Affiliation:
1Institute of Biomedical Sciences, Jiangsu Normal University, Xuzhou 221116, Jiangsu, China
Affiliation:
1Institute of Biomedical Sciences, Jiangsu Normal University, Xuzhou 221116, Jiangsu, China
Affiliation:
2School of Life Sciences, Jiangsu Normal University, Xuzhou 221116, Jiangsu, China
Email: lee_shao@hotmail.com
Affiliation:
2School of Life Sciences, Jiangsu Normal University, Xuzhou 221116, Jiangsu, China
Email: jz_zhao@jsnu.edu.cn
ORCID: https://orcid.org/0000-0002-7444-5091
Explor Drug Sci. 2023;1:64–76 DOI: https://doi.org/10.37349/eds.2023.00006
Received: September 21, 2022 Accepted: December 05, 2022 Published: March 30, 2023
Academic Editor: Juergen Reichardt, James Cook University, Australia
The article belongs to the special issue Exploring Potential Drugs from Natural Products
Aim: In the present study, the natural products levistolide A (LA) and periplogenin (PPG) were studied for their growth inhibitory effects on the development of gastric cancer cells in vitro and, more critically, in vivo, alone or in combination with the therapeutic medication 5-fluorouracil (5-FU).
Methods: Methyl thiazolyl tetrazolium (MTT), also known as 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide assays were used for the cell viability study. Apoptosis was detected by western blot to detect the cleavage of caspase substrate poly (ADP-ribose) polymerase (PARP) and terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labelling (TUNEL) assays. The nude mice bearing gastric cancer cells were used for the anti-cancer activity detection of LA and its combinational treatment effect with 5-FU.
Results: The results in the present study shown that the two compounds were able to inhibit the viability of the cancer cells in a dose- and time-dependent manner by MTT method. They could trigger apoptosis when used alone, and more potently, in combination with 5-FU detected by TUNEL positivity and the cleavage of caspase substrate PARP. In nude mice bearing gastric cancer cells, injection (i.p.) of LA or PPG alone inhibited the growth of the cancer cells. The treatment using one of the compounds in combination with 5-FU inhibited the cancer cell growth at a higher level than the treatment by a compound alone.
Conclusions: LA and PPG could inhibit the growth of the cancer cells, alone or in combination with 5-FU, in vitro and in vivo, suggesting that they are promising investigational drugs for therapeutic development.
Cancer is one of the most common non-communicable diseases and one of the leading causes of death worldwide, which represents a great barrier to increasing life expectancy in today’s communities [1, 2]. According to the statistics from the World Health Organization, an estimated 19.3 million cases of cancer and almost 10.0 million cases of cancer death occurred in 2020. In an increasing number of countries, cancer prevalence is expected to exceed many common diseases such as stroke and coronary heart disease [3]. The economic burden of cancer is substantial in all countries, which causes healthcare spending as well as the loss of productivity due to morbidity and premature death. In the USA, in 2017 alone, the estimated cancer healthcare spending is $161.2 billion, accounting for the approximately 1.8% of the gross domestic product. Specifically, gastric cancer is one of the major cancers, in particular in Eastern Asia. In China, cancers of the lung, stomach, colorectum, liver, and breast account for almost 60% of all cancers diagnosed, amongst which gastric cancer ranks second [4]. The incidence of gastric cancer is 2-fold higher in China than the average incidence worldwide [1], which makes gastric cancer a huge threat to the health of Chinese people and an economic burden to the country. Compared to the USA and UK, China has lower cancer incidence but 30% to 40% higher cancer mortality, among which 36.4% of the cancer-related deaths are from digestive tract cancers that include gastric cancer [5]. The high prevalence of cancer creates a huge market for anti-cancer drugs. In China, the overall average direct expenditure per cancer patient is estimated to be $9,899 in 2020 [6] and the market of anti-cancer drugs has increased 24% every year from 2011 to 2013 when it reaches $50 billion according to the market forecast.
