Affiliation:
1Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, Newark, NJ 07103, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-2628-6217
Affiliation:
2Division of Cardiology, Department of Medicine, University of California San Francisco, San Francisco, CA 94158, USA
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0002-7561-052X
Affiliation:
1Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0000-0002-9155-2059
Affiliation:
1Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, Newark, NJ 07103, USA
ORCID: https://orcid.org/0009-0001-4594-1205
Affiliation:
3Rutgers University–Molecular Design & Synthesis Core, Piscataway, NJ 08854, USA
ORCID: https://orcid.org/0000-0002-7810-4139
Affiliation:
1Department of Cell Biology and Molecular Medicine, Rutgers New Jersey Medical School, Newark, NJ 07103, USA
Email: vatnersf@njms.rutgers.edu
ORCID: https://orcid.org/0000-0003-0521-8634
Explor Endocr Metab Dis. 2025;2:101445 DOI: https://doi.org/10.37349/eemd.2025.101445
Received: July 30, 2025 Accepted: October 20, 2025 Published: November 05, 2025
Academic Editor: Esma R. Isenovic, University of Belgrade, Serbia
The article belongs to the special issue Innovative Strategies for Diabetes and Metabolic Disorders: Current and Future Directions
Adenylyl cyclase 5 knockout (AC5 KO) is a healthful longevity model; not only do the AC5 KO mice live a third longer than wild-type (WT) mice, but they are also protected against obesity, diabetes, heart failure, and exercise intolerance, mediated by anti-apoptosis, cell survival, myocardial biogenesis, and anti-oxidative stress mechanisms. To translate these salutary effects to the clinics, we developed a drug, C90, which recapitulates the AC5 KO model of healthful longevity. We then examined its effects on glucose tolerance and exercise capacity. C90 (30 mg/kg/day) or vehicle was chronically administered to age-matched C57BL/6 mice via an osmotic pump. The WT mice receiving C90 exhibited improved glucose tolerance, following glucose i.v. injection, when compared to the vehicle. Furthermore, the C90-treated mice had a lower fasting glucose level when compared to the vehicle-treated mice (113 ± 6.5 mg/dL vs. 129 ± 4.2 mg/dL, p < 0.05). Additionally, the WT group that received C90 exhibited greater exercise capacity, reflected by longer running distance (384 ± 27 m vs. 253 ± 16 m, p < 0.05) and greater work to exhaustion (18.1 ± 1.5 J vs. 12.4 ± 0.7 J, p < 0.05) than mice receiving vehicle. In view of these findings, C90 is an excellent candidate for clinical development as an effective pharmacological treatment for glucose intolerance and enhancing exercise performance.
Adenylyl cyclase mediates increased sympathetic tone through beta-adrenergic receptor stimulation, resulting in increased cardiac function and exercise performance when stimulated. Importantly, increased exercise capacity is almost always associated with increased sympathetic tone [1–3]. Paradoxically, adenylyl cyclase 5 knockout (AC5 KO) is a healthful longevity model with decreased sympathetic tone [4], and with protection against glucose intolerance [5], obesity [5], heart failure [6–8], and enhanced exercise capacity [9] (Figure 1). The goal of this investigation was to determine whether C90, an AC5 inhibitor, recapitulates the salutary features observed in AC5 KO mice, including enhanced exercise capacity and glucose tolerance. Healthful longevity in AC5 KO mice is mediated by anti-apoptosis, cell survival, myocardial biogenesis, and anti-oxidative stress mechanisms [4–9]. The cellular mechanisms responsible are noted in Figure 1.
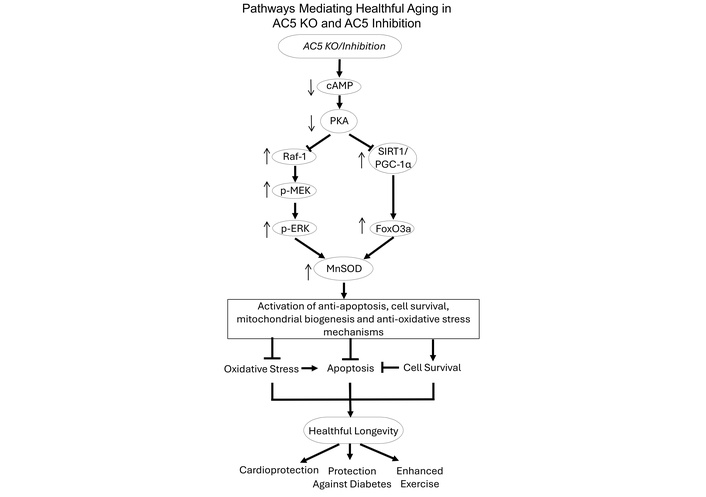
Pathways mediating healthful aging in AC5 KO and AC5 inhibition. The mechanisms mediating the healthful longevity, enhanced exercise performance, cardioprotection, and protection against diabetes in the AC5 KO mice and the AC5 pharmacological inhibitor are shown. Deletion or inhibition of AC5 reduces intracellular cAMP production, which leads to less activation of protein kinase PKA. The reduced activity of PKA leads to increased activity of Raf-1/MEK/ERK and SIRT1/PGC-1α/FoxO3a, ultimately increasing MnSOD, inducing healthful longevity with activation of anti-apoptosis, cell survival, mitochondrial biogenesis, and anti-oxidative stress. AC5 KO: adenylyl cyclase 5 knockout; PKA: protein kinase A; SIRT1: sirtuin 1; PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha; FoxO3a: forkhead box O3.
