile Q4 to in reference to quartile Q1. Yoshitomi et al. [44] confirmed the findings for composite endpoints, reporting that the high NLR group showed a substantial increase in the HR for composite outcomes (HR 1.67, 95% CI 1.02–2.77) compared to the low NLR group. The patients in this study were categorized into four quartiles rather than the two groups reported by Yoshitomi et al. [44], enabling to perform a more comprehensive comparison of NLR groups.
Patients with CKD frequently experience inflammation. The exact mechanism has not been investigated, however, an increase in chronic inflammation, which is likely linked to greater NLR, was assumed to be the major mechanism behind the association between NLR and these poor renal outcomes. It is well recognized that an inflammatory condition in the vascular endothelium increases the likelihood of atherosclerotic plaque formation and calcification foci, both of which increase the risk of cardiovascular events and even death in patients with chronic renal disease [45].
Cox regression hazard model for renal outcomes based on inflammatory status was also explored to improve the reliability of the findings. When compared to non-inflammatory patients, individuals with inflammation had higher composite endpoints and were more likely to die. This study’s findings concurred with those of a study by Solak et al. [46], which demonstrated that NLR > 3.76 was a strong and independent predictor of cardiovascular events in pre-dialysis patients. Based on renal outcomes, survival curves for NLR quartiles were plotted. The results showed that the survival rates were decreasing as patients progressed from the NLR Q1 to Q4. When the survival curves for renal outcomes stratified by malnutrition, hyperphosphatemia, and inflammatory status were displayed, renal survival rates subsequently declined. These findings imply that NLR is a credible mediator of inflammation for predicting a poor prognosis in CKD patients.
According to the study results, the NLR may be a possible predictor of mortality and composite endpoints in patients with chronic renal disease even before they begin dialysis. However, more quantifiable data is needed to corroborate the findings. Additionally, inflammation should be regarded as a common comorbid condition in CKD patients due to its high prevalence. Early identification of inflammation can help delay the progression of CKD and increase a patient’s longevity.
The sample size may be one of the drawbacks of this study because it was carried out at a single location.
ALP: alkaline phosphatase
BMI: body mass index
CI: confidence interval
CKD: chronic kidney disease
CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration
CRP: C-reactive protein
eGFR: estimated glomerular filtration rate
ESRD: end-stage renal disease
HR: hazard ratio
IL-6: interleukin-6
NLR: neutrophil-lymphocyte ratio
PG-SGA: Patient-Generated Subjective Global Assessment
SBP: systolic blood pressure
SD: standard deviation
TG: triglyceride
TNF-α: tumor necrosis factor-α
VLDL: very low-density lipoprotein
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001141_sup_1.pdf.
The authors extend their gratitude towards all the participants and those who carried out the extensive clinical and laboratory work at the clinical site.
IR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Writing—original draft. PT: Conceptualization, Methodology, Supervision, Validation, Writing—original draft. SDC and SJ: Conceptualization, Methodology, Supervision, Validation. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
This study is approved by the Institutional Ethics Committee GMCH & NIPER and Research Committee GMCH and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ishfaq Rashid ... Gautam Sahu
Randa Choueiri ... Vanessa Nseir