Surgery remains an effective method for the treatment of cancer [7], particularly when the disease is at its early stage. Unfortunately, a significant number of patients are diagnosed when cancer has progressed to a late stage and missed out on the optimal window time for the treatment by surgery, in China, approximately 70% of gastric cancer patients are diagnosed with advanced clinical stage [8]. At the end of the 20th century, a combination of surgery with chemotherapy is developed, which significantly prolongs the life of cancer patients. However, chemotherapy also brings serious adverse effects which have negative impacts on patients’ physical health and quality of life [9, 10]. Moreover critically, the outcomes of current chemotherapy are often not satisfactory. For better treatment, methods such as targeted therapy and immunotherapy are further developed, during which the development of traditional chemotherapeutic drugs was nearly completely stopped. Chernecky [10] found that the cell signal transductions for growth empower the cancer cells with superior proliferation potential. Thus, molecules targeting the cell signaling proteins such as the epidermal growth factor receptor (EGFR) or the vascular endothelial growth factor receptor (VEGFR) are generated, which creates the first-generation drugs for the targeted therapy [11]. Compared to traditional chemotherapy drugs, targeted therapy is better tolerated by patients due to fewer adverse effects. However, the overall therapeutic outcome is limited, as strong drug resistance often occurs soon after the use of the drugs [12]. Immunotherapy also brings new hopes to cancer treatment since the 90th of the last century. Rituximab and trastuzumab are monoclonal antibody drugs for treating breast cancer and lymphoma, respectively. However, these therapies are not more effective than traditional chemotherapy [13, 14]. For gastric cancer, more specifically, human EGFR2 inhibitor trastuzumab in combination with the traditional chemotherapeutic drug has entered the phase III clinical trial. Nevertheless, the treatment could only prolong the life of advanced gastric cancer patients only for one month on average [15]. The monoclonal antibody bevacizumab targeted to VEGFR was also used for the advanced gastric cancer treatment combined with the chemotherapy drugs capecitabine/cisplatin, however, it did not show better treatment effectiveness compared to the traditional chemotherapy drugs capecitabine or cisplatin alone [16]. Thus, the traditional method that looks for cancer chemotherapy drugs from the mother nature has returned to the center stage of drug development.
Natural compounds form a huge library for drug development, which has been used for thousands of years, in particular in China. According to the Food and Drug Administration, only about 30% of the new drugs were synthesized in the lab in the past nearly 30 years. Most of the new drugs are natural products or their chemically modified structures. In today’s clinical practice, combinational treatment (i.e., the concomitant use of two drugs) is common in cancer therapy. The drugs in vitro can show combinational effects against cancer cell growth, in either a synergistic or additive manner. Thus, the use of a lower dose of the individual drug can achieve a therapeutic outcome, compared to that in mono-therapy, which lowers the dose-limiting adverse effects and allows more patients for chemotherapy [17]. Furthermore, combination therapy is less susceptible to drug resistance than monotherapy because the constant treatment with a single compound induces cancer cells to recruit alternative salvage pathways [18, 19]. In fact, combination therapy using one clinical drug and an investigational drug such as a natural compound has become one of the concepts in the search for new drugs for the treatment of cancer [20–22].
Verification of the anti-cancer activity of a compound in animal models in vivo is a key step for therapeutic development. In some cases, the anti-cancer effect of a compound in vitro cannot be validated in vivo. Monobut-3, a stable butyric derivative, exhibited a marked growth inhibitory effect on breast cancer cell lines in vitro, but could not inhibit the cancer cell growth in vivo [23]. Mitomycin C exhibited cytotoxicity to cultured neuroblastoma cells but not the tumor in the nude mice mold [24]. In some other cases, the in vivo experiments even show bioactivity that was opposite to that observed under in vitro conditions. Retinoic acid increased apolipoprotein A-I expression in vitro but inhibited its expression in vivo [25]. Nanocrystalline fullerene (C60) inhibits melanoma cell viability in vitro but promotes tumor growth in vivo [26].
The results of this study have shown that the natural compounds levistolide A (LA) and periplogenin (PPG) individually can inhibit the proliferation and cause the apoptosis of HCT116 colon cancer cells in vitro. In the present study, it has been further investigated whether in vitro and in vivo the two compounds could inhibit the growth of gastric cancer cells individually or in combination with the current clinical drug 5-fluorouracil (5-FU).
LA, (product number: 88182-33-6), a chemically defined compound isolated from the traditional Chinese herb Ligusticum chuanxiong Hort. or Angelica sinensis (Oliv.) Diels, was purchased from DESIT biological company, Chengdu, China. The purity and mass detection results were provided as the supplemented data (Figure S1). A 100 mmol/L stock solution of LA was prepared by dissolving the compound in dimethyl sulfoxide (DMSO, product number: D8370; Solarbio life science company, Beijing, China) and was kept frozen at –20℃ until use. PPG, (product number: 514-39-6), another chemically defined compound extracted from Periploca sepium Bunge, was also purchased from DESIT biological company, Chengdu, China. Its purity and mass detection results were also provided as the supplemented data (Figure S2). A 4 mmol/L stock solution of PPG was prepared by dissolving the compound in DMSO and was kept frozen at –20℃ until use. The methyl thiazolyl tetrazolium (MTT, product number: M1205) was obtained from Solarbio life science company, Beijing, China.