The AC5 inhibitor, C90, the focus of this investigation, is a pharmacological analog of the AC5 KO, which we have developed and found to protect against myocardial infarction and acute heart failure in mice, and protects against chronic atherosclerosis in rabbits [10]. C90 has several features making it superior to other AC5 inhibitors, i.e., it is a more specific inhibitor and less toxic, and more soluble, and can be structured for future oral use [10, 11]. Our previous study demonstrated the efficacy and selectivity of C90 as an AC5 inhibitor [10]. C90 reduced forskolin-induced AC activity and cAMP in wild-type (WT) mice, compared with vehicle. However, in AC5 KO, C90 no longer reduced cAMP in response to forskolin, indicating that the mechanism of reduction by C90 involved AC5 inhibition and not just inhibition of any AC isoform [10].
The AC5 KO and the AC5 inhibitor both decrease sympathetic tone [4, 9], making this therapeutic approach more favorable for treating patients with heart failure or coronary artery disease. It would be important to translate these beneficial effects to patients. Whereas all these features in the AC5 KO are important for healthful aging, the enhanced exercise capacity is also therapeutic for most diseases, as well as normal health. Therefore, a pharmacological compound that recapitulates these features would have considerable clinical appeal. With advancing age, it becomes increasingly important to find a pharmacological moiety to combat the deleterious influences of aging and enhance those functions that are beneficial for healthful aging, e.g., improved glucose tolerance and increased exercise capacity. It is our hypothesis that the AC5 inhibitor, C90, will fill this gap by replicating the salutary effects observed in AC5 KO mice.
All experiments were performed on 3–5-month-old male C57BL/6 mice. All mice were housed at Rutgers New Jersey Medical School. All experiments were conducted at Rutgers New Jersey Medical School. For exercise studies, all mice were matched for body weight. Animals were all placed on standard chow and had free access to water for the duration of the study. Animals used in this study were maintained, and all experiments were performed, in accordance with the Guide for the Care and Use of Laboratory Animals (National Research Council, 8th ed., 2011). All animals were kept on a standard 12:12-hour light-dark cycle. These studies were approved by the Institutional Animal Care and Use Committee of Rutgers New Jersey Medical School.
The AC5 inhibitor C90 was synthesized [12] and subcutaneously administered at 30 mg/kg/day or its vehicle via osmotic pumps as previously described [10, 13] for 7 and 14 days for exercise capacity, and for 14 days for the glucose tolerance test. After mice were anesthetized with isoflurane at 2–3% in oxygen, ALZET pumps (Durect, Cupertino, CA) were implanted to deliver C90 or vehicle to mice at a concentration of 30 mg/kg/day for 7 days of treatment (0.5 µL/h, 1007D) and for 14 days of treatment (0.25 µL/h, 1002). The doses were selected from prior work on other AC5 inhibitors [14]. After preparation, the pumps were incubated overnight and implanted subcutaneously onto the back of the mice the following day. Cefazolin (100 mg/mL) was given intramuscularly in the quadriceps after surgery. The mice were allowed to recover for 7 days before exercise testing began [13].
Glucose tolerance test mice (n = 8/group) were fasted for 6 h prior to initiation of the glucose tolerance test [5]. A 50 µL blood sample was drawn from a venous tail puncture at the end of the fasting period for basal glucose measurement with a glucometer (Accu Check, Roche, Indianapolis, IN, USA). A dose of dextrose (50% solution, 1 g/kg body weight) was injected intraperitoneally, and blood was drawn at 15, 30, 60, 90, 120, and 180 min after injection for glucose measurements. The area under the curve (AUC) of the glucose levels over time was calculated and compared in the vehicle and treated groups.
