Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0001-9254-8644
Affiliation:
2Department of Cardiology, Xuzhou Municipal Hospital Affiliated to Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
†These authors contributed equally to this work.
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Affiliation:
1Department of Cardiology, The Affiliated Hospital of Xuzhou Medical University, Xuzhou 221000, Jiangsu, China
Email: xzwenhua0202@163.com
ORCID: https://orcid.org/0000-0002-9457-6662
Explor Med. 2022;3:571–582 DOI: https://doi.org/10.37349/emed.2022.00114
Received: September 24, 2022 Accepted: November 08, 2022 Published: December 28, 2022
Academic Editor: Yingyong Zhao, Northwest University, China
The article belongs to the special issue Disease Diagnosis, Molecular Mechanism and Therapeutic Strategies in Kidney Injury and Fibrosis
Aim: To investigate the relationship between the incidence of contrast-induced acute kidney injury (CI-AKI) and the level of small dense low-density lipoprotein (sd-LDL) and systemic immune-inflammation index (SII) in patients with acute ST-segment elevation myocardial infarction (STEMI) undergoing emergency percutaneous coronary intervention (PCI), and to further compare the predictive values of SII, sd-LDL and their combination for CI-AKI.
Methods: A total of 674 patients were assigned to a training and a validation cohort according to their chronological sequence. The baseline characteristics of the 450 patients in the training cohort were considered as candidate univariate predictors of CI-AKI. Multivariate logistic regression was then used to identify predictors of CI-AKI and develop a prediction model. The predictive values of SII, sd-LDL and their combination for CI-AKI were also evaluated.
Results: Multivariate logistic regression analysis showed that age, left ventricular ejection fraction (LVEF), sd-LDL, uric acid, estimated glomerular filtration rate (eGFR) and SII were predictors of CI-AKI. The area under the curve (AUC) of the prediction model based on the above factors was 0.846 [95% confidence interval (CI) 0.808–0.884], and the Hosmer-Lemeshow test (P = 0.587, χ2 = 6.543) proved the goodness of fit of the model. The AUC combining SII with sd-LDL to predict CI-AKI was 0.785 (95% CI 0.735–0.836), with a sensitivity of 72.8% and a specificity of 79.8%, and was statistically significant when compared with SII and sd-LDL, respectively. The predictive efficiency of combining SII with sd-LDL and SII were evaluated by improved net reclassification improvement (NRI, 0.325, P < 0.001) and integrated discrimination improvement (IDI, 0.07, P < 0.001).
Conclusions: Both SII and sd-LDL can be used as predictors of CI-AKI in STEMI patients undergoing emergency PCI, and their combination can provide more useful value for early assessment of CI-AKI.
Contrast-induced acute kidney injury (CI-AKI) is impairment in renal function that occurs within days following the intravascular injection of iodinated contrast agents. The pathogenesis of CI-AKI has not been fully elucidated, contrast nephrotoxicity, inflammation, oxidative stress and reactive oxygen species production, as well as renal medullary ischemia have been implicated [1]. The incidence of CI-AKI is generally 3–25%, and can be as high as 30% in patients with acute coronary syndrome [2]. The incidence of CI-AKI in patients with ST-segment elevation myocardial infarction (STEMI) who undergo urgent percutaneous coronary intervention (PCI) is four times higher than that in patients with stable angina [3]. However, periprocedural hydration is currently the primary intervention to mitigate risk, and the benefits of other preventive and therapeutic measures have not been proved [4]. Therefore, early and accurate identification of patients at risk for CI-AKI can help to take appropriate intervention in time to reduce morbidity and mortality [5].
Current CI-AKI prediction models [6] usually required subjective variables, including procedural variables, which compromised the ability of these models to be applied to patients with STEMI treated with emergency PCI. Hence, it is particularly important to find quantitative biomarkers that are more readily available and may serve as predictors for CI-AKI scoring models. Systemic immune-inflammation index (SII) as well as small and dense low-density lipoprotein (sd-LDL) are both clinically accessible and inexpensive quantitative biochemical markers. SII is a novel inflammatory index initially used to predict poor clinical outcomes in various cancers and inflammatory diseases [7], and in recent years studies have found that SII is associated with mortality and prognosis in patients with cardiovascular disease [8]. Sd-LDL is more likely to cause atherosclerosis than LDL, and is an independent risk factor for cardiovascular disease [9]. It has been shown that sd-LDL is also an independent risk factor for CI-AKI in STEMI patients undergoing PCI [10]. The present study aimed to investigate the predictive values of SII, sd-LDL and the combination of both on the risk of CI-AKI after PCI in STEMI patients.