The gastric cancer cell line BGC823 was obtained from the National Collection of Authenticated Cell Cultures, Shanghai, China. It was cultured in cultured in Dulbecco’s modified eagle medium (DMEM, product number: 11995; Solarbio life science company, Beijing, China) supplemented with 10% fetal bovine serum (FBS, product number: S9020; Solarbio life science company, Beijing, China) at 37℃ in a humidified incubator (product number: 31111; ThermoFisher science, Shanghai, China) with 5% CO2. All of the experiments were performed while the cell density reached 70–80%. Chemical was added directly into the DMEM medium to the desired working concentration in the in vitro experiments. The samples treated for 48 h were used for cell viability and apoptosis assays, and those treated for 36 h were used for Western blot analysis for evidence of protein cleavage. In the control group, cells were treated with an equal amount of DMSO.
The gastric cancer cells at 70–80% density were collected and seeded into a 96-well plate (product number: 3344; Solarbio life science company, Beijing, China), 6,000 cells per well, 24 h later, LA and PPG were added to the DMEM medium with a step-increasing concentration (LA: 0, 25, 50, 100, 150 μmol/L; PPG: 0, 1, 2, 4, 8, 16, 32 μmol/L), 10 µL MTT reagent (5 mg/mL) was added to each well at the end of treatment and incubated for 4 h at 37°C in an incubator, the medium was removed, and 150 µL DMSO was added to dissolve the Formazan (gentle shaking at room temperature for 10–15 min). The absorbance at 570 nm was recorded on a spectrophotometer (product number: A51119500C; ThermoFisher science, Shanghai, China), which represents the level of cell viability.
The apoptotic cells were visualized by terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labelling (TUNEL) staining using an assay kit (product number: C1091) from Beyotime Biotechnology, Shanghai, China. Cells were seeded on the slides, treated, washed with phosphate buffered saline (PBS, product number: P1022; Solarbio life science company, Beijing, China), and fixed with formaldehyde. Then the slides were immersed in 0.1% Triton X-100 (product number: T8200; Solarbio life science company, Beijing, China) for 2 min at 4℃, followed by 0.3% H2O2 (product number: MM0750; MKbio life science company, Shanghai, China) in methanol (product number: G00005; Innochem company, Beijing, China) for 20 min at room temperature. TUNEL stain was then performed according to the instructions of the manufacturer. The number of TUNEL-positive cells was counted manually in five fields per slide at 400× magnification, 200 cells were counted in each group for statistical analysis.
The cells after treatment were washed with cold PBS twice, lysed with ice-cold lysis buffer [25 mmol/L Tris·HCl (product number: T1010; Solarbio life science company, Beijing, China)], pH 7.4, 150 mmol/L NaCl (product number: S8210; Solarbio life science company, Beijing, China), 1 mmol/L ethylenediaminetetraacetic acid (EDTA, product number: E8040; Solarbio life science company, Beijing, China), 1% nonyl phenoxypolyethoxylethanol (NP-40, product number: N8030; Solarbio life science company, Beijing, China), and 5% glycerol (product number: G8190; Solarbio life science company, Beijing, China) with protease inhibitors cocktail (product number: P1005; Beyotime Biotechnology, Shanghai, China). The lysates were centrifuged at 4℃ for 30 min centrifuged at 13,000 rpm for 30 min and the supernatants were collected. Protein concentration was determined using a protein assay kit (product number: P0010S; Beyotime Biotechnology, Shanghai, China). Total cellular proteins were separated by polyacrylamide gel (product number: P1200; Solarbio life science company, Beijing, China) electrophoresis, blotted onto polyvinylidene fluoride (PVDF; product number: FFP24; Beyotime Biotechnology company, Shanghai, China) membrane, blocked with non-fat milk, washed, incubated with primary antibody [antibody against PPAR (product number: D199347; Sangon Biotech company, Shanghai, China) and β-actin (product number: AA128; Beyotime Life Science company, Shanghai, China)]; both of these two antibodies were diluted by 1:1,000, washed, incubated with secondary antibody (product number: AA128; Beyotime Life Science company, Shanghai, China) goat anti-mouse; diluted by 1:3,000, and washed. The bands were visualized using Image J software and were captured using Chemiluminescence Imaging System (Clinx, Shanghai, China). The antibodies against poly (ADP-ribose) polymerase (PARP) or β-actin were purchased from Beyotime Biotechnology, Shanghai, China. The detailed procedure of Western blot analysis was described previously [27].