Mice (n = 6/group) were exercised on a treadmill (AN5817474, Accuscan Instruments, Columbus, OH, USA) attached to a metabolic chamber to measure maximum exercise capacity. Mice were subjected to a practice trial 3 days before the experiment to adapt to the treadmill testing environment [9, 13, 15].
Food was withdrawn 3 h before exercise testing. All mice were exercised at the same time of day for each experiment. All exercise testing was done by the same investigator, blinded to the treatment group. At the time of the experiment, each mouse was placed on the treadmill with a constant 10% grade. The treadmill started at 4 m/min, and the speed increased incrementally by 2 m/min every 2 min until the mice reached exhaustion. At the end of each treadmill lane was a rod that delivered a shock, which served as negative reinforcement for cessation of running. Work to exhaustion was defined as spending 10 seconds on the rod without attempting to reengage the treadmill belt. The indices of exercise capacity measured were maximal distance and work to exhaustion [9, 13, 15].
In accordance with institutional and federal guidelines for the ethical treatment of animals, euthanasia was performed when animals exhibited signs of severe distress, pain, or illness that could not be alleviated and when continued participation in the study would compromise their welfare. Euthanasia was carried out using approved methods to ensure rapid and painless death.
All data are expressed as mean ± standard error of the mean (SEM). To compare two independent groups, we used the Student’s unpaired t-test, whereas for more than two variables, two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons test was used. p < 0.05 was taken as the level of significance.
Animals were age-matched prior to randomization. Randomization was performed using a computer-generated sequence to assign animals to experimental groups, ensuring balanced distribution across treatment conditions. All measurements listed below were conducted in a blinded manner, with investigators unaware of group assignments during data collection and analysis to minimize bias.
Sensitivity to glucose was measured using a glucose tolerance test in mice treated with C90 (Figure 2A). Following a 14-day treatment, the C90-treated mice exhibited an overall improvement in glucose tolerance (Figure 2B), measured as AUC, when compared to vehicle-treated mice (6,414 ± 890 mg/dL·min vs. 9,658 ± 1,039 mg/dL·min, p < 0.05). Furthermore, C90-treated mice had a lower fasting glucose level when compared to vehicle-treated mice (113 ± 6.5 mg/dL vs. 129 ± 4.2 mg/dL, p < 0.05).
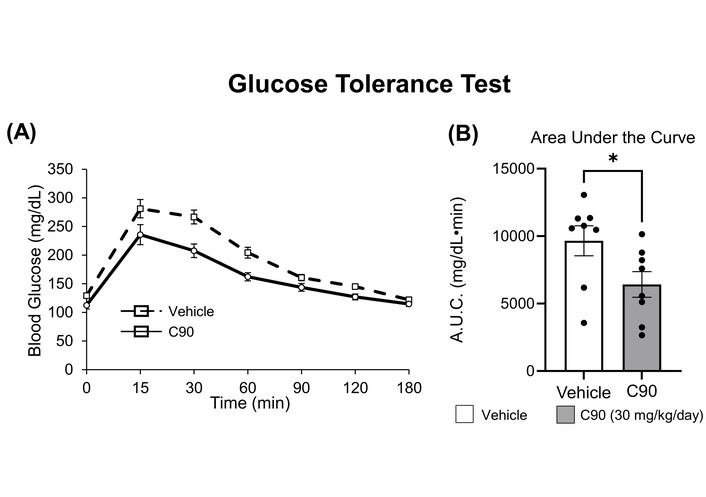
C90 improves glucose tolerance in wild-type (WT) C57BL/6 mice. 3–5-month-old male C57BL/6 were chronically treated with C90 at 30 mg/kg/day via osmotic pumps for 14 days. The glucose tolerance test was examined after 2 weeks of treatment. Compared to the vehicle group, WT mice that received C90 display improved glucose tolerance, as shown in the curve (A) and area under the curve (B). *p < 0.05, by Student’s unpaired t-test. n = 8 in each group.
C57BL/6, male, age-matched mice treated with C90 (30 mg/kg/day), via an infusion pump, exhibited a greater running distance (358 ± 28 m vs. 222 ± 21 m, p < 0.05) (Figure 3A) and a 53% increase in work to exhaustion (p < 0.05) (Figure 3B), when compared to their vehicle-treated counterparts following 7 days of treatment. Similar results were observed after 14 days of treatment with greater running distance (384 ± 27 m vs. 253 ± 16 m, p < 0.05) and greater work to exhaustion (18.1 ± 1.5 J vs. 12.4 ± 0.7 J, p < 0.05).