This retrospective case-control study enrolled consecutive patients with STEMI who underwent emergency PCI from November 2019 to October 2021 in a single tertiary center. Patients with active infection (n = 5), previously proven autoimmune disease (n = 11), known malignancy (n = 16), hematologic system diseases (n = 8), hemodialysis before admission or chronic renal failure [estimated glomerular filtration rate (eGFR) < 30 mL•min–1•1.73 m–2] (n = 28), lack of laboratory parameters (n = 67) were excluded from the study. Finally, 674 consecutive patients were included in this study, and all patients were created in a 2:1 manner according to chronological sequence in the training cohort (n = 450) and the validation cohort (n = 224). The flow chart of the study is shown in Figure 1.
Medical and demographic data of the patients such as age, gender, hypertension, diabetes mellitus, smoking, and blood pressure on admission were collected. Blood samples were taken from each patient upon their admission, including complete blood count, high-sensitivity C-reactive protein (hs-CRP), blood glucose, blood urea, serum creatinine, lipid profile, and myocardial enzyme profiles. Serum creatinine levels were measured continuously in all patients before the procedure and for 72 h after PCI, and all patients who developed CI-AKI were further monitored. All blood specimens were tested at our central testing laboratory. Patients underwent echocardiography within 2 days of admission to measure left ventricular ejection fraction (LVEF).
PCI procedures were performed by experienced cardiac interventionalists. All patients underwent PCI intraoperatively using standard catheters, guidewires, stents, and balloons with a transradial approach in accordance with standard clinical practice. A low-osmolar, non-anionic contrast medium was used during the entire study period. The details of the PCI procedure, the use of intra-aortic balloon pump (IABP) and the contrast volume were determined intraoperatively by the interventionalist at his discretion according to the patient’s condition. Physiological saline was administered intravenously at a rate of 1 mL•kg–1•h–1 (0.5 mL•kg–1•h–1 in case of LVEF < 40% or severe congestive heart failure) for 12 h after contrast exposure. All patients received a single loading dose of 300 mg aspirin and 300 mg clopidogrel or 180 mg ticagrelor immediately on emergency admission, and were subsequently administered a daily dose of aspirin (100 mg/day) and clopidogrel (75 mg/day) or ticagrelor (180 mg/day).
SII was calculated using the formula neutrophil count × platelet count/lymphocyte count which were measured on admission. The neutrophil-to-lymphocyte ratio (NLR) was calculated by dividing the number of neutrophils by the number of lymphocytes. The platelet-to-lymphocyte ratio (PLR) was calculated by dividing the platelet count by the lymphocyte count. The eGFR was calculated using the simplified modification of diet in renal disease (MDRD) formula: eGFR (mL•min–1•1.73 m–2) = 186 × serum creatinine (mg/dL)–1.154 × age (years)–0.203 (female × 0.742). Anemia was defined as hemoglobin concentration < 13g/dL for men and < 12g/dL for women. STEMI was defined as at least one increase in cardiac troponin values above the 99th percentile upper reference limit and new ischemic electrocardiogram changes (significant ST-segment change, development of new Q waves in two or more contiguous electrocardiogram leads, and new left bundle branch block pattern) [11]. CI-AKI was defined as an absolute increase in serum creatinine level 0.5mg/dL (≥ 44.2 μmol/L) and/or ≥ 25% increase within 72 h after contrast exposure and excluding other causes of renal damage [12].
