Affiliation:
1Independent Researcher, Shenzhen 518000, Guangdong, China
Email: huilinghuangsz@163.com
ORCID: https://orcid.org/0009-0009-0482-1897
Affiliation:
2Department of Obstetrics, Dongguan Huangjiang Hospital, Dongguan 523000, Guangdong, China
ORCID: https://orcid.org/0009-0008-3518-7684
Affiliation:
3Department of Surgery, Dongguan Huangjiang Hospital, Dongguan 523000, Guangdong, China
ORCID: https://orcid.org/0009-0007-7572-6542
Explor Endocr Metab Dis. 2025;2:101443 DOI: https://doi.org/10.37349/eemd.2025.101443
Received: June 28, 2025 Accepted: September 24, 2025 Published: October 23, 2025
Academic Editor: Peter Schwarz, Medical Faculty Carl Gustav Carus, Germany
The article belongs to the special issue Innovative Strategies for Diabetes and Metabolic Disorders: Current and Future Directions
Background: Metabolic dysfunction-associated fatty liver disease (MAFLD) and type 2 diabetes mellitus (T2DM) frequently coexist, showing a bidirectional relationship. MAFLD increases the risk of T2DM, while T2DM independently raises the likelihood of MAFLD.
Methods: A comprehensive review was carried out on recent systematic reviews and meta-analyses by searching databases including PubMed, Embase, Web of Science, and the Cochrane database of systematic reviews, covering studies from inception to February 2025. Additionally, manual searches of reference lists were conducted. Inclusion criteria involved systematic reviews and meta-analyses of randomized controlled trials (RCTs) evaluating treatment effects on health outcomes in individuals with T2DM and MAFLD.
Results: The search yielded 19 meta-analyses and 112 health outcomes from 622 unique articles. Most analyses focused on treatment effects on endocrine metabolic outcomes (n = 28), lipid metabolic indicators (n = 26), liver health indicators (n = 34), and body composition indicators (n = 24). High-quality evidence indicates that high-intensity interval training improves insulin resistance and low-density lipoprotein cholesterol levels. High-quality evidence also indicates sodium-glucose cotransporter-2 (SGLT-2) inhibitors improved liver proton density fat fraction and fatty liver index, while glucagon-like peptide-1 receptor agonists (GLP-1RAs), particularly liraglutide, enhanced subcutaneous adipose tissue (SAT). Moderate-quality evidence shows that dipeptidyl peptidase-4 (DPP-4) inhibitors enhanced insulin resistance and GLP-1RAs benefited triglycerides, aspartate transaminase, liver fat, and visceral adipose tissue. SGLT-2 inhibitors improved controlled attenuation parameter, body mass index (BMI), SAT, visceral fat mass, and moderate-intensity continuous training improved triglycerides and high-density lipoprotein cholesterol. Fifty-six outcomes were rated as low-quality evidence, and five as very low-quality.
Discussion: GLP-1RAs, SGLT-2 inhibitors, DPP-4 inhibitors, exercise, and Chinese Herbal Medicines benefited liver health, glycemic control in T2DM with MAFLD, and impacted body composition and lipid metabolism.
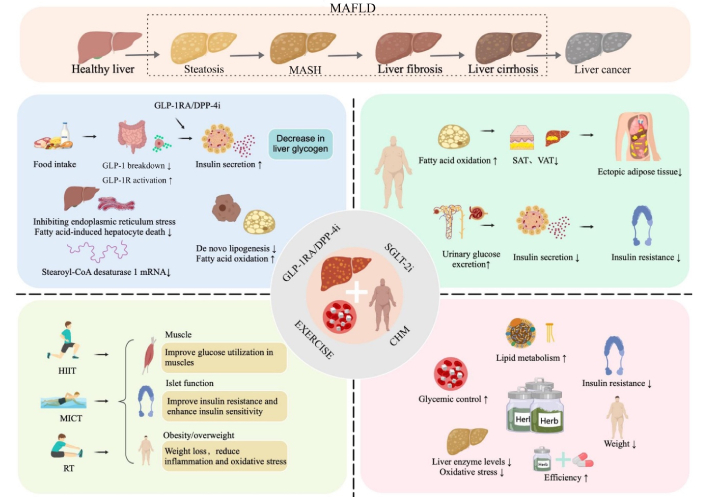
In 2025, the International Diabetes Federation (IDF) reported that 589 million adults aged 20–79 had diabetes globally in 2024. Projections indicate this figure will rise to 853 million by 2050 [1]. Type 2 diabetes mellitus (T2DM), characterized by abnormal glucose metabolism and insulin resistance, accounts for 90% of these cases, with impaired insulin regulation as its primary pathophysiological feature [2, 3]. Metabolic dysfunction-associated fatty liver disease (MAFLD), formerly known as non-alcoholic fatty liver disease (NAFLD), is the leading cause of liver-related diseases globally, affecting approximately 30% of the population [4]. Its prevalence has surged from 391.2 million in 1990 to 882.1 million in 2017 [5]. Prevalence rates of MAFLD differ across regions, with Asia at 31.6%, Europe at 32.6%, and North America at an estimated 47.8%, and patient numbers are rising [6]. MAFLD is a chronic liver disease excluding causes like heavy alcohol use, viruses, and drugs. Diagnosis involves imaging or histological evidence of hepatic steatosis (> 5%), with clinical manifestations including MAFLD and metabolic dysfunction-associated steatohepatitis (MASH), potentially progressing to fibrosis, cirrhosis, and liver cancer [7]. Key risk factors include alcohol consumption, obesity, T2DM, and metabolic syndrome. MAFLD is increasingly recognized as the hepatic manifestation of metabolic syndrome, prevalent among individuals with obesity and diabetes [8].
MAFLD and T2DM frequently co-occur in clinical settings, with a well-documented bidirectional relationship [9]. Individuals with MAFLD are at increased risk for developing T2DM, while T2DM independently elevates the risk of MAFLD [10]. Studies report MAFLD prevalence among T2DM patients at 69.4–78% [11]. The underlying pathogenesis involves insulin resistance, oxidative stress, and mitochondrial dysfunction [12], which contribute to the progression of MASH and further exacerbate insulin resistance. Dyslipidemia and obesity are contributing factors to the coexistence of T2DM and MAFLD, with insulin resistance being central to the pathogenesis of both conditions [2, 13].
The comorbidity of T2DM and MAFLD poses a significant global health challenge. Lifestyle interventions, particularly dietary changes and weight loss, remain pivotal in MAFLD management [14]. Key to this approach is enhancing insulin sensitivity and reducing body weight, as these factors are closely linked to decreased liver fat and improved liver histology, underscoring their inclusion in MAFLD treatment guidelines [15]. Nevertheless, only 3% to 6% of individuals achieve sustained long-term weight loss through lifestyle modifications alone [16]. Currently, no internationally approved medications exist for MAFLD, irrespective of T2DM status [17]. Recent evidence supports the efficacy of traditional Chinese medicine (TCM) in treating T2DM and MAFLD, particularly in improving liver enzymes and blood lipid levels. Our study integrates TCM with conventional drug treatments and lifestyle interventions, offering a comprehensive strategy for managing T2DM complicated by MAFLD. Interventions focusing on weight reduction, fat content decrease, and insulin sensitivity improvement are essential for managing MAFLD in T2DM patients [18].
