Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, Punjab 160062, India
ORCID: https://orcid.org/0000-0001-8816-3987
Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, Punjab 160062, India
Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, Punjab 160062, India
Email: ptiwari@niper.ac.in
ORCID: https://orcid.org/0000-0003-0442-7880
Affiliation:
2Department of General Medicine, Government Medical College and Hospital, Chandigarh 160030, India
ORCID: https://orcid.org/0000-0001-8831-8002
Affiliation:
3Department of Biochemistry, Government Medical College and Hospital, Chandigarh 160030, India
Affiliation:
1Department of Pharmacy Practice, National Institute of Pharmaceutical Education and Research, S.A.S. Nagar, Punjab 160062, India
Explor Med. 2022;3:249–259 DOI: https://doi.org/10.37349/emed.2022.00089
Received: March 26, 2022 Accepted: May 18, 2022 Published: June 23, 2022
Academic Editor: Yingyong Zhao, Northwest University, China
The article belongs to the special issue Disease Diagnosis, Molecular Mechanism and Therapeutic Strategies in Kidney Injury and Fibrosis
Aim: Hyperuricemia as a putative risk factor for chronic kidney disease (CKD) progression remains controversial and debatable. This systematic review aims to explore the prevalence of hyperuricemia among CKD patients worldwide.
Methods: This study was conducted in accordance with the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines by using the existing literature from online databases such as MEDLINE/PubMed, ScienceDirect, Google Scholar, Cochrane library and grey literature. The effect size with corresponding 95% confidence interval (CI) was calculated to assess the pooled prevalence of hyperuricemia in chronic kidney patients. The subgroup analysis based on gender and geography was also carried out by utilizing comprehensive meta-analysis, version 2.0.
Results: Twenty-three studies containing 212,740 participants were eligible for quantitative synthesis. The pooled prevalence of 43.6% (35.2–52.4%) hyperuricemia was reported in patients with CKD globally. In India, 38.4% of prevalence was observed. The gender specific prevalence (9 studies) was reported as 67.4% (60.9–73.3%) in case of male patients and 32.6% (26.7–39.1%) in female patients with 95% CI.
Conclusions: The prevalence of hyperuricemia was reported to be reasonably high among CKD patients worldwide. During the management of CKD, this high prevalence demands more prudent attention for this clinical complication which possibly can lead to positive renal outcomes.
Chronic kidney disease (CKD) is a major public health problem worldwide due to its high prevalence, morbidity and mortality. CKD has a globally estimated prevalence of 13.4% (11.7–15.1%), and the patients with advanced CKD like end-stage renal disease who require renal replacement therapy (RRT) are estimated between 4.902 and 7.083 million [1].
In the United States, 37 million people or 15% of adults, that is more than 1 in 7, are estimated to have CKD and as many as 9 in 10 adults with CKD are totally unaware of their illness [2, 3]. In the United States, the CKD is slightly more dominant in females (14%) than male (12%) population [2].
Asia accounts for 434.3 million adults suffering from CKD ranging from 350.2 to 519.7 million with 95% confidence interval (CI). Out of 434.3 million, 65.6 million (42.2–94.9) of population belong to advanced CKD category. China occupies the major share 159.8 million (95% CI 146.6–174.1) of adults living with CKD while India has 140.2 million (95% CI 110.7–169.7) of adult CKD population. Both China and India are the big giants having 69.1% of CKD prevalence all across the Asia [4].
CKD progression is panic and inevitable. In India, the true burden of end stage renal disease (ESRD) is unknown, the reason being a lack of universal access to RRT, with few dedicated centers for care, and absence of a renal registry. In India, a majority of the patients (90%) requiring RRT attain mortality because of inability to afford care, and even the worst condition is that the people who start the RRT around 60% of them stop the treatment for the financial reasons [5]. A session of hemodialysis costs approximately US $9–45 (without costs for allotted space and machines), but expenses incurred by the patient vary from region to region [5].
Due to challenges in access to care, most of the CKD cases remain undiagnosed. Around 50% of patients with advanced CKD are first seen when the estimated glomerular filtration rate (eGFR) is < 15 mL/min per 1.73 m2 [6]. This sobering number warrants the need for robust screening programs for those at risk for CKD.
Cardiovascular disease (CVD) is the major cause of the early morbidity and mortality sustained by patients with CKD. CKD is also an important predictor of premature deaths and disability-adjusted life years among CVD. The management of CKD is challenging as it is associated with various complications like malnutrition, hyperuricemia, inflammation, anemia, etc.