All statistical analyses were performed using SPSS version 26.0 (SPSS Inc., Chicago, IL, USA) and the statistical software package R, version 4.0.3 (https://cran.r-project.org). Categorical variables are expressed as the number of patients in that group and percentages, and are compared using the Chi-square or Fisher’s exact test. Continuous variables are expressed as mean ± standard deviation for normally distributed data and median (interquartile range) otherwise and are compared using the t-test or Mann-Whitney U test as appropriate. Natural logarithm-transformed values were used for the statistical analyses of SII levels as the original values were skewed. Logistic regression was used to determine the associated factors of CI-AKI in STEMI patients undergoing emergency PCI. Significant univariable predictors (P < 0.05) subjected to Spearman’s correlation analysis and those with significant covariance with SII were excluded. The remaining predictors were included in the multivariable analysis and a binary logistic regression model was established using the forward likelihood ratio method. The logistic regression model discriminative power was evaluated using the area under the receiver operating characteristic (ROC) curve (AUC), calibration power was evaluated by Hosmer-Lemeshow test, and the predictive value of the model for CI-AKI patients was further validated by validation cohort. In addition, ROC curves analysis was conducted and the Youden index was used to determine the best cutoff values of SII, sd-LDL and their combination for predicting CI-AKI. The AUCs of the three groups were compared using the DeLong test [13]. All tests were two-tailed, with a P value < 0.05 considered statistical significance.
There was no significant difference in baseline characteristics between the training and validation cohorts (Table S1). There were 347 males and 103 females in the training cohort with a mean age of 63.45 ± 12.63 years, and 103 (22.9%) patients developed CI-AKI after PCI. Baseline characteristics are summarized in Table 1. Age, female proportion, sd-LDL, blood urea, serum creatinine, uric acid, neutrophil count, hs-CRP, NLR, PLR and SII were higher than those in the non-CI-AKI group, and LVEF, high-density lipoprotein cholesterol (HDL-C), eGFR, and lymphocyte count were lower than those in the non-CI-AKI group, with statistically significant differences (P < 0.05); there was no statistically significant difference between the two groups in any other variables (P > 0.05) (Table 1).
Comparison of baseline characteristics in the training cohort
| Variables | CI-AKI (n = 103) | Non-CI-AKI (n = 347) | t/Z/χ2 value | P value |
|---|---|---|---|---|
| Demographics and clinical features | ||||
| Age, years | 65.87 ± 11.53 | 62.73 ± 12.87 | 2.36 | 0.019 |
| Female gender, n (%) | 33 (32.04) | 70 (20.17) | 6.34 | 0.012 |
| History of smoking, n (%) | 39 (37.86) | 160 (46.10) | 2.19 | 0.139 |
| Hypertension, n (%) | 46 (44.66) | 147 (42.36) | 0.17 | 0.679 |
| Diabetes mellitus, n (%) | 28 (27.18) | 102 (29.39) | 0.19 | 0.664 |
| Anemia, n (%) | 18 (17.48) | 81 (23.34) | 1.59 | 0.207 |
| Systolic pressure, mmHg | 129.01 ± 21.02 | 126.88 ± 50.98 | 0.41 | 0.679 |
| Diastolic pressure, mmHg | 79.72 ± 13.01 | 80.46 ± 51.02 | –0.15 | 0.883 |
| LVEF, % | 50.51 ± 6.16 | 52.37 ± 5.98 | –2.74 | 0.006 |
| Procedural features | ||||