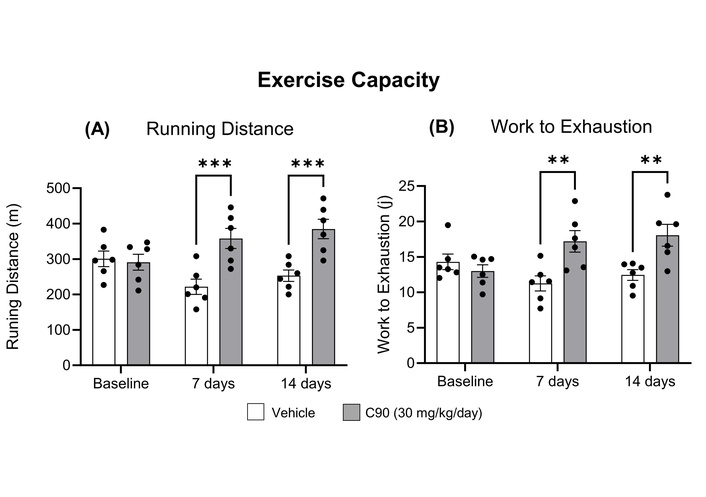
C90 enhances exercise capacity in wild-type (WT) C57BL/6 mice. 3–5-month-old male C57BL/6 were chronically treated with C90 at 30 mg/kg/day via osmotic pumps for 14 days. Maximal exercise capacity was tested at baseline and after 1 and 2 weeks of treatment. WT mice that received C90 showed longer running distance (A) and greater work to exhaustion (B), when compared to the vehicle group, after 1 and 2 weeks of C90 treatment. **p < 0.01, ***p < 0.001 by two-way ANOVA with Sidak’s multiple comparisons test. n = 6 in each group.
After 14-day treatment, no significant differences in body weight were observed between the vehicle and C90 groups.
The results of this investigation further confirm the similarity between the AC5 inhibitor, C90, and the genetic deletion of AC5, as in the AC5 KO mice. We previously found that C90 protects against myocardial ischemia [10], and in this investigation, we found that C90 improves glucose tolerance and exercise performance, which are also observed in the AC5 KO mice [5, 9]. These salutary features of C90 would likely improve healthful longevity and protect against the decline in cardiovascular fitness, development of metabolic syndrome and diabetes, and reduced exercise capacity in the aging population. Exercise reduces cardiovascular risk and improves longevity, and is an independent predictor of all-cause mortality [16–18]. Reduced exercise or functional capacity is a central feature of heart failure, as noted in the New York Heart Association functional classification system [19] and recent reviews [20, 21], used by cardiologists to risk-stratify patients with heart failure, as exercise intolerance strongly correlates with higher mortality [22]. Improving exercise tolerance would be a major therapeutic advance for all patients with heart failure, and potentially all elderly patients and those with other diseases, e.g., obesity and diabetes, that limit their ability to exercise. Therefore, a new therapy for improving exercise tolerance would have almost universal applicability to the aging population and to patients with a wide range of diseases. Furthermore, improving exercise tolerance would also be applicable to the younger, healthy population that routinely exercises for fitness and for long-term healthful longevity. Improved glucose tolerance helps prevent age-related diseases like type 2 diabetes and cardiovascular conditions, supporting longer health span and better metabolic resilience in older adults [23]. Therefore, developing a small molecule AC5 inhibitor that improves exercise capacity and glucose tolerance would be extremely useful clinically.
The pharmacological inhibitor of AC5, C90, is novel in that it improves glucose tolerance and exercise capacity, major features required for healthful longevity. Our previous study has shown C90’s efficacy and selectivity as an AC5 inhibitor [10]. Aging [24] and diseases such as diabetes mellitus [25], heart failure [26], and obesity, among other conditions, negatively impact exercise tolerance. Large-scale clinical trials have successfully focused on reducing mortality and morbidity associated with those disease processes. Yet there is modest progress in improving the quality of life of those patients, which is highly dependent on their functional capacity. There is a significant interest in developing new interventions that enhance exercise tolerance. Still, no FDA-approved pharmacologic treatments are available that can directly improve exercise tolerance.