Institutional approval was obtained for the experimental protocol. Nude mice (BALB/c nu/nu mice, 6 weeks old, average 18 g) were purchased from the Institute of Laboratory Animals Science, Beijing, China. The gastric cancer cells (1×107 cells/mL, 200 µL) were injected subcutaneously into the lower right flank of each mouse. Mice were randomly assigned and divided into different experimental groups: control, treatment with LA, treatment with PPG, treatment with 5-FU (product number: HY-90006; MedChemExpress company, Shanghai, China), treatment with LA plus 5-FU, and treatment with PPG plus 5-FU. There were 5 mice in each group. The chemicals were given by i.p.: LA (15 mg/kg per day), PPG (6 mg/kg per day), and 5-FU (20 mg/kg per day). All of these three compounds were solved by 30% DMSO plus 70% normal saline. When two kinds of compounds were given combined, they were mixed first and then injected into the mice. The drug treatment continued for 21 days, and the body weight of each mouse was measured on days 3 and 21. At the end of treatment (day 21), the animals were sacrificed after anesthesia, and the tumors were dissected and measured with a vernier caliper to calculate their volume (the dead mice did not been measured).
The in vitro studies were performed in triplicate. All data were represented as mean ± SD. Statistical analysis was performed using the Statistical Product and Service Solutions (SPSS) computer analysis software package. The means of different groups were compared using ANOVA analysis. A P-value < 0.05 was considered to be statistically significant. * P < 0.05; ** P < 0.01; *** P < 0.001, compared with the control group; # P < 0.05; ## P < 0.01, compared with the groups treated with the single compound.
The chemical structures of LA and PPG were shown in Figure 1A and B, respectively. When LA or PPG was added to the cell culture, they were able to inhibit the viability of the gastric cancer cells in a dose- and time-dependent manner (Figure 1C and D). 5-FU was used as the positive control (Figure 1E). The values of half maximal inhibitory concentration (IC50) were 75 µmol/L, 4 µmol/L, and 200 µmol/L for LA, PPG, and 5-FU, respectively. To investigate whether LA or PPG had combinational effects when used with a current clinical drug, the cell viability was compared amongst the four experimental groups, the control, treatment with a natural compound, treatment with 5-FU, and treatment with 5-FU plus LA or PPG. The results indicated that the cell viability in the samples treated with LA or PPG in combination with 5-FU was significantly lower than that in the samples treated with a single compound (Figure 1F and G).
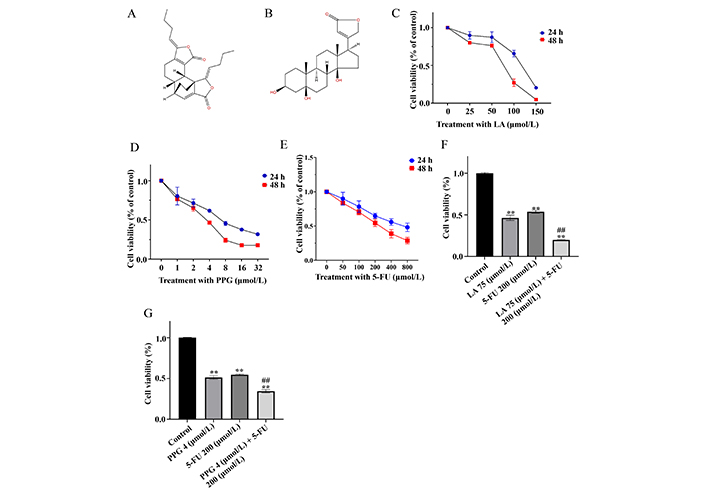
LA and PPG inhibit the viability of the gastric cancer cells. (A, B) The chemical structures of LA (A) and PPG (B); (C–E) LA (C), PPG (D) and 5-FU (E) inhibited the viability of the cancer cells in a dose- and time-dependent manner; (F, G) the combinational effect of LA (F) or PPG (G) with 5-FU to inhibit the viability of gastric cancer cells. ** P < 0.01, compared with the controls; ## P < 0.01, compared with the samples treated with a single compound
To test whether LA and PPG inhibit the cancer cell viability via the induction of apoptosis, TUNEL staining assays were performed to assess the level of the apoptotic cells. The results indicated that LA or PPG was able to increase the number of TUNEL-positive cells (Figure 2A and B). Again, the apoptosis occurred at a higher level in the samples treated with LA or in combination with PPG than that in the samples treated with a single compound (Figure 2C and D). To further support that the compounds triggered apoptosis, the activation of caspase-3 and its substrate PARP were detected using Western blot analysis. Indeed, the treatment of LA or PPG was able to cause the cleavage of PARP. Compared to the samples treated with a single compound, those treated in combination with LA or PPG had a higher level of PARP cleavage (Figure 3). The cleaved caspase-3 fragment was not visualized in our Western blots under experimental conditions.