| Door-to-balloon time, min | 60.23 ± 15.61 | 58.67 ± 12.25 | 0.78 | 0.413 |
| Intra-aortic balloon pump, n (%) | 4 (3.88) | 11 (3.17) | 0.13 | 0.755 |
| Culprit coronary artery | ||||
| Left anterior descending, n (%) | 48 (46.60) | 156 (44.96) | 0.09 | 0.768 |
| Left circumflex, n (%) | 23 (22.33) | 71 (20.46) | 0.17 | 0.682 |
| Right coronary artery, n (%) | 31 (30.10) | 119 (34.29) | 0.63 | 0.428 |
| Left main, n (%) | 1 (0.97) | 1 (0.29) | 0.84 | 0.406 |
| Total length of stent, mm | 25.37 ± 12.61 | 23.65 ± 10.47 | 0.97 | 0.296 |
| Total time of procedure, min | 64.12 ± 40.61 | 59.36 ± 38.47 | 0.92 | 0.355 |
| Contrast volume, mL | 126.00 (102.00, 135.00) | 122.00 (87.00, 136.00) | 1.43 | 0.156 |
| Laboratory values | ||||
| Total cholesterol, mmol/L | 4.40 ± 0.93 | 4.44 ± 1.08 | –0.56 | 0.578 |
| Triglycerides, mmol/L | 1.20 (0.91, 1.81) | 1.18 (0.86, 1.78) | 1.04 | 0.299 |
| HDL-C, mmol/L | 1.00 ± 0.24 | 1.06 ± 0.23 | –2.35 | 0.019 |
| LDL-C, mmol/L | 2.77 ± 0.77 | 2.75 ± 0.88 | 0.15 | 0.882 |
| Sd-LDL, mmol/L | 1.278 ± 0.512 | 0.879 ± 0.546 | 6.61 | < 0.001 |
| Lipoprotein(a), mg/L | 301.00 ± 182.56 | 276.15 ± 209.61 | 1.08 | 0.280 |
| Prealbumin, g/L | 0.223 ± 0.049 | 0.223 ± 0.049 | –0.01 | 0.990 |
| Albumin, g/L | 38.71 ± 3.50 | 38.73 ± 4.47 | 0.04 | 0.965 |
| Total bilirubin, μmol/L | 16.79 ± 7.46 | 15.59 ± 8.45 | 1.43 | 0.155 |
| Direct bilirubin, μmol/L | 6.12 ± 2.74 | 5.54 ± 2.75 | 1.78 | 0.076 |
| Basal blood glucose, mmol/L | 6.24 (5.25, 7.54) | 6.00 (5.18, 7.61) | –0.56 | 0.578 |
| Glycosylated hemoglobin, % | 6.74 ± 1.49 | 6.60 ± 1.53 | 0.80 | 0.427 |
| Blood urea, mmol/L | 5.99 ± 1.93 | 5.54 ± 1.83 | 1.97 | 0.025 |
| Serum creatinine, μmol/L | 65 (56, 75) | 55 (46, 67) | 4.95 | < 0.001 |
| Uric acid, μmol/L | 318.72 ± 92.37 | 273.14 ± 77.03 | 4.56 | < 0.001 |
| eGFR, mL·min–1·1.73 m–2 | 112.58 ± 31.23 | 127.82 ± 37.69 | –4.14 | < 0.001 |
| Lactate dehydrogenase, U/L | 606 (524, 845) | 596 (455, 755) | –0.57 | 0.624 |
| Creatine kinase, U/L | 335 (139, 790) | 309 (94, 653) | –0.78 | 0.435 |
| CK-MB, ng/mL | 19.45 (4.22, 62.92) | 17.90 (2.85, 43.23) | –0.64 | 0.552 |
| Hs-cTn, ng/L | 199.00 (41.67, 738.80) | 193.39 (20.93, 480.40) | –0.59 | 0.593 |
| White blood cell count, × 109/L | 10.39 ± 2.91 | 9.90 ± 2.92 | 1.49 | 0.136 |
| Neutrophil count, × 109/L | 8.69 ± 2.90 | 7.46 ± 2.84 | 3.84 | < 0.001 |
| Lymphocyte count, × 109/L | 1.00 (0.70, 1.50) | 1.40 (1.00, 2.00) | –5.20 | < 0.001 |
| Monocyte count, × 109/L | 0.51 ± 0.36 | 0.54 ± 0.27 | –0.98 | 0.328 |
| Platelet count, × 109/L | 207.80 ± 61.32 | 208.00 ± 57.44 | –0.03 | 0.976 |
| Hemoglobin, g/L | 137.95 ± 15.15 | 139.45 ± 18.11 | –0.87 | 0.387 |
| Red blood cell distribution width, % | 12.98 ± 3.17 | 12.70 ± 0.88 | 0.88 | 0.382 |
| Platelet distribution width, % | 15.18 ± 2.35 | 15.78 ± 11.25 | –0.53 | 0.599 |
| Hs-CRP, mg/L | 3.20 (1.30, 9.90) | 2.60 (1.00, 6.30) | 2.12 | 0.034 |
| NLR | 8.10 (5.88, 11.43) | 5.36 (3.20, 8.86) | –5.11 | < 0.001 |
| PLR | 191.33 (141.25, 291.67) | 144.38 (98.64, 208.75) | –4.42 | < 0.001 |
| SII, × 109/L | 1642.79 (1122.94, 2353.75) | 959.47 (599.15, 1535.60) | –6.04 | < 0.001 |
| Medication | ||||
| Aspirin, n (%) | 103 (100) | 347 (100) | - | 1 |
| Clopidogrel/ticagrelor, n (%) | 103 (100) | 347 (100) | - | 1 |
| Statins, n (%) | 102 (99.03) | 343 (98.85) | 0.02 | 0.877 |
| Beta-blockers, n (%) | 89 (86.41) | 293 (84.44) | 0.24 | 0.624 |