Hyperuricemia is one of the most serious complications of CKD. Serum uric acid (UA) is a metabolic product of purine nucleotides and a normal component of urine. It possesses slight solubility in water and comfortably forms crystals. The human body comprises a total body pool of exchangeable UA (1200 mg and 600 mg) in men and women, respectively [7]. Hyperuricemia is technically an elevated UA level (upper limit 6.8 mg/dL, and anything over 7 mg/dL) in the blood and followed by symptoms. This elevated level occurs either due to increased production, or decreased excretion of UA, and/or a combination of both processes.
CKD is extremely prevalent worldwide but early identification of patients who are at high risk of entering into ESRD is very challenging. The patients with CKD develop many complications like malnutrition, proteinuria, inflammation and hyperuricemia, which leads to higher risk of comorbidities, medical cost and mortality.
The elevated UA level in the blood is a recognized risk factor of CKD progression [8], but it remains controversial and debatable due to insufficient evidence. Several studies report that hyperuricemia is an independent risk factor for incident cohort; however, some reported there is no statistically significant relationship between hyperuricemia and incident CKD [9–14]. The results from interventional studies have underlined that allopurinol a urate lowering drug delays the CKD progression [15, 16], and few other evidences demonstrate that hyperuricemia may potentiate the susceptibility to hypertensive kidney damage through disrupted auto-regulation of glomerular hemodynamics [17].
The prevalence of hyperuricemia is poorly characterized in this chronic illness all across the globe. Also, a very little is known about gender differences in case of hyperuricemia associated CKD. In light of this, this study aims to explore the prevalence of hyperuricemia among CKD patients worldwide.
To identify the desired articles, a systematic literature search was performed by using MEDLINE/PubMed, ScienceDirect, and Cochrane library databases. The search methods were extended manually to find out the relevant articles missed out in primary search. This meta-analysis has adopted the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [18]. The study protocol has been registered (PROSPERO: CRD42021230385) at PROSPERO which is the International Prospective Register of Systematic Reviews.
The literature search was carried out on 25th December, 2020 (mentioned in PROSPERO protocol) after the discussion with a technical expert for conducive inclusion sensitivity. The literature search on PubMed/MEDLINE databases utilized advanced filters and Medical Subject Headings (MeSH) terms: (“Prevalence”[MeSH Terms] OR “epidemiology”[MeSH Terms] OR “incidence”[MeSH Terms]) AND (“hyperuricemia”[MeSH Terms] OR “gout”[MeSH Terms] OR “uric acid”[MeSH Terms] OR “HUA”[Title/Abstract] OR “HU”[Title/Abstract]) AND (“renal insufficiency, chronic”[MeSH Terms] OR “Chronic Renal Insufficiencies”[Title/Abstract] OR “Chronic Kidney Diseases”[Title/Abstract] OR “Chronic Kidney Insufficiency”[Title/Abstract] OR “renal disease chronic”[Title/Abstract] OR “disease chronic renal”[Title/Abstract] OR “Diabetic Kidney Disease”[Title/Abstract]). Similar search strategy was applied to other databases. The study characteristics and quality assessment scores are represented in Tables S1 to S3.
The published articles featuring CKD patients reporting the prevalence of hyperuricemia from all age groups were selected for quantitative analysis. There has been no restriction on the year of publication, dialysis modality or stages of CKD. The study design was confined to observational studies only. The exclusion criteria set for eligibility were quite diverse, such as any study with disputed discrepancies in data or date repetition or lack of desired data or language barrier was left out.
The outcomes of the study include pooled estimate reported as effect size of hyperuricemia prevalence in patients with CKD worldwide and the pooled estimate of subgroups based on geography, and gender.
After the primary screening of articles on the basis of title and abstract by two independent authors, the articles were assessed for duplication by cross referencing. The data from eligible articles were retrieved by two authors (IR, PK) on the basis of journal name, title of the article, abstract, publication year, study population, sample size, outcomes, and study location, etc. Any sort of disagreement with the data was amicably resolved by the other three authors (PT, SD, and SJ). The articles with incomplete data and language barriers were left out. The data extracted was preserved in Microsoft excel spreadsheet under column headings: study title, journal name, year of study, study design, 1st author’s name, study location, diagnostic criteria for hyperuricemia, sample size, number of patients with hyperuricemia, and the number of patients with normal uric acid levels.