Numerous recent meta-analyses of randomized controlled trials (RCTs) have explored the therapeutic effects of various treatments for T2DM complicated by MAFLD. However, flawed study designs, varied evaluation metrics, and inconsistent findings have hindered clear conclusions. Thus, a thorough evaluation of existing evidence on treatment impacts is essential before developing management strategies. This study evaluated evidence quality, bias potential, and research validity using the Grading of Recommendations, Assessment, Development, and Evaluations (GRADE) framework to assess outcome indicators and determine the strength of associations according to evidence classification criteria. The methodological quality of the meta-analyses included was assessed using the A Measure Tool to Assess Systematic Reviews (AMSTAR) tool. Publication bias was evaluated through Egger’s test for outcomes with ten or more studies, ensuring a comprehensive review of meta-analyses in the field.
We systematically retrieved, extracted, and analyzed data from published systematic reviews and meta-analyses examining the relationships between clinical management strategies and health outcomes in patients with T2DM complicated by MAFLD [19, 20]. Treatment effects were evaluated using indicators of liver health, lipid metabolism, blood glucose control, and body composition changes, and synthesized in the meta-analysis. Systematic reviews lacking meta-analyses were excluded from this umbrella review, which was prospectively registered on PROSPERO (CRD420251036455).
We conducted a comprehensive search of the PubMed, Embase, Web of Science, and Cochrane systematic reviews databases for systematic reviews and meta-analyses of RCTs, covering the period from inception to February 2025. Our search adhered to the Scottish Intercollegiate Guidelines Network’s literature search guidelines, using a combination of Medical Subject Headings and keywords: (Therapeutics) AND (Non-alcoholic Fatty Liver Disease) AND (Diabetes Mellitus, Type 2) AND (Systematic Review OR Meta-Analysis). Two authors (HH and SS) independently performed electronic searches, screened titles and abstracts, and identified meta-analyses meeting the inclusion criteria through full-text review. Discrepancies were resolved by a third author (DL). Additionally, we manually reviewed reference lists of all included articles to identify any studies that might have been overlooked.
We included systematic reviews and meta-analyses of RCTs to assess the impact of various treatments on health outcomes in T2DM with MAFLD. Eligibility required meta-analyses comparing treatment strategies using weighted mean differences (WMD) or standardized mean differences (SMD). For studies reporting multiple interventions and outcomes, we extracted data separately for each. If multiple studies on the same treatment and outcomes were published over 24 months apart, we selected the most recent study, typically with the largest sample size, for data extraction. For studies published within the same 24-month period, we prioritized the meta-analysis with the most RCTs. If the number of trials was identical, we selected the meta-analysis of higher quality [21, 22].
Exclusion criteria for these evaluations included meta-analyses of observational studies, studies on pre-diabetic and non-diabetic groups, meta-analyses focused solely on MAFLD or T2DM, articles published only in abstract form (lacking detailed data extraction lists), non-English studies, and research involving animals or cell cultures.
Two reviewers (HH and SS) independently extracted data from each eligible study, including the first author’s name, publication year, treatment modalities [such as exercise, glucagon-like peptide-1 receptor agonists (GLP-1RAs), sodium-glucose cotransporter-2 (SGLT-2) inhibitors, dipeptidyl peptidase-4 (DPP-4) inhibitors, Chinese Herbal Medicine (CHM)], outcomes, number of studies, cases and controls, study design (RCTs), and estimated overall effects [mean differences (MD), WMD or SMD with 95% confidence interval (CI)]. Additionally, they extracted information on effect models (random and fixed), heterogeneity models (I2 statistic and P-value of Cochran’s Q test), and publication bias (P-value of Egger’s test or funnel plot). Disagreements were resolved by a third author (DL).
Two reviewers (HH and SS) employed AMSTAR to evaluate the methodological quality of the articles. AMSTAR is a reliable tool for assessing systematic reviews and meta-analyses [23]. Additionally, using the GRADE, we assessed the evidence for each health outcome, categorizing it as “high”, “moderate”, “low”, or “very low” quality [24]. Furthermore, we classified the evidence into four categories: Class I (convincing evidence), Class II (highly suggestive evidence), Class III (suggestive evidence), Class IV (weak evidence), and NS (not significant) [24]. Detailed criteria for evidence classification are presented in Table 1.
Evidence classification criteria.
| Evidence class | Description |
|---|---|
| Class I: convincing evidence | > 1,000 cases (or > 20,000 participants for continuous outcomes); statistical significance at P < 10−6 (random effects); no evidence of small study effects and excess significance bias; 95% prediction interval excluded null value; no large heterogeneity (I2 < 50%) |
| Class II: highly suggestive evidence | > 1,000 cases (or > 20,000 participants for continuous outcomes), statistical significance at P < 10−6 (random effects), and the largest study with a 95% confidence interval excluding null value |
| Class III: suggestive evidence | > 1,000 cases (or > 20,000 participants for continuous outcomes) and statistical significance at P < 0.001 |
| Class IV: weak evidence | Remaining significant associations with P < 0.05 |
| NS: not significant | P > 0.05 |
To integrate studies with varying measurement units, we extracted raw data for significant effect sizes, standardized them using the SMD, and reanalyzed each study with RevMan 5.3. Results were expressed as SMD with 95% CI. The I2 statistic and Cochran’s Q test P-value were recalculated. A random-effects model was applied if I2 exceeded 50%; otherwise, a fixed-effects model was used. For non-significant effect sizes, results were taken directly from the original studies. We recalculated Egger’s regression test P-value for analyses with at least 10 studies [25]. Each meta-analysis detailed indicators, case numbers, and participant information from the original studies. For Class I or II classifications, we performed a sensitivity analysis when sufficient data allowed evaluation of evidence credibility changes after excluding certain studies. When reanalysis was not possible, we extracted summary data to assess heterogeneity and publication bias. A P-value < 0.10 indicated significant heterogeneity, while other tests used a significance threshold of P < 0.05. Evidence synthesis was conducted with RevMan 5.3, and Stata 15.1 was used for Egger’s test and sensitivity analysis.
Figure 1 illustrates the literature search and selection process [26]. A systematic search identified 622 unique articles, from which 19 meta-analyses of RCTs were selected based on the inclusion criteria [2, 3, 18, 27–42]. These analyses yielded 112 unique outcomes. The results encompassed changes in endocrine and metabolic indicators [fasting blood glucose (FBG) and postprandial blood glucose (PBG), glycated hemoglobin A1c (HbA1c), homeostasis model assessment of insulin resistance (HOMA-IR)], lipid metabolism indicators [triglyceride (TG), low-density lipoprotein cholesterol (LDL-C), total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C)], liver health indicators (liver enzymes, steatosis, fibrosis), and body composition indicators [body weight, body mass index (BMI), waist circumference, waist-to-hip ratio (WHR), fat distribution]. Forest plots revealed significant associations between treatment strategies and these indicators: endocrine and metabolic (Figure 2), lipid metabolism (Figure 3), liver health (Figure 4), and body composition (Figure 5). Detailed relationships between clinical treatment strategies and outcomes are in Tables S1–7.