| Nitrates, n (%) | 51 (49.51) | 117 (51.01) | 0.07 | 0.790 |
| Diuretics, n (%) | 48 (46.60) | 148 (42.65) | 1.26 | 0.127 |
| Calcium channel blockers, n (%) | 15 (14.56) | 41 (11.82) | 0.55 | 0.458 |
| ACEI/ARB, n (%) | 73 (70.87) | 215 (61.96) | 2.74 | 0.098 |
| Heparin, n (%) | 85 (82.52) | 281 (80.98) | 0.13 | 0.724 |
LDL-C: low-density lipoprotein cholesterol; CK-MB: creatine kinase-myocardial band; Hs-cTn: high-sensitive cardiac troponin; ACEI: angiotensin converting enzyme inhibitor; ARB: angiotensin receptor blocker; -: no statistically significant
Spearman correlation analysis of statistically significant predictors in univariate analysis showed that SII was positively correlated with neutrophil count, NLR and PLR (r = 0.681, 0.831, 0.805, P < 0.001) and negatively correlated with lymphocyte count (r = –0.709, P < 0.001). To prevent covariance among factors in the regression analysis from affecting the stability of the regression model, neutrophil count, lymphocyte count, NLR and PLR, which had significant correlation with SII, were excluded from the regression analysis. The other predictors with statistical significance in the univariate analysis (age, gender, LVEF, HDL-C, sd-LDL, blood urea, serum creatinine, uric acid, eGFR, and SII) were included in the multivariate regression analysis. The results showed that increasing age, decreased LVEF, increased sd-LDL, increased uric acid, decreased eGFR, and increased SII were risk factors for the development of CI-AKI in STEMI patients undergoing emergency PCI (P < 0.05) (Table 2).
Predictors of CI-AKI after emergency PCI in patients with STEMI in training cohort
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| Odds ratio, 95% CI | P value | Odds ratio, 95% CI | P value | |
| Age, years | 1.021 (1.002–1.039) | 0.028 | 1.042 (1.016–1.068) | 0.001 |
| Gender | 0.491 (0.302–0.797) | 0.004 | ||
| LVEF, % | 0.950 (0.914–0.986) | 0.007 | 0.933 (0.893–0.976) | 0.002 |
| HDL-C, mmol/L | 0.967 (0.940–0.994) | 0.020 | ||
| Sd-LDL, mmol/L | 3.532 (2.302–5.421) | < 0.001 | 5.356 (3.188–8.996) | < 0.001 |
| Blood urea, mmol/L | 1.032 (1.006–1.059) | 0.010 | ||
| Serum creatinine, μmol/L | 1.052 (1.020–1.085) | < 0.001 | ||
| Uric acid, μmol/L | 1.014 (1.007–1.021) | < 0.001 | 1.012 (1.004–1.021) | 0.005 |
| eGFR, mL·min–1·1.73 m–2 | 0.994 (0.991–0.997) | < 0.001 | 0.995 (0.991–0.998) | 0.002 |
| Neutrophil count, × 109/L | 1.152 (1.069–1.242) | < 0.001 | - | - |
| Lymphocyte count, × 109/L | 0.531 (0.378–0.746) | < 0.001 | - | - |
| Hs-CRP, mg/L | 1.007 (0.991–1.010) | 0.170 | - | - |
| NLR | 1.120 (1.074–1.167) | < 0.001 | - | - |
| PLR | 1.005 (1.003–1.008) | < 0.001 | - | - |
| lnSII × 109/L | 2.512 (1.768–3.570) | < 0.001 | 2.471 (1.661–3.676) | < 0.001 |
CI: confidence interval; lnSII: natural logarithm-transformed of SII; -: no multivariate regression analysis was included
A CI-AKI prediction model was developed based on the above factors. The ROC curves showed that the training cohort and validation cohort AUCs were 0.846 (95% CI 0.808–0.884) and 0.878 (95% CI 0.826–0.931), respectively, and the prediction model had some accuracy (Figure 2). The Hosmer-Lemeshow test showed that both the training cohort (P = 0.587, χ2 = 6.543) and the validation cohort (P = 0.831, χ2 = 4.280) were statistically significant, indicating that the prediction model had a good calibration ability (Figure 3).