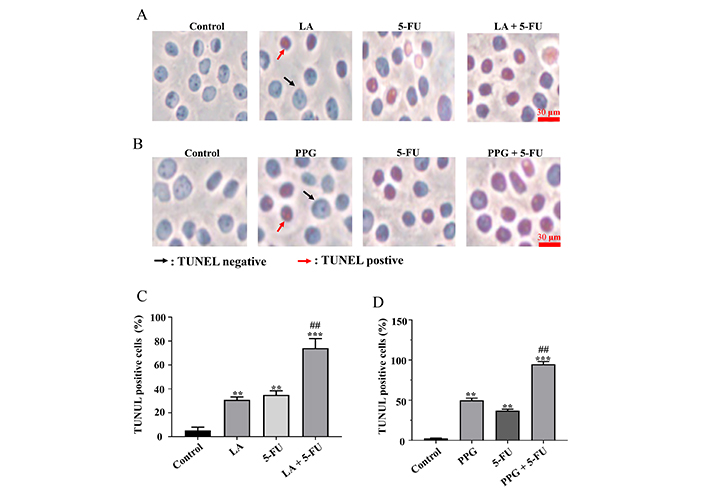
LA and PPG increase the percentage of TUNEL-positive cells. (A, B) In samples treated with LA, LA combined with 5-FU (A), or PPG and PPG combined with 5-FU; (B) TUNEL staining and visualization of TUNEL-positive cells were performed; (C, D) the statistical analysis of TUNEL-positive cells in samples that were given either LA (C) or PPG (D) by themselves or in combination with 5-FU; LA: 75 μmol/L; 5-FU: 200 μmol/L; PPG: 4 μmol/L. A total of 200 cells were counted in each group for statistical analyze in the TUNEL assays. ** P < 0.01, compared with the controls; *** P < 0.001, compared with the controls; ## P < 0.01, compared with the samples treated with a single compound
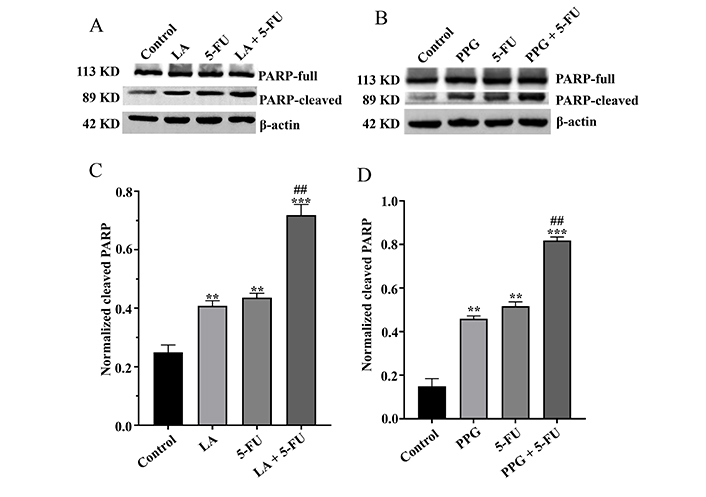
LA and PPG cause the cleavage of PARP. (A) The samples treated with LA, 5-FU, and their combination promoted PARP cleavage; (B) the samples treated with PPG and 5-FU as well as their combination promoted PARP cleavage; (C) digitalized cleaved PARP in the samples treated with LA and 5-FU as well as their combination; (D) digitalized cleaved PARP in the samples treated with PPG and 5-FU as well as their combination. LA: 75 μmol/L; 5-FU: 200 μmol/L; PPG: 4 μmol/L; ** P < 0.01, compared with the controls; *** P < 0.001, compared with the controls; ## P < 0.01, compared with the samples treated with a single compound
Next, the growth inhibition effects of LA and PPG were assessed on gastric cancer cells in vivo in nude mice. Indeed, both LA and PPG, when used alone, were able to inhibit the growth of the cancer cell in vivo. The inhibition rates were higher in the mice treated in combination with a LA or PPG than that in the mice treated with only one compound (Figure 4).