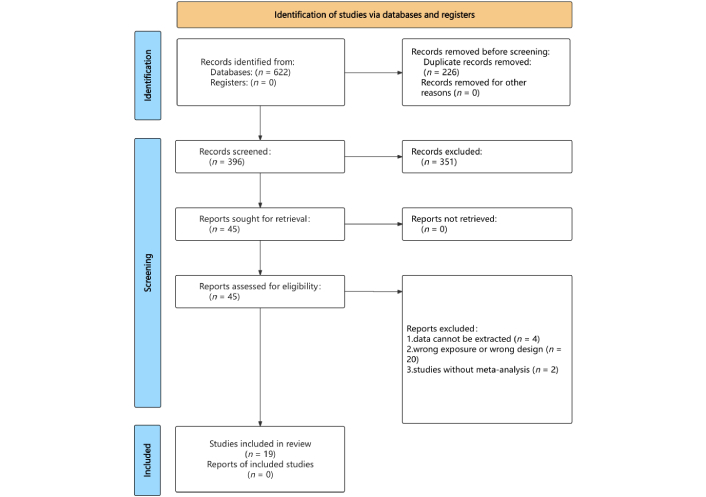
Flowchart of the systematic search and selection process. Adapted from [26]. © Author(s) (or their employer(s)) 2019. CC BY.
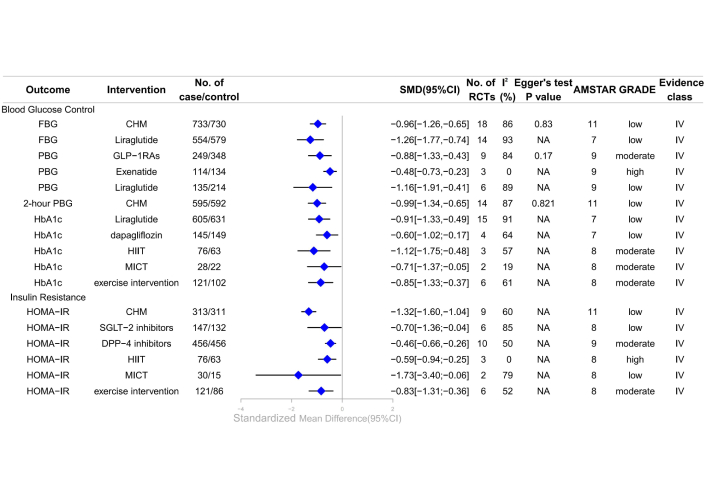
Forest plot of clinical management strategies significantly improving endocrine and metabolic indicators in patients with T2DM complicated with MAFLD. CHM: Chinese Herbal Medicine; HOMA-IR: homeostasis model assessment of insulin resistance; FBG: fasting blood glucose; DPP-4: dipeptidyl peptidase-4; SGLT-2: sodium-glucose cotransporter 2; GLP-1RAs: glucagon-like peptide-1 receptor agonists; PBG: postprandial blood glucose; HbA1c: glycated hemoglobin A1c; HIIT: high-intensity interval training; MICT: moderate-intensity continuous training; AMSTAR: A Measure Tool to Assess Systematic Reviews; CI: confidence interval; NA: not available; RCTs: randomized controlled trials; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; SMD: standardized mean differences; T2DM: type 2 diabetes mellitus; MAFLD: metabolic dysfunction-associated fatty liver disease.
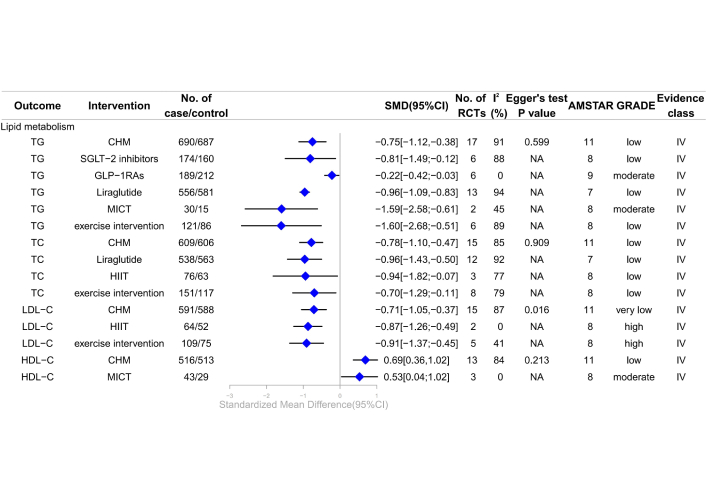
Forest plot of clinical management strategies significantly improving lipid metabolism indicators in patients with T2DM complicated with MAFLD. TG: triglyceride; TC: total cholesterol; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; CHM: Chinese Herbal Medicine; HIIT: high-intensity interval training; MICT: moderate-intensity continuous training; SGLT-2: sodium-glucose cotransporter 2; GLP-1RAs: glucagon-like peptide-1 receptor agonists; AMSTAR: A Measure Tool to Assess Systematic Reviews; CI: confidence interval; NA: not available; RCTs: randomized controlled trials; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; SMD: standardized mean differences; T2DM: type 2 diabetes mellitus; MAFLD: metabolic dysfunction-associated fatty liver disease.
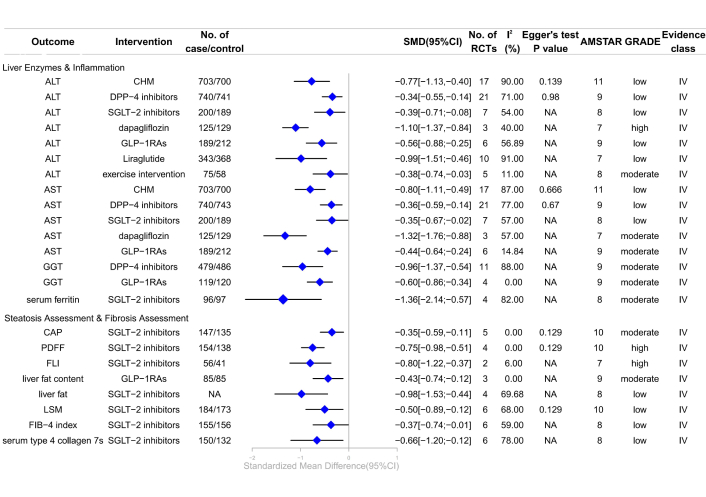
Forest plot of clinical management strategies significantly improving hepatic health indicators in patients with T2DM complicated with MAFLD. CHM: Chinese Herbal Medicine; DPP-4: dipeptidyl peptidase-4; SGLT-2: sodium-glucose cotransporter 2; GLP-1RAs: glucagon-like peptide-1 receptor agonists; ALT: alanine aminotransferase; AST: aspartate aminotransferase; GGT: gamma-glutamyl transferase; LSM: liver stiffness measurement; FLI: liver fat index; PDFF: proton density fat fraction; CAP: controlled attenuation parameter; FIB-4: fibrosis 4; AMSTAR: A Measure Tool to Assess Systematic Reviews; CI: confidence interval; NA: not available; RCTs: randomized controlled trials; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; SMD: standardized mean differences; T2DM: type 2 diabetes mellitus; MAFLD: metabolic dysfunction-associated fatty liver disease.