The treatment of type 2 diabetes and the underlying pathophysiologic mechanism, i.e., insulin resistance, has rapidly evolved. There is currently a broad array of drugs as part of the pharmacologic armamentarium to treat diabetes beyond the older compounds such as insulin, sulfonylureas, biguanides, and thiazolidinediones [27]. Some drugs that have revolutionized diabetes management include the glucagon-like peptide 1 receptor agonists, which have an effective antidiabetic treatment but also have shown an effect on weight loss [28] and reduction of cardiovascular events [29, 30]. Additionally, there are the sodium-glucose co-transporter 2 inhibitors (SGLT-2i) that have a mild effect on diabetes [31, 32]. The improved glucose tolerance found in the AC5 KO mice [5] and in the current data for the AC5 inhibitor (Figure 2) is critical for protection against diabetes and aging, even without diabetes.
Given the broader effects of the AC5 inhibitor, including glucose metabolism, exercise tolerance, obesity prevention, ischemic cardiomyopathy, and longevity prolongation, this drug is unique and an attractive novel treatment for combating the factors impairing healthful longevity. For translation to patients, more chronic studies, more cellular mechanistic studies, more toxicology, larger animal, and sex studies need to be done. The next goal will be to also conduct a longer-term study in a diabetic disease model.
It is interesting that the inhibition of AC5 improves exercise performance, since AC mediates increased beta-adrenergic receptor signaling, which is central to improved exercise. The mechanism is complex but involves selective differences in AC5 from other AC isoforms. In part, the answer comes from the fact that a selective cardiac specific AC5 KO mouse does not improve exercise performance, whereas the total body AC5 KO mouse and the skeletal muscle-specific AC5 KO mouse both increase exercise performance [9]. Therefore, there must be other mechanisms that compensate for the minor contribution of AC5’s increase in total AC activity, e.g., increased mitochondrial biogenesis and reduced oxidative stress [9, 33], which also mediate increased exercise capacity. The AC5 KO and AC5 inhibitor mechanisms mediating healthful longevity are shown in Figure 1. The AC5 KO model of healthful aging, specifically the enhanced exercise capacity, is mediated by upregulated antioxidant (MnSOD), the sirtuin 1/peroxisome proliferator-activated receptor gamma coactivator 1-alpha (SIRT1/PGC-1α) pathway, and mitochondrial biogenesis [9]. The protection against oxidative stress is mediated by reducing cAMP and protein kinase A (PKA), which in turn activates the Raf/MEK/ERK pathway, which increases MnSOD [4] and regulates cardiomyopathy through the AC5, SIRT1, PGC-1α, FOXO3a, and MnSOD pathways [7]. In addition, the improved glucose tolerance is derived from genes regulating mitochondrial biogenesis [5]. A future goal is to determine if the mechanisms mediating the salutary effects of C90 are the same as those for the AC5 KO. In addition, extensive studies on more chronic effects, other models of disease, and toxicology will be needed before a clinical trial can commence. Additional positive features are the statistics showing minimal variability and that no adverse effects of C90 treatment were observed in this study.
The novel AC5 inhibitor, C90, is highly water-soluble, and the toxicology studies performed to date also indicate that this compound is an excellent candidate to advance for human treatment, as it appears to be safe. A critical positive feature of this AC5 inhibitor is that it decreases sympathetic tone while improving exercise capacity, which would be particularly helpful to patients with heart failure and myocardial ischemic disease, where increased sympathetic tone, usually accompanying increased exercise performance, is deleterious.
AC5 KO: adenylyl cyclase 5 knockout
AUC: area under the curve
PGC-1α: peroxisome proliferator-activated receptor gamma coactivator 1-alpha
SIRT1: sirtuin 1
An abstract related to this study was published in the American Heart Association’s 2022 Scientific Sessions (https://www.ahajournals.org/doi/10.1161/circ.146.suppl_1.13767). The publication of this abstract and its data were not influenced by any funding or other interests.
DEV: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Conceptualization, Writing—review & editing. CAB: Formal analysis, Investigation, Methodology, Writing—review & editing. MO: Data curation, Formal analysis, Investigation, Methodology, Visualization, Writing—original draft, Writing—review & editing. JZ: Data curation, Formal analysis, Project administration, Visualization, Writing—original draft, Writing—review & editing. JYR: Methodology, Resources, Writing—review & editing. SFV: Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Conceptualization, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflict of interest.
The animal study protocol was approved by the Institutional Animal Care and Use Committee of Rutgers New Jersey Medical School (protocol code PROTO201900086, 10/11/2022).
Not applicable.
Not applicable.
Data will be made available upon reasonable request.
Study supported by National Institutes of Health grants [R21AG075656]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1246
Download: 28
Times Cited: 0
Huiling Huang ... Dongsheng Li
Huiling Huang ... Dongsheng Li
Maria-Kalliopi Spanorriga ... Konstantinos Tsioufis
