Affiliation:
1Department of Pharmacology and Toxicology, University of Texas Medical Branch, Galveston, TX 77550, USA
†These authors contributed equally to this work.
Email: rahuldilawari1@gmail.com; radilawa@utmb.edu
ORCID: http://orcid.org/0000-0002-9208-6045
Affiliation:
2Department of Pediatrics, Washington University School of Medicine, St. Louis, MO 63110, USA
†These authors contributed equally to this work.
ORCID: http://orcid.org/0000-0002-6518-0714
Affiliation:
3National Agri-Food Biotechnology Institute (NABI), Sahibzada Ajit Singh Nagar 140308, Punjab, India
ORCID: http://orcid.org/0000-0002-5030-7567
Explor Drug Sci. 2025;3:1008110 DOI: https://doi.org/10.37349/eds.2025.1008110
Received: February 11, 2025 Accepted: April 08, 2025 Published: May 09, 2025
Academic Editor: Jean-Marc Sabatier, Aix-Marseille University, France
Microbial pathogens with antibiotic resistance have become challenging to manage in the last few decades. The situation is alarming and a threat to humankind. Despite emerging new drug candidates, there are numerous strategies for efficient antibiotic delivery for treating such infections; antibiotic-resistant bacteria are still not eradicated adequately from the infected hosts. Recently, antimicrobial peptides (AMPs) have emerged as saviors in overcoming antibiotic resistance against bacteria. AMPs are being produced naturally as well as synthetically. Irrespective of the source of production, AMPs have shown higher specificity and lower toxicity to the host. Such functions have been attributed to distinct structures, functions, and varied mechanisms of action. This review highlights sources, structural and physiological characteristics, action mechanisms, and biological potency towards clinical applications. Most recently, AMPs have also been explored in treating cancers and tumors. Despite the entry of a few AMPs into clinical trials, there are limitations associated with their usage, like shorter half-life, protease cleavage, and toxicity. There is an urgent need to produce AMPs with intensified activity, biocompatibility, and lesser toxicity. This review sheds light on these aspects and the future of AMPs for the betterment of the human race.
Host defense peptides (HDPs), previously known as antimicrobial peptides (AMPs), are small molecular weight peptides and are present in all strata of life, i.e., prokaryotes (bacteria) to eukaryotes (fungi, plants, and animals including invertebrates and vertebrates). They play a crucial role in immunity to the host [1] by acting against bacteria, fungi, parasites, and viruses [2, 3]. AMPs exert broad-spectrum bioactivity with less toxicity and minute chances of resistance development. To date, the AMP database [data repository of antimicrobial peptides (DRAMP)] has reported a total of 22,499 entries, out of which 6,105 are natural and synthetic AMPs, 16,110 patent candidates, and 96 are in drug development (preclinical and clinical stage). AMPs from the different kingdoms are also categorized, such as 2,519 from animals, 826 from plants, 6 from fungi, 431 from bacteria, 4 from archaea, and 7 from the protozoan kingdom [4]. AMPs produced by bacteria (also called bacteriocins) become non-favorable to other’s survival as they compete for the nutrients present in the same niche. Lantibiotics and non-lantibiotics are two categories of bacteriocins. Lantibiotics contain non-natural amino acids like nisin produced by Lactococcus lactis; it was the first lantibiotic to be isolated and characterized well in 1947 [5]. Nisin has a nanomolar (nM) minimum inhibitory concentration (MIC) against a myriad of Gram-positive bacteria and has thus been used as a food preservative also for the last 6 decades [6]. Another famous lantibiotic is paenibacillin produced by Paenibacillus spp., which is tolerant to high temperature and proteinase K degradation, making it a potential candidate in the health sector at the industrial scale [7]. Mersacidin is another clinically important bacteriocin resistant to Gram-positive bacteria [8]. Eukaryotic examples are also flooded with AMPs with beneficial attributes. The hemolymph of Hyalophora cecropia (silk moth) contained AMPs named cecropins [9]. Cecropins are positively charged and amphipathic in nature and possess broad-spectrum anti-microbial activity against Gram-positive and Gram-negative bacteria and fungi. It went on to discover more eukaryotic AMPs like defensins from mammalian macrophages [10] and sacrotoxins from Sarcophaga peregrine, a flesh fly [11]. Since plants do not possess B cells and T cells as in mammals for the innate and adaptive immune response against foreign pathogens; AMPs play a key role in giving protection against bacteria and fungi. AMPs produced by plants are cysteine-rich and disulfide linkages. Plant flowers, tubers, seeds, and leaves produce AMPs. Well-characterized AMPs from plant sources come under thionins [12], plant defensins [13], and cyclotides [14]. Invertebrates are like plants in terms of immune response, i.e., they lack adaptive and innate immune responses. Nearly all invertebrates produce AMPs; however, insects and marine invertebrate AMPs (oysters, shrimps, horseshoe crabs) have been studied the most [15–17]. Horseshoe crabs produce tachyplesin and polyphemusin AMPs, which have antibacterial and antifungal activity [18]. Also, polyphemusin possesses antiviral properties, especially against the human immunodeficiency virus (HIV) [19]. Vertebrate AMPs have innate and adaptive immunity arsenal. Still, a variety of vertebrates produce AMPs like magainins from the skin of Xenopus laevis, a frog [20], crypt peptides from mouse paneth cells [21], granules of white blood cells, epithelial tissues of mouth, lungs, skin, and bodily fluids [22–25]. Cathelicidins and defensins are AMP subcategories produced by vertebrates. Regulation of AMPs occurs by posttranslational modifications (PTMs) like proteolysis, amidation, ADP-ribosylation, glycosylation, and phosphorylation. Anticancer treatment medicines pose resistance to patients undergoing pain; AMPs serve as an anticancer treatment option. AMPs may both promote and inhibit cancer growth [26]. AMPs produced by vertebrates also possess immunomodulatory and anti-inflammatory effects [27]. AMP has also a role in wound healing [28]. Pathogenic bacterial diseases have been treated by antibiotics for a long time, but drug resistance due to long-term use, extensive utilization, and single target of antibiotics is one of the most recent unsolved challenges for clinical infection management [29, 30]. Here comes the AMP, which acts against all ordeals because it acts on multiple targets on the plasma membrane and intracellular targets of drug-resistant pathogens [3, 31, 32]. A smaller number of AMPs enter the clinical stage with therapeutic efficacy because of innate defects in the natural AMPs. Further exploration of AMPs for structural characteristics, and mechanism of action of AMPs may improve stability, activity, targeting, and lesser cytotoxicity. This review elaborates on the origin, characteristics, and mechanism of action and bioactivity of AMPs so that readers have comprehensive latest knowledge and understanding of AMPs along with updates on clinical development and AMPs applications.
Eukaryotes have an evolutionary conserved mechanism of AMP production in response to pathogen invasion. AMP production takes less time and energy as compared to antibody synthesis and moves to target relatively in less time as compared to immunoglobulins; also, some eukaryotes, like insects, lack lymphocyte-based immune arsenal and here, smaller peptide synthesis combat bacterial infections [33]. Very first discovered AMP was from chrysalis (silkworm) and from micro-organisms [34], plants [35], invertebrates [36], fish [37], amphibians [38], reptiles [39], birds [40], and mammals [41]. Nisin was the first AMP from bacteria to be isolated, and it is cytotoxic to other bacterial communities present in the same environment [5]. This feature of nisin is exploited at an industrial scale by using it as a food preservative [42–44]. Mammalian intestinal microbiota bacterial communities also produce AMPs [45, 46]. Mushrooms like Coprinopsis cinerea produce AMP named copsin, which is bactericidal against Enterococcus faecium (E. faecalis) and Listeria monocytogenes (L. monocytogenes) [47]. AMPs derived from plants like thionins, defensins, and cyclotides prevent pathogenic microbe invasion. Such AMPs are rich in disulfide linkages, which allows structural stabilization [48]. Since invertebrates do not possess a lymphocyte-based immune system, they possess innate immune responses present in hemolymph, skin, mucosa, etc., to resist pathogens [49]. Hyalophora cecropia’s hemolymph has cecropins, which are antibacterial against Gram-positive and harmful bacteria [50]. Drosocin from the intestine of Drosophila is effective against (Pseudomonas entomophila) P. entomophila [50]. To date, 1,770 AMPs have been reported from vertebrates (fish, amphibians, reptiles, birds, and mammals). Fishes produce hepcidins and piscidins [51]. Frog-like Paa yunnanensis’s skin has cathelicidin-PV, which is antibacterial and antifungal to even drug-resistant candidates [52, 53]. AMPs from mammals fall under cathelicidins and defensins [54]. Crotalicidin and fragments from rattlesnakes have antibacterial, antifungal, and anti-tumor activity [55]. Cathelicidins from mammals have conserved cathelin domain [56], stored in nonfunctional form encapsulated in granules of neutrophils and macrophages and they become functional upon leukocyte activation and processing [54]. LL-37 is the only cathelicidin found in humans [57] and gets accessory help from moonlighting protein GAPDH to lessen M.tb (Mycobacterium tuberculosis) burden intracellularly [25].