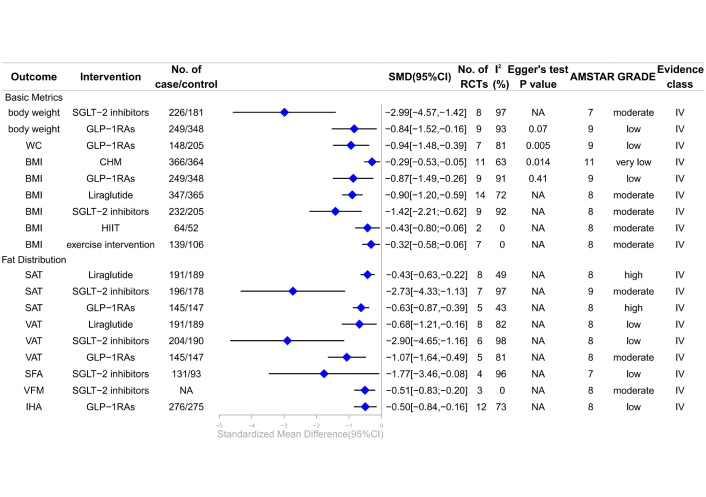
Forest plot of clinical management strategies significantly improving body composition indicators in patients with type 2 diabetes mellitus complicated with non-alcoholic fatty liver disease. CHM: Chinese Herbal Medicine; HIIT: high-intensity interval training; SGLT-2: sodium-glucose cotransporter 2; GLP-1RAs: glucagon-like peptide-1 receptor agonists; BMI: body mass index; WC: waist circumference; IHA: intrahepatic adipose; VFM: visceral fat mass; VAT: visceral adipose tissue; SAT: subcutaneous adipose tissue; SFA: subcutaneous fat areas; AMSTAR: A Measure Tool to Assess Systematic Reviews; CI: confidence interval; NA: not available; RCTs: randomized controlled trials; GRADE: Grading of Recommendations, Assessment, Development, and Evaluations; SMD: standardized mean differences.
The majority of the meta-analyses examined associations between various treatments and endocrine and metabolic outcomes (n = 28), followed by lipid metabolism (n = 26), liver health (n = 34), and body composition (n = 24). Treatments included CHM (n = 10), DPP-4 inhibitors (n = 4), GLP-1RAs (n = 41), SGLT-2 inhibitors (n = 30), and exercise interventions (n = 27). Of the 112 associations, 73 were statistically significant, and 39 were not. After SMD conversion, five outcomes changed from statistically significant to non-significant: LDL-C (liraglutide), liver fat score (NAFLDFS) (SGLT-2 inhibitors), liver-to-spleen attenuation ratio (SGLT-2 inhibitors), and alanine aminotransferase (ALT) [high-intensity interval training (HIIT) and moderate-intensity continuous training (MICT)]. Using GRADE and evidence classification criteria, most outcomes were of “moderate” or “low” quality at evidence levels IV or NS. Specifically, 9 outcomes (8%) were rated as high-quality evidence, 42 outcomes (37.5%) as moderate-quality, 56 outcomes (50%) as low-quality, and 5 outcomes (4.5%) as very low-quality.
A meta-analysis revealed that GLP-1RAs significantly reduced PBG compared to non-GLP-1RAs treatments or placebo [SMD –0.88, 95% CI –1.33 to –0.43; moderate; IV (the quality of evidence is expressed as “GRADE, evidence class”)] [41]; specifically, exenatide and liraglutide demonstrated substantial improvements in PBG compared to other drugs or placebo [SMD –0.48, 95% CI –0.73 to –0.23 (high; IV) and SMD –1.16, 95% CI –1.91 to –0.41 (low; IV), respectively] [41]. Additionally, exercise interventions significantly enhanced HbA1c levels compared to controls (SMD –0.85, 95% CI –1.33 to –0.37; moderate; IV). HIIT was more effective than MICT, with SMDs of –1.12 (95% CI –1.75 to –0.48) and –0.71 (95% CI –1.37 to –0.05), respectively (both moderate quality; IV) [28]. This meta-analysis determined that exercise interventions improved HOMA-IR (SMD –0.83, 95% CI –1.31 to –0.36) (moderate; IV), and HIIT significantly enhanced HOMA-IR (SMD –0.59, 95% CI –0.94 to –0.25) (high; IV) [28]. Additionally, DPP-4 inhibitors showed a significant improvement in HOMA-IR compared to placebo (SMD –0.46, 95% CI –0.66 to –0.26) (moderate; IV) [38]. However, the review found no significant association between GLP-1RAs and changes in HbA1c or HOMA-IR, nor between exenatide and changes in FBG or HbA1c [41], nor between liraglutide [41], dapagliflozin [42] and HOMA-IR improvement, all with moderate evidence (Figure 2, Table S1).
A meta-analysis of 18 RCTs revealed that CHM significantly improved FBG (SMD –0.96, 95% CI –1.26 to –0.65) and 2-hour PBG (SMD –0.99, 95% CI –1.34 to –0.65) (both low quality; IV) [27]. Liraglutide showed improvements in FBG (SMD –1.26, 95% CI –1.77 to –0.74) and HbA1c (SMD –0.91, 95% CI –1.33 to –0.49) (both low quality; IV) [2]. Dapagliflozin was effective in reducing HbA1c (SMD –0.60, 95% CI –1.02 to –0.17) (low; IV) [35]. Improvements in HOMA-IR were noted with CHM, MICT, and SGLT-2 inhibitors (SMD –1.32, 95% CI –1.60 to –1.04 [27]; SMD –1.73, 95% CI –3.40 to –0.06 [28]; SMD –0.70, 95% CI –1.36 to –0.04 [32], respectively). However, SGLT-2 inhibitors did not significantly affect FBG or HbA1c (low; NS) [29], and dapagliflozin did not significantly impact FBG (low; NS) [35]. GLP-1RAs and exenatide showed no significant association with FBG and HOMA-IR improvement (low; NS) [41], respectively (Figure 2, Table S1).
A separate study reported that exercise interventions significantly enhance lipid metabolism, notably improving LDL-C levels (SMD –0.91, 95% CI –1.37 to –0.45) (high; IV) [28]. HIIT specifically improved LDL-C levels (SMD –0.87, 95% CI –1.26 to –0.49) (high; IV), while MICT significantly affected TG and HDL-C levels (SMD –1.59, 95% CI –2.58 to –0.61) (moderate; IV) for TG and SMD 0.53, 95% CI 0.04 to 1.02, (moderate; IV) for HDL-C [28]. A meta-analysis of six RCTs showed that GLP-1RAs significantly improved TG levels (SMD –0.22, 95% CI –0.42 to –0.03) (moderate; IV) [18]. The review indicated moderate-quality evidence that MICT was not associated with changes in TC and LDL-C (moderate; NS) [28], resistance training (RT) was not linked to HDL-C alterations (moderate; NS) [28], and dapagliflozin had no effect on LDL-C (moderate; NS) (Figure 3; Table S2) [35].