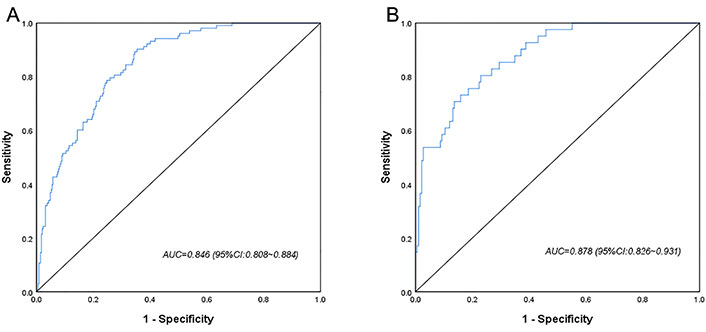
ROC curves for the logistic regression model in the training cohort (A) and the validation cohort (B)
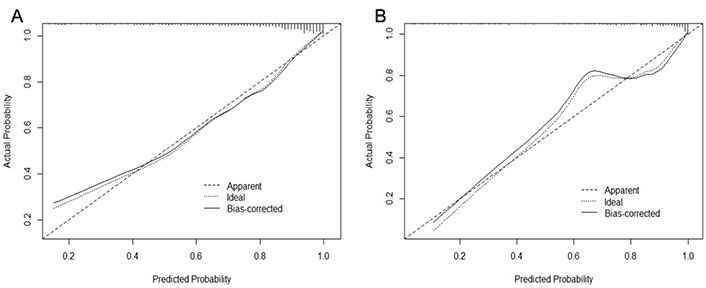
The calibration plot for the logistic regression model in the training cohort (A) and the validation cohort (B)
The ROC curves for SII, sd-LDL and the combination of both to predict the incidence of CI-AKI in STEMI patients after emergency PCI were plotted (Figure 4). The ROC statistical analysis showed that SII above 1,179.07 × 109/L had 73.8% sensitivity with 62.5% specificity for the development of CI-AKI (AUC = 0.696, 95% CI 0.639–0.753, P < 0.001), and sd-LDL more than 1.147 mmol/L had 66.0% sensitivity with 79.0% specificity for the development of CI-AKI (AUC = 0.721, 95% CI 0.662–0.781, P < 0.001). The AUC for the combined prediction of CI-AKI was 0.785 (95% CI 0.735–0.836, P < 0.001), with a sensitivity of 72.8% and a specificity of 79.8%. Further comparison of the AUC of the three groups using the DeLong test showed that the difference was not statistically significant when SII was compared with sd-LDL (Z = 0.579, P > 0.05). Conversely, SII or sd-LDL was statistically significant compared with the combination of both (Z = 2.825, 3.191, P < 0.05). Furthermore, the combination of SII and sd-LDL showed better outcomes in predicting CI-AKI than SII, as measured by improved net classification improvement (NRI, 0.325, P < 0.001) and integrated discrimination improvement (IDI, 0.07, P < 0.001). These show that the combination of SII with sd-LDL may have better predictive efficacy compared to SII and sd-LDL.