Another important class of AMPs belonging to mammals is defensins, which are further subdivided into α-, β-, and θ-defensins based on disulfide bond arrangement [58, 59]. β-defensins are predecessors for α- and θ-defensins [60]. Humans possess both except θ-defensins. Birds, reptiles, and cattle produce β-defensins. Non-human primates produce θ-defensins from leukocytes and bone marrow [54, 60]. Both cathelicidin and α-defensins are trimmed to C-terminal peptides possessing antimicrobial activity after getting cleaved by elastase, metalloproteinase [61]. AvBD9, a β-defensins isolated from quail, was antibacterial to 11 species of bacteria, including Gram-positive and Gram-negative [62]. Mammals have mucus layers where AMPs are abundant and prevent the colonization of bacteria, fungi, and parasites [63, 64], as well as in mast cells and granulocytes [65]. α-defensins are secreted from neutrophils, macrophages, and Paneth cells, while β-defensins are harbored in leukocytes and epithelial cells of the skin, respiratory, digestive, and genitourinary tract, urine, heart, and skeletal muscle [66]. Similar to defensins, most cathelicidins are stored in granules of epithelial and immune cells in humans [54, 67] along with mucus, blood, urine, sweat, and tears [63, 68–70]. Therefore, extensive structural and physiochemical characterization of AMPs from different sources can identify novel AMPs with substantial clinical and industrial potential. Directed evolution, machine learning, and sequence-based encoding are a few key approaches that can accelerate the AMP discovery process [71]. Various AMPs and antimicrobial proteins produced at varied sites in human tissue after contracting microbial pathogens are enlisted in Table S1.
Based on amino acid sequences, peptide net charge, protein structure, and origins, AMPs are separated into multiple subgroups. Most anti-microbial peptides have a net charge of +2 to +9 and are made up of 10–100 amino acids [72]. Data on the bioactivities, toxicities, chemical modifications, amino acid sequences, and three-dimensional structures of peptides having antimicrobial properties can be found in the vast, publicly accessible Database of Antimicrobial Activity and Structure of Peptides (DBAASP, https://dbaasp.org/). Over 15,700 entries (8,000 more than the previous version) are included in the most recent version 3.0 (DBAASP v3) [73]. Anionic AMPs, with a net charge range of −1 to −8, comprise the first class of AMPs and have five to seventy amino acid residues [74]. While tiny molecules encoded by genes make up a minor portion of anionic AMPs, most anionic AMPs are peptide fragments following proteolysis. Among their structural characteristics are cyclic cysteine knots and α-helical peptides seen in certain amphibians [75]. Similar to bigger proenzymes’ capacity to neutralize charges, they appear to use metal ions and the negatively charged elements of the microbial membrane to create salt bridges and interact with bacteria [76, 77]. For instance, it was shown that in the presence of zinc ions, the ovine pathogen Mannheimia haemolytica was susceptible to the antibacterial effects of ovine pulmonary surfactant-associated anion peptide (SAAP), the first anionic AMP ever identified with 5−7 aspartate residues [78]. The surfactant solution’s bactericidal activity was significantly reduced when 0.14 mol/L NaCl and EDTA were added, but it was recovered when ZnCl2 was added again. Furthermore, the α-helical anionic AMP maximin H5’s amidated C-terminal fragment establishes an intra-peptide hydrogen bond with the peptide’s N-terminal portion, which is crucial for maintaining the tipped α-helix shape [75]. The cationic α-helical AMPs comprise the second subgroup. These short peptides, typically less than 40 amino acids long, exhibit amidation at the C-terminus and have a net charge ranging from +2 to +9 [79]. In aqueous solutions, the structure of these peptides is disordered; however, in the presence of liposomes or liposomes A, phospholipid vesicles, sodium dodecyl sulfate (SDS) micelles, and trifluoroethanol, all or parts of the molecules convert into α-helical structure [80]. Moreover, these AMPs usually contain more than 50% hydrophobic amino acids, which encourages the development of an amphiphilic structure during target-cell interaction [81]. The majority of cathelicidins are amphiphilic α-helical AMPs [82], among which LL-37, cecropins, and magainins have all been thoroughly investigated. The only human cathelicidin of an active fragment that is liberated from hCAP18 in neutrophils with a neutral pH and a net charge of +6 is LL-37, which is produced by serine protease 3 [83, 84]. When LL-37 is exposed to water at a concentration of 15 mmol/L, its circular dichroism displays a disordered structure; but, in the presence of HCO3−, SO42−, or CF3CO2−, it changes into an α-helical structure [85]. The peptide concentrations directly correlate with the structural transformation