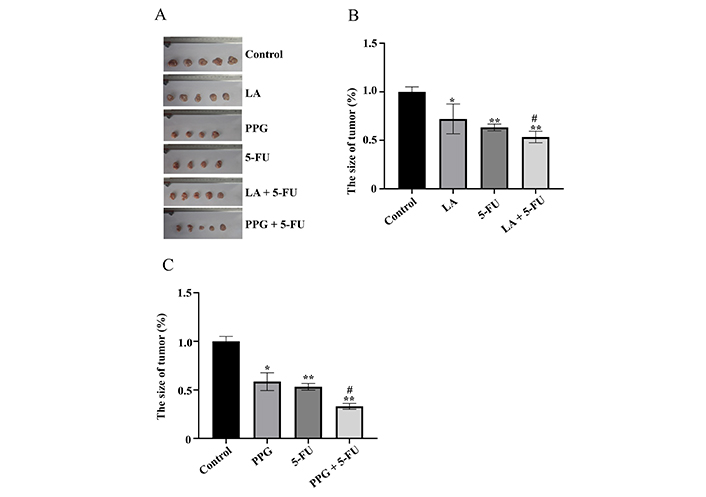
LA and PPG inhibit the growth of the cancer cells in vivo in nude mice. (A) The images of the tumors from the mice treated with LA and PPG as well as their combination with 5-FU; (B) the size of the tumors from the mice treated with LA as well as its combination with 5-FU; (C) the size of the tumors from the mice treated with PPG as well as its combination with 5-FU. LA: 15 mg/kg per day; 5-FU: 20 mg/kg per day; PPG: 6 mg/kg per day. The untreated gastric cancer-bearing mice was used as control. Five mice were used in each group. One mouse was dead during the study in PPG and 5-FU treatment group respectively. * P < 0.05 and ** P < 0.01, compared with the controls; # P < 0.01, compared with the samples treated with a single compound
Chemotherapy was often accompanied by undesirable adverse effects, largely due to dose-limiting toxic effects which can result in weight loss. The body weight of the mice treated with LA or PPG was higher than that of mice treated with 5-FU (Figure 5).
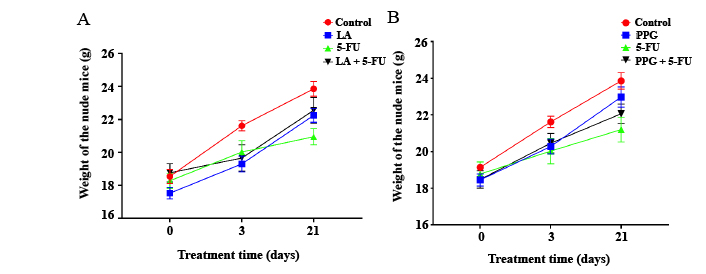
The body weight of nude mice in experimental groups with different treatments. (A) The body weight of nude mice treated with LA and 5-FU only, as well as their combination; (B) the weight of nude mice treated with PPG and 5-FU only, as well as their combination. LA: 15 mg/kg per day; 5-FU: 20 mg/kg per day; PPG: 6 mg/kg per day
The results in this study showed that LA or PPG alone had anti-cancer activity in vitro and in vivo. This confirms the findings of the anti-cancer effects of the two compounds under in vitro conditions [2, 28]. Moreover, there were obvious combinational effects of LA or PPG when used together in vitro and in vivo (Figure 1E and F; Figure 4A–C), implicating that the natural products can be used for combinational treatment with a current clinical drug, at least, which is a better way to start clinical trials than the use of LA or PPG alone. At the cellular level, it seemed that LA and PPG inhibited the cancer cell growth through the induction of apoptosis of the gastric cancer cells (Figure 2A and B), which is supported by the cleavage of PARP which is a substrate of caspase-3 (Figure 3A and B). Under some experimental conditions, cleavage of caspase-3, which indicates its activation, was not detected. This might be due to the poor quality of antibodies or other technical difficulties. It should be mentioned that PPG has been previously shown to inhibit the proliferation of various cancer cell line cells which include PC3, U937, HCT-8, Bel-7402, BGC823, A549, and A2780 cell lines in vitro [29]. In adriamycin-resistant leukemia cell line k562/dox, LA induced cell apoptosis in vitro by inhibiting multi-drug resistance protein 1 (MDR1) expression [30]. The shared shortcoming of these studies was that the anti-cancer effect of LA and PPG was shown only under in vitro conditions, which is overcome by this study which confirmed the anti-cancer effects of the compounds in vivo. At the molecular level, it has reported that LA-induced colon cancer cell apoptosis by activating reactive oxygen species (ROS)-endoplasmic reticulum (ER) stress pathways [28]. In breast cancer cells, Chen and his colleagues [31] found that LA significantly enhanced adriamycin- or vincristine-induced G2/M arrest and apoptosis by inhibiting P-glycoprotein’s function. It is also showed that PPG induced colon cancer cell apoptosis through activating ROS-ER stress pathways by triggering binding immunoglobulin protein (BIP)-phosphorylation of eukaryotic initiation factor-2α (eIF2α)- C/EBP homologous protein (CHOP) and inositol-requiring transmembrane kinase endoribonuclease-1α (IRE1α)-apoptosis signal-regulating kinase 1 (ASK1)-c-Jun N-terminal Kinase (JNK) signaling pathways [2]. In nasopharynx cancer cells, PPG induced cancer cells apoptosis by inhibiting mitogen-activated protein kinase signaling pathway (MAPK) [32]. PPG was also found to inhibit esophageal squamous cell carcinoma growth by inhibiting signal transducer and activator of transcription 3 (STAT3) function [33]. PPG could directly bind with STAT3, therefore, inhibits STAT3 dimerization, nuclear import, and transcription activities [34]. The two compounds can inhibit the growth and cause apoptosis of various cancer cells. Different signal transduction pathways might be involved in the initiation of apoptosis; however, ER stress appears to be a shared pathway.