A meta-analysis of CHM in T2DM patients with MAFLD revealed significant improvements in blood lipid metabolism. CHM notably reduced TG (SMD –0.75, 95% CI –1.12 to –0.38) (low; IV), TC (SMD –0.78, 95% CI –1.10 to –0.47) (low; IV), and LDL-C (SMD –0.71, 95% CI –1.05 to –0.37) (very low; IV), while increasing HDL-C (SMD 0.69, 95% CI 0.36 to 1.02) (low; IV) [27]. Evidence of low to very low quality indicated that SGLT-2 inhibitors reduced TG (SMD –0.81, 95% CI –1.49 to –0.12) (low; IV) [32], and liraglutide improved TG (SMD –0.96, 95% CI –1.09 to –0.83) (low; IV), TC (SMD –0.96, 95% CI –1.43 to –0.50) (low; IV) [2]. Exercise interventions may reduce TG (SMD –1.60, 95% CI –2.68 to –0.51) (low; IV) and TC (SMD –0.70, 95% CI –1.29 to –0.11) (low; IV) [28]. HIIT also showed a potential reduction in TC (SMD –0.94, 95% CI –1.82 to –0.07) (low; IV) [28]. However, exercise interventions were not linked to changes in HDL-C (low; NS), with HIIT showing no association with TG and HDL-C (low; NS), RT showing no association with TC (low; NS) [28], and dapagliflozin showing no association with TG (low; NS) [35]. Very low-quality evidence indicated no association between liraglutide and HDL-C (very low; NS) [2], LDL-C (very low; NS) (Figure 3; Table S2) [2].
This meta-analysis demonstrated that SGLT-2 inhibitors significantly improved liver enzyme levels and markers of inflammation, steatosis, and fibrosis in patients with T2DM and MAFLD. SGLT-2 inhibitors significantly reduced serum ferritin (SMD –1.36, 95% CI –2.14 to –0.57) (moderate; IV) [32], controlled attenuation parameter (CAP) (SMD –0.35, 95% CI –0.59 to –0.11) (moderate; IV) [36], liver proton density fat fraction (PDFF) (SMD –0.75, 95% CI –0.98 to –0.51) (high; IV) [36], and liver fat index (FLI) (SMD –0.80, 95% CI –1.22 to –0.37) (high; IV) [39]. Additionally, a meta-analysis of three RCTs indicated that dapagliflozin significantly improved ALT (SMD –1.10, 95% CI –1.37 to –0.84) (high; IV) and aspartate aminotransferase (AST) (SMD –1.32, 95% CI –1.76 to –0.88) (moderate; IV) [35]. GLP-1RAs significantly improved AST (SMD –0.44, 95% CI –0.64 to –0.24) (moderate; IV), gamma-glutamyl transferase (GGT) (SMD –0.60, 95% CI –0.86 to –0.34) (moderate; IV), and liver fat content (SMD –0.43, 95% CI –0.74 to –0.12) (moderate; IV). Exercise interventions effectively enhanced ALT (SMD –0.38, 95% CI –0.74 to –0.03) (moderate; IV) [28]. DPP-4 inhibitors significantly improved GGT (SMD –0.96, 95% CI –1.37 to –0.54) (moderate; IV) [38]. However, GLP-1RAs showed no significant association with the fibrosis 4 (FIB-4) index or NAFLD fibrosis score (NFS) (moderate; NS), and liraglutide was not associated with alkaline phosphatase (ALP) (moderate; NS) (Figure 4; Table S3) [3].
Low-quality evidence indicated that CHM significantly improved ALT (SMD –0.77, 95% CI –1.13 to –0.40) (low; IV) and AST (SMD –0.80, 95% CI –1.11 to –0.49) (low; IV) [27]. GLP-1RAs, including liraglutide, significantly improved ALT [SMD –0.56, 95% CI –0.88 to –0.25 (low; IV) [18]; liraglutide: SMD –0.99, 95% CI –1.51 to –0.46 (low; IV)]. SGLT-2 inhibitors significantly improved ALT (SMD –0.39, 95% CI –0.71 to –0.08) (low; IV) [32], AST (SMD –0.35, 95% CI –0.67 to –0.02) (low; IV) [32], liver fat (SMD –0.98, 95% CI –1.53 to –0.44) (low; IV) [40], FIB-4 index (SMD –0.37, 95% CI –0.74 to –0.01) (low; IV) [32], and liver stiffness measurement (LSM) (SMD –0.50, 95% CI –0.89 to –0.12) (low; IV) [36], and serum type 4 collagen 7S (SMD –0.66, 95% CI –1.20 to –0.12) (low; IV) [32]. DPP-4 inhibitors significantly improved ALT (SMD –0.34, 95% CI –0.55 to –0.14) (low; IV), AST (SMD –0.36, 95% CI –0.59 to –0.14) (low; IV) [38]. However, liraglutide showed no significant association with AST (very low; NS) [2], GGT (low; NS) [3], or liver fat content (low; NS) [3], and SGLT-2 inhibitors showed no association with GGT (low; NS) [32], liver-to-spleen attenuation ratio (low; NS) [36], NAFLDFS (low; NS) [39]. HIIT and MICT showed no significant association with ALT [(moderate; NS), (low; NS), respectively] (Figure 4; Table S3) [28].
Recent studies demonstrated that GLP-1RAs significantly reduced subcutaneous adipose tissue (SAT) (SMD –0.63, 95% CI –0.87 to –0.39) (high; IV) [34], and visceral adipose tissue (VAT) (SMD –1.07, 95% CI –1.64 to –0.49) (moderate; IV) [34]. Liraglutide significantly improved BMI (SMD –0.90, 95% CI –1.20 to –0.59) (moderate; IV) and SAT (SMD –0.43, 95% CI –0.63 to –0.22) (high; IV) [3]. Our findings indicated that SGLT-2 inhibitors significantly decreased body weight (SMD –2.99, 95% CI –4.57 to –1.42) (moderate; IV) [29], BMI (SMD –1.42, 95% CI –2.21 to –0.62) (moderate; IV) [32], SAT (SMD –2.73, 95% CI –4.33 to –1.13) (moderate; IV) [31], and visceral fat mass (VFM) (SMD –0.51, 95% CI –0.83 to –0.20) (moderate; IV) [40]. Exercise interventions also reduced BMI (SMD –0.32, 95% CI –0.58 to –0.06) (moderate; IV), with HIIT showing a pronounced effect (SMD –0.43, 95% CI –0.80 to –0.06) (moderate; IV) [28]. This review revealed no significant association between GLP-1RAs and WHR (moderate; NS) [41], and no association between MICT or RT and BMI (moderate; NS) (Figure 5; Table S4) [28].
Evidence of low quality suggested that GLP-1RAs may reduce waist circumference (SMD –0.94, 95% CI –1.48 to –0.39) (low; IV) [41], body weight (SMD –0.84, 95% CI –1.52 to –0.16) (low; IV) [41], BMI (SMD –0.87, 95% CI –1.49 to –0.26) (low; IV) [41], and intrahepatic adipose (IHA) (SMD –0.50, 95% CI –0.84 to –0.16) (low; IV) [34]. Liraglutide significantly decreased VAT (SMD –0.68, 95% CI –1.21 to –0.16) (low; IV) [3], while SGLT-2 inhibitors showed significant reductions in VAT (SMD –2.90, 95% CI –4.65 to –1.16) (low; IV) [32] and subcutaneous fat areas (SFA) (SMD –1.77, 95% CI –3.46 to –0.08) (low; IV) [29]. CHM showed potential in reducing BMI (SMD –0.29, 95% CI –0.53 to –0.05) (very low; IV) [27]. The review indicated that liraglutide and exenatide do not significantly affect body weight (low; NS), and exenatide does not affect BMI (low; NS) (Figure 5; Table S4) [41].