The present study evaluated the value of SII and sd-LDL in predicting the risk of CI-AKI after emergency PCI in STEMI patients. This study found that elevated preprocedural SII (> 1179.07 × 109/L) and sd-LDL (> 1.147 mmol/L) levels in STEMI patients were associated with the development of CI-AKI, and the combination of SII and sd-LDL can be used to assess the risk of CI-AKI in STEMI patients. Additionally, the present study showed that other predictors of development CI-AKI following PCI were advanced age, high uric acid levels, lower LVEF, and lower eGFR. The prediction model for the risk of developing CI-AKI based on these six indicators as predictors showed good discrimination and calibration.
The development of CI-AKI, associated with prolonged hospital stay, treatment costs and short- and long-term mortality, has been an important limitation of angiography with contrast, especially in high-risk patients like STEMI [14]. The occurrence of CI-AKI is currently included as one of the metrics for quality outcomes of PCI [15]. Therefore, proper risk stratification and prevention of CI-AKI after PCI are important from a clinical perspective. Over the past few decades, many different risk scores and markers have been introduced to predict CI-AKI after PCI. The Mehran score [6], which incorporates eight scoring variables including age, diabetes, hypotension, anemia, congestive heart failure, contrast media volume, IABP, and eGFR, or serum creatinine, has had repeated validations. However, it included perioperative variables and excluded patients with acute myocardial infarction. This study constructed a CI-AKI prediction model for STEMI patients undergoing emergency PCI. The predictors were all clinically available quantitative indicators, among which the predictive values of preprocedural SII and sd-LDL levels for CI-AKI were rarely reported. The model showed good interpretability and high predictive accuracy.
Similar to previous studies [16–18], this study found that there was no significant difference in white blood cell count and platelet count between the group with and without CI-AKI, while lymphocyte count was lower, neutrophil count, hs-CRP, NLR and PLR were higher in the CI-AKI group. SII is an indirect inflammatory index that combines peripheral blood platelet count, neutrophil count and lymphocyte count, which can reflect the immune and inflammatory status of patients more comprehensively. It was used to predict mortality in patients with malignancy and acute disease [7], and Esenboğa et al. [19] found that elevated SII was independently associated with no recurrent flow in patients with STEMI undergoing PCI. In a recent study, lnSII > 5.91 × 109/L was one of the independent predictors of the development of CI-AKI after PCI in STEMI patients [20]. The pathophysiological mechanisms between SII and CI-AKI have not been fully elucidated. Previous studies have shown that ischemia, nephrotoxicity and endotoxin-induced AKI are associated with an increase in renal infiltrating neutrophils [21]. When the inflammatory activity of the body increases, the release of large amounts of anti-inflammatory factors leads to apoptosis of lymphocytes and impairment of the body’s immune and antioxidant defenses, resulting in endothelial dysfunction [18]. In addition, platelet activation can activate pro-inflammatory cytokines, leading to collective microcirculation disorders and increased platelet destruction during severe inflammatory responses, resulting in reduced renal blood flow and oxygen supply [22]. Hence, elevated SII levels may influence the development of CI-AKI through inflammatory pathways and overactive coagulation pathways.
Clinical studies have found that sd-LDL is more likely to be phagocytic and transformed into foam cells by macrophages than LDL, and it is difficult to identify and remove, ultimately causing atherosclerosis [23]. Previous studies have also confirmed that oxidized HDL (ox-HDL) and LDL undergo oxidative changes during the progression of renal disease, promoting the formation of small lipoproteins and an increase in oxidized LDL (ox-LDL), with reduced clearance and increased levels of sd-LDL favoring its entry into the arterial wall, leading to renal and vascular damage, causing glomerular obstruction and microcirculatory disturbances, and promoting renal ischemia and hypoxia [24]. In addition, oxidized lipoproteins are more likely to bind to glomerular basement membrane through polyanionic glycosaminoglycans. After glomerular basement membrane damage, a large amount of lipoproteins enter glomerular endothelial cells, which are then presented to foam cells derived from thylakoid cells, macrophages and vascular smooth muscle cells, further aggravating glomerular basement membrane thickening, extracellular matrix expansion, glomerulosclerosis, capillary stenosis and even blockage [25]. Therefore, the renal hemodynamic alterations caused by elevated serum sd-LDL levels may be significant.