LA is rich in Ligusticum chuanxiong Hort. and Angelica sinensis (Oliv.) Diels and belongs to the family of Apiaceae, and is often used in the form of formulas for treating cancers (Chinese Pharmacopoeia Commission, 2010). For example, in a study, Ligusticum chuanxiong Hort., Radix Paeoniae Rubra, Rheum palmatum L., and Rehmannia glutinosa (Gaetn.) Libosch. ex Fisch. et Mey. were used as formulas to treat ovarian cancer. Compared with the patients (n = 101) who were not treated with this formula, the patients (n = 101) who used the formula had a lower mortality rate; the mortality rate was reduced by 55% [35]. Chen used Ligusticum chuanxiong Hort., Ostrea gigas Thunberg, Ranunculus ternatus Thunb, Juglans regia, and Fritillaria cirrhosa D. Don for the treatment of malignant lymphoma for three months, during which the size of the tumors was significantly reduced [36]. In addition, Chen also used Ligusticum chuanxiong Hort., Bupleurum L., Radix Paeoniae Rubra, Citrus reticulata Blanco, and Glycyrrhizae to treat advanced esophageal cancer, and found that the size of cancer was significantly reduced after treatment for three months, and in addition, Chen also used Ligusticum chuanxiong Hort., Bupleurum L., Cynanchum otophyllum Schneid., and others to treat neck reticulum sarcoma and found that the treatment inhibited the tumor growth; tumors eventually became undebatable [36]. In 2011, Wang used the formula that had Angelica Sinensis (Oliv.) Diels, Glyptostrobus pensilis (Staunt.) Koch, Fritillaria cirrhosa D. Don, and Hedyotis diffusa Willd. for treating cancers in the bladder, prostate, and cervix for three months, and found that the formula significantly reduced the size of the bladder cancer as well as blocked the progress of prostate and cervical cancers [37]. Liu used Angelica Sinensis (Oliv.) Diels, Cynanchum otophyllum Schneid., Rhizoma Curcumae Aeruginosae, Glycyrrhiza uralensis Fisch., Hedyotis diffusa Willd., and Salvia chinensis Benth. to treat gastric cancer [38]. After one year of treatment, the size of the tumors was significantly reduced. Du used the formula of Angelica sinensis (Oliv.) Diels, Citri Reticulatae Pericarpium, and other herbs to treat cervical cancer for three months, and found that the treatment stopped the development of cancer, as evidenced by the reduction of tumor size [39]. When Angelica Sinensis (Oliv.) Diels and Ligusticum chuanxiong Hort. were used with Psoralea corylifolia Linn. for treating fibroosteoma, in a report, the 2–4 years of treatment progressively reduced the tumor size, and the tumors eventually became undetectable. There was no single case of relapse in the follow-up of 33 years. Date back to at least Ming and Qing Dynasties, Ligusticum chuanxiong Hort. and Angelica Sinensis (Oliv.) Diels had already often been used in formulas for the treatment of cancers, with recorded positive therapeutic outcomes. Similarly, PPG is abundant in Streptocaulon Juventas (Lour.) Merr., which is also used for the treatment of cancer in China. Liang has used Streptocaulon juventas (Lour.) Merr., Selaginella uncinata, Asarum sagittarioides C. F. Liang, Tribulus terrester L., and Lobelia chinensis L. to treat lung cancer [40]. After one year of treatment, the size of primary and metastatic tumors was reduced or even disappeared. For the patients whose tumors became undetectable, there was no recurrence after follow-up for one year. Zhao and Cui [41] used Streptocaulon juventas (Lour.) Merr. in combination with FOLFOX4 chemotherapy to treat advanced lung cancer for three months. In 80 patients, this combinational treatment significantly inhibited the growth of lung tumors. At the cellular level under the in vitro conditions, extracts or compounds of the Ligusticum chuanxiong Hort., Angelica sinensis (Oliv.) Diels and Streptocaulon juventas (Lour.) Merr. are shown to inhibit the growth of various cancer cells [42, 43]. The results in the present study showed that LA and PPG were able to inhibit the growth of the gastric cancer cells in vitro and in vivo (Figure 1C and D; Figure 4A), which provides the experimental evidence that Ligusticum chuanxiong Hort., Angelica sinensis (Oliv.) Diels and Streptocaulon juventas (Lour.) Merr. have anti-cancer effects, which shows that the reason for using these herbs for the treatment of cancers is sound scientifically. Our results also support the view that natural products provide a major resource for drug development.