A Newcastle-Ottawa Scale (NOS) [19] was employed to critically appraise the qualified studies for quality assessment by a couple of authors, independently. The NOS allocates a score of maximum 9 points on the basis of selection, comparability and outcomes of study populations. This scale is frequently used in the prevalence based meta-analysis studies. The study quality scores were predefined as poor (0–3), fair (4–6), or good (7–9). The studies having quality score less than 50% were excluded from the analysis. Any sort of controversy between the authors with respect to quality of studies was firmly determined by formal consensus from all the authors.
The statistical analysis was performed by using licensed comprehensive meta-analysis version 2.0. The random effect model was preferred over fixed effect model to determine the pooled estimate based on the methods of DerSimonian and Laird [20].
The variability due to heterogeneity was estimated by I squared (I2) statistic. The values of I2 > 50% and P < 0.10 were taken as significant determinants for heterogeneity. The funnel plot was used to assess the publication bias. The presence of symmetry reflects absence of publication bias, whereas asymmetry reflects publication bias. The effect size of studies was taken as the overall prevalence and prevalence across all subgroups gender (male and female), and geography (comparison of prevalence in India with other countries). Meta-regression analysis was also conducted to assess the effects of different covariates (gender, mean age, systolic blood pressure, diastolic blood pressure and geography) in predicting the prevalence of hyperuricemia (significant at P < 0.05).
One hundred forty-nine studies were identified. The primary screening based on title and abstract excluded 71 studies due to irrelevance with the predefined eligibility criteria.
Based on full text screening out of 78 articles only 23 studies containing 212,740 participants met the inclusion criteria (Figure 1). From the 23 studies, only 9 have reported gender specific data with respect to prevalence of hyperuricemia in patients with CKD.
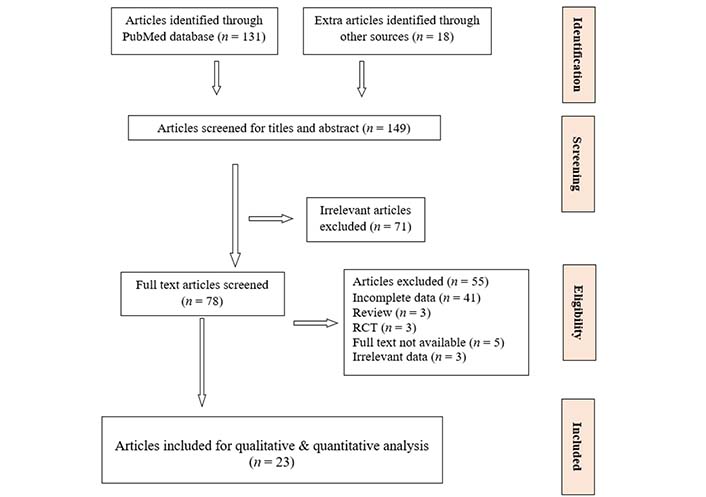
Systematic review PRISMA flowchart of research articles screened, excluded and included in meta-analysis. PRISMA: Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT: randomized controlled trials
An extremely high prevalence 43.6% (95% CI) of hyperuricemia ranging from 35.2% to 52.4% was observed in CKD patients [17, 21–42] (Figure 2). The heterogeneity and publication bias were performed for all the 23 studies included for quantitative analysis. A significant heterogeneity was observed (P < 0.05, I2 = 99.6%), however, the heterogeneity decreased slightly in the subgroup analysis. Egger’s test for a regression intercept gave a P-value of 0.007, while funnel plot shows asymmetry which indicates a publication bias is present.
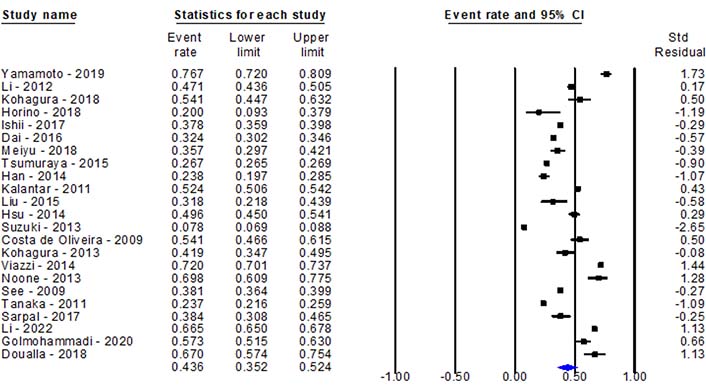
Pooled prevalence of hyperuricemia among chronic kidney disease patients worldwide. Std: standard
The prevalence of hyperuricemia among CKD patients was also analyzed for different subgroups such as geography, and gender (Figure 3 and Figure 4).