Subgroup analyses on HOMA-IR and ALT were conducted based on treatment duration of either ≥ 24 weeks or < 24 weeks. For HOMA-IR, CHM administered for ≥ 24 weeks showed greater efficacy in reducing insulin resistance compared to < 24 weeks (–1.47 [–1.84, –1.11] vs. –1.28 [–1.64, –0.93]). Conversely, DPP-4 inhibitors were more effective within 24 weeks than beyond (–0.51 [–0.75, –0.26] vs. –0.44 [–0.71, –0.17]). SGLT-2 inhibitors did not show significant results in either time frame (Figure S1). Regarding ALT, CHM, DPP-4 inhibitors, and SGLT-2 inhibitors all demonstrated greater reductions when used for ≥ 24 weeks (CHM: –1.21 [–2.36, –0.06] vs. –0.67 [–1.03, –0.31]; DPP-4 inhibitors: –0.36 [–0.61, –0.11] vs. –0.30 [–0.67, 0.07]; SGLT-2 inhibitors: –0.46 [–0.68, –0.24] vs. 0.04 [–1.03, 1.11]) (Figure S2).
We reanalyzed the heterogeneity of 77.7% of the results using random or fixed-effect models. This reanalysis indicated that about 64.4% of these results exhibited significant heterogeneity (I2 > 50% or P < 0.10 in Cochran’s Q test). Factors such as environment, region, race, gender, age, study quality, study design, sample size, follow-up duration, and adjustment for confounders explained most of this heterogeneity. Of the 25 results not reanalyzed, approximately 44% demonstrated significant heterogeneity.
In our reanalysis, Egger’s test was applied to 18.6% of the results, revealing publication bias in two cases: LDL-C (CHM) (P = 0.016) and BMI (CHM) (P = 0.014). The other results either showed no significant publication bias or lacked a publication bias assessment.
The median AMSTAR score across all outcomes was 8 (range 7–11) (Table S5), with detailed scores for each outcome in Table S6. This study focused exclusively on meta-analyses of RCTs, extracting data solely from these trials. Most evidence was assessed as “moderate” or “low” quality, with 9 pieces rated “high” and 5 “very low”. In two meta-analyses of RCTs, five pieces of evidence [BMI (CHM), LDL-C (CHM, liraglutide), AST (liraglutide), HDL-C (liraglutide)] were downgraded to "very low" quality due to risks of bias, inconsistency, indirectness, imprecision, or publication bias (Table S5). Table S7 details the GRADE classifications for each outcome. Of the 112 outcomes, 73 (65.18%) were rated as Class IV, while 39 (34.82%) were deemed non-significant (Table S5).
Multiple treatments, including GLP-1RAs, SGLT-2 inhibitors, DPP-4 inhibitors, exercise, and CHM, were found to be effective for T2DM complicated by MAFLD. This umbrella review of 19 meta-analyses (encompassing 112 distinct outcomes) revealed positive associations between various treatments and endocrine and metabolic outcomes (specifically FBG, PBG, HbA1c, and HOMA-IR) in T2DM with MAFLD. Lipid metabolism benefits were noted, with improvements in TC, TG, LDL-C, and HDL-C. Liver health indicators also showed positive changes, including liver enzymes (AST, ALT, GGT), inflammatory markers (serum ferritin), and measures of steatosis and fibrosis (CAP, PDFF, FLI, liver fat content, LSM, FIB-4 index, and serum type 4 collagen 7s). Additionally, favorable outcomes were observed in body weight, waist circumference, BMI, and fat distribution indicators (SAT, VAT, SFA, VFM, IHA).
Among the 112 associations, converting to SMD altered the statistical significance of five health outcomes from significant to non-significant. This change likely resulted from large within-group standard deviations, which increased the uncertainty of the SMD estimate. An umbrella review seeks to thoroughly assess all evidence in a specific field, and the primary studies involved often employ varied measurement methods. SMD addresses this clinical heterogeneity and was chosen as the pooled effect size out of methodological necessity. Given the diverse measurement units in the included studies, SMD offers a unified, comparable indicator. Although the significance level of individual studies changed, this approach maintains the comparability of the overall analysis and consistency in interpretation.
Our review demonstrates beneficial links between GLP-1RAs and various indicators of blood glucose control, lipid metabolism, liver enzymes, and body composition, notably PBG [41], TG [2, 18], TC [2], body weight [41], waist circumference [41], SAT [3, 34], VAT [3, 34], and IHA [34]. These improvements are primarily due to GLP-1RAs. MAFLD pathogenesis involves complex interactions among environmental factors, obesity, microbiota changes, and genetic predispositions, resulting in disrupted lipid homeostasis in hepatocytes and excessive TG accumulation [43]. Lipotoxicity and inflammation are key pathogenic factors in MASH [44]. GLP-1RAs, gut-derived incretin hormones, promote weight loss and enhance insulin sensitivity. In vitro, these drugs directly target human hepatocytes, mitigating steatosis by reducing de novo lipogenesis (DNL) and increasing fatty acid oxidation [45, 46]. Through AMP-activated protein kinase (AMPK), GLP-1RAs modulate insulin signaling components, enhancing hepatocyte insulin sensitivity and reducing hepatic steatosis [47]. Additionally, they downregulate stearoyl-CoA desaturase 1 (Scd1) mRNA and other genes involved in fatty acid synthesis [48], further curtailing DNL. In patients with MAFLD, mechanistic studies link GLP-1RAs to improvements in DNL, β-oxidation, and insulin resistance at the systemic, adipose tissue, and hepatic levels [49, 50]. GLP-1RAs may reduce liver fat by promoting weight loss [51], which is closely linked to improved insulin sensitivity [52]. Additionally, GLP-1RAs protect hepatocytes from ischemia-reperfusion injury by mitigating necrosis and apoptosis [53], and prevent fatty acid-induced hepatocyte death by reducing endoplasmic reticulum stress [54]. Thus, GLP-1RAs represent a promising therapeutic strategy for patients with T2DM and MAFLD. Animal studies indicate that GLP-1 analogs, such as liraglutide, enhance hepatic insulin sensitivity and decrease steatosis and fibrosis [54, 55]. Meta-analyses reveal that GLP-1RAs treatment improves hepatic steatosis, liver function, lipid profiles, glucose levels, inflammation, and insulin sensitivity [37]. A subgroup analysis within a meta-analysis further shows significant reductions in FBG and HOMA-IR levels in T2DM and MAFLD patients treated with GLP-1RAs for over 24 weeks compared to other treatments [41]. In preclinical mouse models of steatohepatitis, GLP-1RAs reduced liver enzymes and oxidative stress, downregulated genes involved in fatty acid synthesis, and improved liver histology [48, 56]. Ji et al. [57] demonstrated that two weeks of liraglutide treatment in mice on a high-fat diet led to reductions in body weight, blood glucose, liver function markers, hepatic steatosis, and bridging fibrosis. The beneficial effects of liraglutide are partly due to its inhibition of the advanced glycation end products (RAGE)/nicotinamide adenine dinucleotide phosphate (NADPH) oxidase signaling pathway, which is associated with reactive oxygen species (ROS) formation. This mechanism was corroborated by in vitro experiments in the same study [57, 58].