Drug discovery and development is a sophisticated procedure, which can not only be time-consuming but also costly. More than 90% of drug developments failed, as the candidates cannot reach the market for clinical use. In extreme cases, licensed drugs can even be withdrawn from the market due to various reasons [44]. To increase the probability of success, researchers should set up a good plan which includes the selection of proper candidates, rigorous protocols for the validation of bioactivities in vitro and in vivo as well as clinical studies. The intellectual property and availability as well as the price of drug candidates should also be taken into consideration. Screening is a common method for drug discovery; the development of taxol is an example [45]. The question is how to increase the efficiency of screening. In this study, the approach is to create a databank that lists herbs and animals which are often used in treating cancer in traditional Chinese Medicine (TCM). The known chemical constituents of the herbs and animals, which have not been reported to have anti-cancer effects, are retrieved from the available literature. The compounds are then used for screening for cancer cell growth inhibition activity. Lately, 36 compounds were screened and found that four of them which include the LA and PPG were able to inhibit the growth of colon or gastric cancer cells in vitro, giving a success rate of 11%, which indicates that the screening approach is effective.
5-FU is a fluoropyrimidine available in the clinic for the treatment of a variety of solid cancers including those in the gastrointestinal tract [46]. It can be incorporated into RNA and DNA of cells and inhibit the thymidylate synthase which is an enzyme involved in nucleotide synthesis [47]. Although the therapeutic benefits of 5-FU have been shown over decades, it must be applied in high doses weekly, causing gastrointestinal toxicity and myelotoxicity [46]. We believe that combinational treatment using 5-FU and a natural product could be an option.
In conclusion, our results show that LA and PPG could inhibit the growth of gastric cancer cells in vitro and in vivo when used alone or in combination with 5-FU. The two compounds are quality candidates for chemotherapeutic development for the treatment of cancers, at least gastric cancer. The results help justify the use of Ligusticum chuanxiong Hort., Angelica sinensis (Oliv.) Diels and Streptocaulon juventas (Lour.) Merr. for the treatment of cancers.
5-FU: 5-fluorouracil
DMEM: Dulbecco’s modified eagle medium
DMSO: dimethyl sulfoxide
ER: endoplasmic reticulum
FOLFOX4: 5-fluorouracil, leucovorin, and oxaliplatin
LA: levistolide A
MTT: methyl thiazolyl tetrazolium
PARP: poly (ADP-ribose) polymerase
PPG: periplogenin
STAT3: signal transducer and activator of transcription 3
TUNEL: terminal deoxynucleotidyl transferase-mediated deoxyuridine triphosphate (dUTP) nick-end labelling
The supplementary material for this article is available at: https://www.explorationpub.com/uploads/Article/file/10086_sup_1.pdf.
We appreciate the methods direction provided by Doctor Ye from the life science school of Jiangsu Normal University.
JLG: Conceptualization, Methodology, Investigation. HMH: Formal analysis, Investigation, Methodology. SCL: Funding acquisition, Writing—review & editing. JZZ: Project administration, Writing—original draft.
The authors declare that they have no conflicts of interest.
The nude mice assays have been approved by the Ethics Committee of Jiangsu Normal University.
Not applicable.
Not applicable.
The data are available from the corresponding authors (jz_zhao@jsnu.edu.cn).
The study was supported by grants to Dr. Shao Lee [No. 9212418120, 9212430401]. Dr. Shao Chin Lee contributes to the study design and the report writing.
©The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 3451
Download: 35
Times Cited: 0
Maria G. Ciulla ... Kamal Kumar
Rozita Takjoo ... Norelle L. Daly
Miguel García-Castro ... Juan Manuel López-Romero
Anton Kolodnitsky ... Vladimir Poroikov
Atri Das ... Shantanabha Das
Galana Siro, Atanas Pipite
Maya G. Pillai, Helen Antony