The countries which have reported more than two studies were acceptable for subgroup analysis. Iran comprised two studies which had reported highest 54.9% (34.7–73.6%) pooled prevalence of hyperuricemia in patients with CKD [29, 41].
Japan occupies the highest share of prevalence studies (7) on hyperuricemia in patients with CKD. In Japan, the pooled prevalence was found to be 27.9% (19.7–37.9%) [16–19, 22, 28, 32] while Taiwan China and Mainland China had reported 51.5% (35.2–67.6%) and 36.8% (24.2–51.4%) of prevalence respectively [17, 22, 26, 30, 31, 37, 40] (Figure 3).
The country-based estimation of hyperuricemia prevalence was extended to countries which have reported only one study. A study conducted in the United Kingdom (only study conducted among pediatric patients) had a prevalence of 69.8% (60.9–77.5%) [36], The United States [21] had a prevalence of 76.7% (72.0–80.9%), Italy [35], Brazil [33] and Cameroon [42] had a prevalence of 72.0% (44.6–89.1%), 54.1% (26.3–61.5%) and 67.0% (57.4–75.4%), while India [39] and South Korea [28] had reported a prevalence of 38.4% (15.7–67.5%) and 23.8% (8.8–50.5%) respectively.
The gender specific prevalence was reported in 9 studies out of 23 studies enrolled for the analysis (Figure 4). Notably, there is a remarkable difference between males and females. The results demonstrate that gender may also be closely associated with hyperuricemia, as the prevalence in case of male patients 67.4% (60.9–73.3%) is considerably higher than female patients 32.6% (26.7–39.1%) with 95% CI (Figure 4) [21, 23, 25, 29, 32, 34, 39–41].
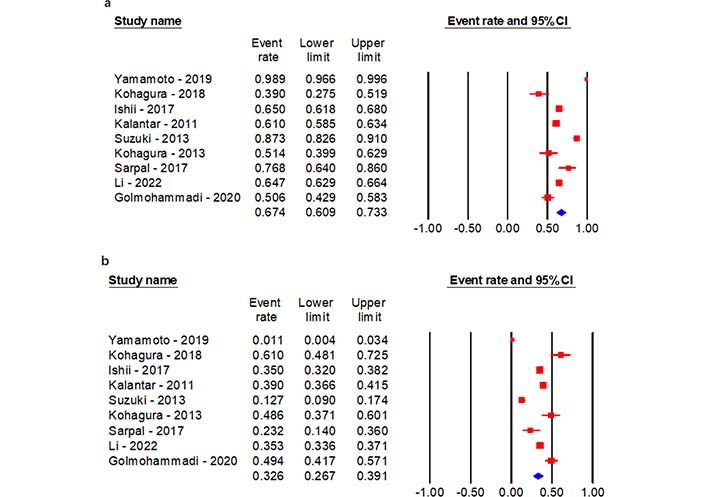
Gender specific prevalence of hyperuricemia in CKD patients. (a) Males; (b) females
The publication bias was quite evident from the funnel plot. A P-value of 0.05 was reported by performing Egger’s test for regression (P < 0.007) and the I2 statistic was found to be 99.5% which indicates a massive publication bias. No major difference in the heterogeneity was observed while assessing the heterogeneity for the different subgroups like gender (I2 = 99.4%), and geography (I2 = 99.6%). There was also no difference observed in the heterogeneity between the studies while performing one-study-removed sensitivity analysis.
The quality assessment was done on the basis of NOS [19] components like selection, comparability and outcomes of study population. A maximum of 9 points were allocated for the quality of studies. Out of total 23 studies, the majority of studies (12) had scored 8 points/stars, 8 studies scored 7 points/stars and 2 studies scored 9 points/stars. Only 1 study had scored 6 points/stars. Fourteen studies were having good quality while nine studies have reported fair quality (Table S2).
The meta-regression analysis was attempted to determine the effect of different covariates (average age, mean systolic and diastolic blood pressure and geography) on prevalence. The average age which was reported had shown significant association (P < 0.001, R2 = 0.00) as was geography (P = 0.009, R2 = 0.52), with the prevalence of hyperuricemia in CKD patients. There was no significant association between gender and dependent variable (prevalence). Here R2 quantifies the proportion of variance likely explained by these covariates.
This systematic review provides a comprehensive overview of the current literature pertaining to the prevalence of hyperuricemia associated with CKD patients worldwide. In CKD patients, hyperuricemia is believed to be an independent risk factor for composite renal outcomes (commencement of dialysis, doubling of creatine from the baseline or reduction in eGFR).