We observed that SGLT-2 inhibitors positively impact patients with T2DM complicated by MAFLD, notably improving HOMA-IR [32], TG [32], CAP [36], PDFF [36], and FLI [39]. Abnormal fat distribution is prevalent in patients with T2DM, with adipose tissue categorized into VAT, SAT, and ectopic adipose tissue (fat deposits in the liver, epicardium, pancreas, and skeletal muscle) [31]. Preclinical studies indicate that SGLT-2 inhibitors significantly alter energy metabolism by enhancing fat oxidation [35]. This metabolic shift confers several benefits, including the reduction of hepatic ectopic fat, decreased body weight and fat mass, and inhibited release of pro-inflammatory cytokines from adipocytes. Research shows a strong correlation between adipose tissue reduction and BMI, with more pronounced reductions in VAT, SAT, and ectopic fat observed in patients with a higher BMI [31]. In patients with T2DM, SGLT-2 inhibitors significantly reduce VAT, SAT, and ectopic hepatic fat. Particularly in young patients with T2DM, MAFLD, and a high BMI, administration of these inhibitors for 16–40 weeks may yield more pronounced and sustained reductions in VAT and SAT [31]. Treatment with SGLT-2 inhibitors enhances urinary glucose excretion and lowers circulating glucose levels, potentially increasing lipolysis in adipose tissue and TG catabolism in the liver to offset glucose loss [32]. Concurrently, this treatment reduces insulin levels, improves insulin resistance, and diminishes the stimulation of fat regeneration [59]. Treatment with SGLT-2 inhibitors reduces the expression of lipogenic genes such as acetyl-CoA carboxylase 1 (Acc1), Scd1, and fatty acid synthase (Fasn) [60], thereby mitigating hepatic lipidosis by decreasing DNL. Improved insulin sensitivity can also downregulate sterol regulatory element-binding protein 1c (SREBP-1c) [61]. SGLT-2, which is encoded by the SLC5A2 gene, is expressed in pancreatic islet α-cells. Inhibition of this sodium-glucose cotransporter elevates serum glucagon levels, which promotes hepatic fatty acid β-oxidation; reduces free radical production; inhibits pro-oxidants; enhances the antioxidant system; alleviates endoplasmic reticulum stress [60], and shifts carbohydrate metabolism toward fatty acid metabolism, thereby reducing hepatic fat deposition [62]. A study in animal models demonstrated that empagliflozin treatment ameliorated hepatic steatosis, inflammation, and fibrosis in mice with MASH and diabetes, thereby slowing liver disease progression [62]. SGLT-2 inhibitors significantly reduce liver fibrosis, indicating their potential as a therapeutic strategy for preventing advanced MAFLD and MASH [32]. Beyond glycemic control, SGLT-2 inhibitors also confer beneficial effects on body weight, blood pressure, insulin resistance, subclinical inflammation, oxidative stress, and hyperuricemia. Incorporating SGLT-2 inhibitors into the dietary and lifestyle management of patients with T2DM and MAFLD may help prevent disease progression and associated comorbidities.
We identified a positive association between DPP-4 inhibitor use and improvements in liver enzyme levels (AST, ALT) and HOMA-IR in patients with T2DM and MAFLD [38]. DPP-4 inhibitors are oral antidiabetic agents that prevent the degradation of GLP-1 and glucose-dependent insulinotropic polypeptide by the DPP-4 enzyme. These incretins, released in response to food intake, are crucial for glucose regulation by enhancing insulin secretion, suppressing glucagon secretion, and reducing hepatic glucose production [38]. Notably, these inhibitors significantly affect mRNA levels of hepatic AMPK and the expression of lipogenic genes. This dual mechanism reduces DNL in the liver, enhances pancreatic β-cell function, and increases insulin sensitivity in adipocytes, leading to decreased fat oxidation, reduced hepatic TG accumulation, and suppressed DNL [63, 64]. These inhibitors also mitigate oxidative stress and improve cognitive dysfunction in diabetic mice [65, 66]. In fructose-fed rats with metabolic syndrome, sitagliptin effectively reduced hepatic steatosis, decreased β-cell apoptosis, and improved insulin sensitivity [67]. Saxagliptin significantly improves endocrine and metabolic functions, as demonstrated by decreased levels of liver enzymes (ALT, AST) and blood lipids (TG, TC) after three months of treatment [38]. A subgroup analysis revealed that in the Asian cohort, ALT and AST levels were notably reduced in the treatment group. In contrast, studies from Europe and the United States exhibited significant variability in effectiveness, suggesting a geographical influence on transaminase levels [38]. Plasma DPP-4 enzyme activity correlates with steatosis, inflammation, and the histological severity of MASH in patients with MAFLD [68, 69]. Research indicates a strong positive correlation between serum sDPP-4 levels and liver enzymes ALT and GGT in both individuals with T2DM and healthy controls [70]. Sagara et al. [71] demonstrated a significant positive correlation between serum sDPP-4 levels and CAP, LSM, and FAST scores as measured by transient elastography (FibroScan). Notably, patients with an LSM ≥ 8.0 kPa exhibited significantly higher serum sDPP-4 levels compared to those with an LSM < 8.0 kPa [71].
Exercise interventions markedly improve HbA1c, HOMA-IR, TG, TC, LDL-C, HDL-C, AST levels, and BMI in patients with T2DM and MAFLD [28]. MICT notably improves HOMA-IR, TG levels, and HDL-C [28]. Blood glucose control and systemic insulin resistance are pivotal factors in the progression of MAFLD [72, 73]. Meta-analysis findings reveal that physical activity lowers HbA1c levels and improves HOMA-IR and ALT levels in patients with MAFLD and T2DM [28]. Subgroup analysis revealed that HIIT and MICT are more effective than RT in improving blood glucose and ALT levels. This disparity likely arises because HIIT and MICT engage multiple large muscle groups continuously, whereas RT targets isolated muscle groups intermittently [74]. A 5–10% weight loss is an effective strategy for preventing the progression of MAFLD and T2DM [75]. Oh et al.’s study [76] demonstrated that exercise can ameliorate metabolic disturbances and reduce inflammation and oxidative stress, highlighting its benefit for patients with MAFLD, independent of weight loss. Notably, HIIT significantly reduced body weight. Hypertriglyceridemia, an independent predictor of MAFLD [77], was significantly reduced in the MICT group [28]. Lifestyle modifications involving diet and exercise can lower the risk of MAFLD in patients with obesity by reducing ALT levels. Gradual weight loss is crucial, as rapid loss may exacerbate steatosis and increase the risks of liver failure and inflammation [2]. Cysteine dioxygenase type 1 (Cdo1) plays a crucial role in cysteine catabolism and taurine synthesis and is highly expressed in liver tissue [78]. In patients with MAFLD, hepatic Cdo1 expression is notably reduced compared to non-MAFLD individuals [79]. Liver-specific Cdo1 knockout (Cdo1LKO) diminishes the beneficial effects of exercise on MAFLD, whereas liver-specific overexpression (Cdo1LTG) ameliorates the condition in mice [79]. Exercise enhances hepatic Cdo1 expression via the cAMP/PKA/CREB signaling pathway. Both liver Cdo1 and exercise may mitigate MAFLD through the Cdo1-Camkk2-AMPK axis [79]. Dietary interventions boost lipophagy by inhibiting the Akt/mTOR/ULK1 pathway, while exercise activates lipophagy through the AMPK/ULK1 pathway [80]. Exercise-induced muscle secretion of FGF21 facilitates liver lipophagy via an AMPK-dependent mechanism, improving MAFLD [80]. FGF21-mediated AMPK-dependent lipophagy represents a potential therapeutic target for MAFLD-induced aging and lipid metabolic disorders [80]. Research by Zhu et al. [81] indicates that exercise-induced irisin mitigates inflammation in MAFLD by competitively binding to MD2, revealing irisin’s novel role as a TLR4 pathway antagonist.