The present study showed that advanced age was a risk factor of CI-AKI, which is consistent with other risk scores [14]. The reason may be related to the progressive decline in residual renal function of patients as vascular stiffness increases with age and endothelial function decreases. This study suggests that patients with lower LVEF are more likely to develop CI-AKI, which may be due to organ hypoperfusion caused by chronic ischemia and hypoxia. Long-term low cardiac output exacerbates organ injury, further leading to impaired renal function and poor prognosis [26]. Previous studies have shown a strong relationship between baseline renal function and the risk of CI-AKI, which is further increased after a decrease in baseline renal function [27]. This study also found that with decreased eGFR, patients had a significantly increased risk of CI-AKI. This study indicates that elevated uric acid levels are associated with the occurrence of CI-AKI. When serum uric acid elevated, it was related with vascular endothelial cell dysfunction, inflammation, and renin-angiotensin-aldosterone system (RAAS) activation. Several studies have reported an association between hyperuricemia and the incidence of CI-AKI [28]. In addition, the new Mehran score suggests that contrast dose and hemoglobin are also independent risk factors for CI-AKI [14]. It has been shown that estrogen inhibits erythropoietin production, which inhibits apoptosis, promotes renal tubular epithelial cell regeneration, and ameliorates ischemia- and hypoxia-induced renal injury [29]. Hence, erythropoietin deficiency in hypoglobulinemia patients may lead to further deterioration of renal function [30]. However, the present study did not yield similar results, possibly due to a bias from the significant gender imbalance caused by the larger proportion of male patients in this study. There was no statistical difference between contrast dose and CI-AKI in our study cohort. Previous studies have shown that complications and hemodynamic instability after contrast agent exposure are more associated with AKI than high-dose contrast agent, and that reducing the dose of contrast agent within a certain range does not reduce the risk of CI-AKI [31].
This study also had several limitations that require attention. First, this was a single-center retrospective study, and some patients were excluded due to missing variables, which may be subject to selection bias. Second, the present study failed to compare with other CI-AKI scoring systems, such as the Mehran score. Therefore, multi-center and expanded sample sizes are still needed to further confirm the predictive value of SII and sd-LDL in the development of CI-AKI after emergency PCI in patients with STEMI.
Collectively, the occurrence of CI-AKI after emergency PCI in STEMI patients was associated with age, LVEF, sd-LDL, uric acid, eGFR, and SII levels. The combination of SII and sd-LDL has a high predictive value for CI-AKI after PCI. It provides a reference possibility for clinicians to rapidly identify high-risk and low-risk patients for enhanced or simplified acute kidney injury prevention measures.
AUC: area under the curve
CI: confidence interval
CI-AKI: contrast-induced acute kidney injury
eGFR: estimated glomerular filtration rate
HDL-C: high-density lipoprotein cholesterol
hs-CRP: high-sensitivity C-reactive protein
LVEF: left ventricular ejection fraction
NLR: neutrophil-to-lymphocyte ratio
PCI: percutaneous coronary intervention
PLR: platelet-to-lymphocyte ratio
ROC: receiver operating characteristic
sd-LDL: small and dense low-density lipoprotein
SII: systemic immune-inflammation index
STEMI: ST-segment elevation myocardial infarction
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/1001114_sup_1.pdf.
GS: Conceptualization, Validation, Investigation, Visualization, Writing—original draft, Writing—review & editing. HH: Methodology, Software, Formal analysis, Data curation, Writing—review & editing. ZW: Software, Validation, Investigation. HQ: Investigation. YZ: Investigation. DZ: Investigation. YD: Investigation. YL: Writing—review & editing. WL: Conceptualization, Methodology, Formal analysis, Writing—review & editing, Supervision.
The authors declare that they have no conflicts of interest.
The study protocol was approved by the hospital local Ethics Committee (ethics number: XYFY2021-KL024-01).
All selected patients provided written informed consent for PCI.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Ishfaq Rashid ... Gautam Sahu
Randa Choueiri ... Vanessa Nseir