Subgroup analysis based on geography stated that the results reported from Iran had the highest pooled prevalence 54.9% (34.7–73.6%) of hyperuricemia in comparison to Taiwan China 51.5% (35.2–67.6%) followed by Mainland China and Japan 36.8% (24.2–51.4%) and 27.9% (19.7–37.9%) respectively. This study also reveals that there is a scarcity of data with respect to pediatric age group across the globe. A study conducted by Noone et al. [36] happens to be the only single study reporting the hyperuricemia scenario 69.8% (60.9–77.5%) among CKD patients of pediatric age group. Noone et al. [36] from the United Kingdom also stated clearly a significant association was observed between elevated serum UA levels and renal dysfunction.
In the countries where only one study pertaining to hyperuricemia among CKD was published, the geographical stratification of results revealed that the United States exhibits the soaring prevalence of hyperuricemia 76.7% (72.0–80.9%) in the adult age group [21]. This was followed by Italy, Cameroon and Brazil which have a prevalence of 72.0% (44.6–89.1%), 67.0% (57.4–75.4%), and 54.1% (26.3–61.5%). The lowest prevalence was found in South Korea 23.8% (8.8–50.5%). The India scenario regarding hyperuricemia in CKD patients was better than Italy, Brazil and Iran. Prevalence of 38.4% (15.7–67.5%) has been observed in India. A strong association of advanced age, male gender, hypertension, diabetes and higher degree of renal dysfunction (ESRD) with hyperuricemia has been reported by Sarpal et al. [39] from India. Iseki et al. [43] have reported the same findings that elevated serum UA levels are associated with a greater incidence of ESRD. Li et al. [44] from Taiwan China had also reported that the prevalence of UA stones was predominantly higher in patients with advanced CKD. Liu and co-workers [45] from China had also come up with the same findings that a long-term exposure to relatively elevated serum UA, regardless of being within the normal range, elevates risk of renal function decline by nearly 2 folds. While Japan had reported increased serum UA levels were significantly associated with the incidence of albuminuria [46].
Scarce information is known about gender specific prevalence of hyperuricemia in patients with CKD. An interaction between gender and hyperuricemia in CKD patients was recognized in 6 studies. The overall prevalence was found to be 40.0% (18.9–65.6%). The pooled prevalence in case of males 25.7% (11.3–48.5%) was found to be two times higher as compared to females 7.6% (2.6–20.6%) with 95% CI. That males are predominantly affected with hyperuricemia also was reported by Yamamoto et al. [21] and Yang et al. [47].
This exhaustive meta-analysis of observational studies substantiates that hyperuricemia is deep rooted in CKD patients. A global prevalence of (31.7–46.5%) hyperuricemia among CKD patients was observed using the existing literature which has been weighed by quality and rigorous methodology. The elevation of serum UA is quite evident throughout all regions in the world, where it has been measured, but a considerable geographic variation exists.
In light of this, the future research should focus on intervention strategies which are deliverable at scale to improve the renal outcomes thus delaying the CKD progression. Also, the impact of these interventions and the associated costs needs to be evaluated simultaneously. The prevalence of hyperuricemia in patients with CKD needs to be further evaluated as it is poorly characterised with respect to CKD stages, dialysis, diabetic status and geography.
The prevalence of hyperuricemia was reported to be reasonably high among CKD patients worldwide. The pooled prevalence in Taiwan China was quite higher as compared to Mainland China, and Japan. The male patients have dominated the proportion of prevalence. During the management of CKD, this high prevalence demands more prudent attention for this clinical complication which possibly can lead to positive renal outcomes.
To the best of our knowledge, this is the first worldwide estimation of hyperuricemia in CKD patients. In subgroup analysis, a significant difference in prevalence was noticed between males and females. As this review involves cross-sectional, cohort and case control studies, the variation due to study designs (observational) was beyond our control.
CI: confidence interval
CKD: chronic kidney disease
ESRD: end stage renal disease
I2: I squared
MeSH: Medical Subject Headings
NOS: Newcastle-Ottawa Scale
PROSPERO: International Prospective Register of Systematic Reviews
RRT: renal replacement therapy
UA: uric acid
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/100189_sup_1.pdf.
The authors are extremely grateful to mentor institute for providing access to comprehensive meta-analysis software package.
Conception and design of the study: PT, SD, SJ, IR; methodology: IR, PT; data extraction: IR, PK, GS; statistical analysis: IR, PK, GS; draft of the manuscript: IR, PK; supervision: PT, SD, SJ. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All datasets generated for this study are included in the manuscript and the supplementary files.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