TCM has been shown to positively affect indicators such as blood lipids, liver enzymes, blood glucose, and BMI, with notable improvements in AST and ALT levels [27]. In patients with MAFLD, elevated levels of ALT and AST signal liver inflammation and damage [82]. Research indicates that CHM can effectively regulate glucose and lipid metabolism, improve insulin resistance and hemorheology, repair liver histopathological damage, inhibit oxidative stress, and slow the progression of T2DM with MAFLD [27]. TCM has gained recognition as a complementary treatment for T2DM with MAFLD. Rooted in the principles of syndrome differentiation, Chinese herbal formulas target various pathological mechanisms, such as insulin resistance, lipid dysregulation, and liver inflammation. Clinical studies demonstrate that TCM preparations, such as Danning Tablets, effectively treat MAFLD by mediating lipids, reducing inflammation, combating oxidation, alleviating insulin resistance, preventing apoptosis, and countering endothelial dysfunction and fibrosis, thus safeguarding the liver [83]. The network meta-analysis revealed that combining Huazhi Rougan granules with Western medicine significantly enhanced clinical efficacy and improved blood lipid levels. Danning Tablets alone exhibited the highest clinical efficacy, while Huazhi Rougan granules notably elevated ALT and AST levels in MAFLD patients [84]. TCM offers distinct scientific and clinical advantages in modulating the AMPK signaling pathway via the synergistic action of multiple components and targets. Unlike dietary or exercise interventions, TCM formulations, which include flavonoids, phenols, alkaloids, terpenoids, polysaccharides, saponins, lignans, and natural extracts, specifically activate AMPK while enhancing metabolic outcomes through the cross-regulation of pathways such as SIRT1/AMPK and Nrf2/AMPK [85]. By optimizing compatibility, as seen in the synergy between Bupleuri Radix and Paeoniae Radix Alba in Sini San, TCM formulations can significantly mitigate the dose-dependent toxicity associated with AMPK activation, thereby improving clinical safety [85]. Combining Western medicine with CHM may enhance lipid and glucose metabolism, liver function, and insulin resistance, reduce body weight, and improve overall treatment efficacy [27], offering a promising approach for managing T2DM complicated by MAFLD.
This review provides a comprehensive summary of evidence from prior meta-analyses of RCTs. These analyses reveal that treatments such as GLP-1RAs, SGLT-2 inhibitors, DPP-4 inhibitors, exercise interventions, and CHM positively affect liver health, blood glucose control, body composition, and lipid metabolism in T2DM with MAFLD. The study employed a systematic approach, with two authors independently handling literature retrieval, study selection, and data extraction. To integrate studies with varying measurement units, we extracted raw data for significant effect sizes, standardized them using the SMD, and reanalyzed each study with RevMan 5.3 and assessed heterogeneity and publication bias in each meta-analysis. We employed AMSTAR, GRADE, and evidence classification criteria to evaluate the methodological quality, strength, and classification of each outcome, respectively. Both the GRADE score and evidence classification criteria are essential for evaluating evidence and formulating recommendations. High-quality evidence, such as the impact of HIIT on HOMA-IR and dapagliflozin on ALT reduction, strongly supports the recommendation of these interventions in most clinical scenarios. Moderate-quality evidence, like the effect of exercise on ALT reduction, should be considered after evaluating patient values, preferences, comorbidities, and economic factors. For interventions supported by low or very low-quality evidence, such as the effect of CHM on FBG, further research is needed before broader implementation. These areas offer promising avenues for future investigation, but currently, a cautious and individualized approach is advised.
This study has several limitations. First, the exclusive use of English databases may introduce bias by omitting studies in other languages. Second, we relied solely on published data, disregarding unpublished or forthcoming evidence-based data. Third, the study focused on data extracted from systematic reviews and meta-analyses, excluding original research data not covered in these reviews.
This umbrella review identified five therapeutic interventions and 112 outcomes from the included meta-analyses, with 73 showing significant associations and 39 showing no significant associations. Most outcomes were deemed “moderate” or “low” quality, with evidence levels of IV or NS. Nine outcomes were supported by high-quality evidence and 42 by moderate-quality evidence. The findings suggest that GLP-1RAs, SGLT-2 inhibitors, DPP-4 inhibitors, exercise intervention, and CHM are beneficially linked to improvements in liver enzymes, blood lipids, body weight, and insulin resistance in patients with T2DM and MAFLD. These interventions may serve as effective treatments for this condition. The study’s results contribute to better prevention and treatment strategies, potentially enhancing therapeutic efficacy, delaying liver cirrhosis and cancer progression, and reducing the global burden of MAFLD-related diseases.
ALT: alanine aminotransferase
AMPK: AMP-activated protein kinase
AMSTAR: A Measure Tool to Assess Systematic Reviews
AST: aspartate aminotransferase
BMI: body mass index
CAP: controlled attenuation parameter
Cdo1: cysteine dioxygenase type 1
CHM: Chinese Herbal Medicine
CI: confidence interval
DNL: de novo lipogenesis
DPP-4: dipeptidyl peptidase-4
FBG: fasting blood glucose
FIB-4: fibrosis 4
FLI: liver fat index
GGT: gamma-glutamyl transferase
GLP-1RAs: glucagon-like peptide-1 receptor agonists
GRADE: Grading of Recommendations, Assessment, Development, and Evaluations
HbA1c: glycated hemoglobin A1c
HDL-C: high-density lipoprotein cholesterol
HIIT: high-intensity interval training
HOMA-IR: homeostasis model assessment of insulin resistance
IHA: intrahepatic adipose
LDL-C: low-density lipoprotein cholesterol
LSM: liver stiffness measurement
MAFLD: metabolic dysfunction-associated fatty liver disease
MASH: metabolic dysfunction-associated steatohepatitis
MICT: moderate-intensity continuous training
NAFLD: non-alcoholic fatty liver disease
NAFLDFS: liver fat score
PBG: postprandial blood glucose
PDFF: proton density fat fraction
RCTs: randomized controlled trials
RT: resistance training
SAT: subcutaneous adipose tissue
Scd1: stearoyl-CoA desaturase 1
SFA: subcutaneous fat areas
SGLT-2: sodium-glucose cotransporter 2
SMD: standardized mean differences
T2DM: type 2 diabetes mellitus
TC: total cholesterol
TCM: traditional Chinese medicine
TG: triglyceride
VAT: visceral adipose tissue
VFM: visceral fat mass
WHR: waist-to-hip ratio
WMD: weighted mean differences
The supplementary figures and tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/101443_sup_1.pdf.
HH: Writing—original draft, Writing—review & editing, Conceptualization, Methodology, Formal analysis, Investigation, Resources, Supervision. SS: Formal analysis, Investigation, Conceptualization. DL: Formal analysis, Investigation, Conceptualization. All authors read and approved the final manuscript.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The original contributions presented in the study are included in the article/supplementary materials. Further inquiries can be directed to the corresponding author.
The authors received no funding from an external source.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 1982
Download: 30
Times Cited: 0
Huiling Huang ... Dongsheng Li
Dorothy E. Vatner ... Stephen F. Vatner