efficiency. Furthermore, there exists a correlation between the degree of α-helix and LL-37’s antibacterial efficacy against both Gram-positive and Gram-negative bacteria. The cationic β-sheet AMPs comprise the third subgroup. Usually, the peptides have two to eight cysteine residues that form one to four intramolecular disulfide bond pairs [86]. These peptides’ biological activities and structural stability depend on their disulfide links. For instance, they stay active when they mutate to hydrophobic amino acids (alanine and leucine excepted) but become inactivated when cysteines are substituted with acidic amino acids [87]. Nevertheless, the antibacterial action and cytotoxicity of mouse defensins, hBD-3 (human beta-defensin 3), and human neutrophilic peptide 1 (HNP 1) are not dependent on their disulfide links or structure [61, 74]. Defensins make up the majority of the β-sheet AMPs [86]. As previously stated, the characteristic intervals between the six cysteine and disulfide bond types are used to categorize the mammalian defensins into two groups: α-defensins and β-defensins [88]. Mammalian defensins exhibit remarkably comparable tertiary structures despite differences in their covalent structures [89]. Regarding α-defensins, they create a cyclic structure by combining cysteine with disulfide bonds near the amino terminus and a three-stranded chain by hydrogen bonding with the β-hairpin [89]. Amphipathic α-defensins’ ability to destroy bacterial membranes by interaction with phosphatidyl chains is dependent on hydrophobic amino acids and their positive charge [90]. Moreover, the contact between cationic α-defensin residues and negatively charged molecules on the bacterial surface, or between hydrophobic residues and the bacterium, maybe the main mediator of membrane breakdown and bacterial killing. The chicken Gga-AvBD11’s N-terminal domain primarily mediates and amplifies its antibacterial and antiparasitic properties, although the full-length protein is necessary for antiviral activity [91]. The end-to-end cyclized tetracyclic peptides known as θ-defensins are connected by three disulfide bridges between their antiparallel β-sheets [92]. The ability of β-defensins to maintain their activity at high salt concentrations is attributed to their cyclic structure, which also contributes to their antibacterial capabilities by reducing the microbicidal activities that occur when the cyclic structure is lost [93]. According to recent research, defensins’ stability and structure are mostly determined by the quantity and location of disulfide bonds, while their capacity to bind membranes and inhibit bacteria is dependent on their cyclic backbone [94]. The fourth grouping consists of extended cationic amino acid polymers (AMPs) that do not have typical secondary structures and contain specific amino acids such as arginine, proline, tryptophan, glycine, and histidine [81]. Only hydrogen bonding and the van der Waals force, which interacts with the membrane lipids, can stabilize their structures. Tryptophan (38%) and proline (23%) are abundant in indolicidin [95], histatin-8 (33.3%) and histidine are abundant in histatin-8 [96], and PR-39 (typically 24%) and proline (49%) are abundant in PR-39 [97]. Proline (53.2%) and phenylalanine (19%) are abundant in prophenin-1 [98]. Antimicrobial protein fragments make up the fifth subgroup. There are proteins found in nature that, when broken down, offer a broad range of bactericidal properties. An essential part of the innate immune system’s defense against foreign infections is lysozyme, the first antimicrobial protein ever found [99–101]. Its extracellular portion has an α-helix and β-sheet structure and 130 amino acids. Other membrane-active and DNA-binding proteins have also been shown to have a helix-loop-helix (HLH) region, as observed in the lysozyme of humans and chickens [102]. When applied to both Gram-positive and Gram-negative bacteria as well as the fungus Candida albicans (C. albicans), the HLH peptide exhibits potent bactericidal properties. A sleep-inducing gene encoding the NEMURI protein, which has an arginine-rich region and immunomodulatory properties as well as a potent bactericidal activity similar to kanamycin, was discovered by Toda et al. [103] in fruit flies. The amino terminal copper and nickel (ATCUN) binding motif is notably present in certain AMPs. The sequence H2N-XXH is found at the N-terminus, where any amino acid other than proline can be the XX [104]. The motif is highly affinized for Cu2+ and Ni2+ [105]. The Cu2+-ATCUN complex has the ability to create reactive oxygen species (ROS), which can damage proteins, lipids, and nucleic acids [106, 107].
Antibacterial activity: AMPs work against bacteria through membrane- or non-membrane-mediated mechanisms. As was previously mentioned, the presence of particular anionic components in the plasma membranes of bacteria and fungus, such as mannan in fungi, lipoteichoic acid in Gram-positive bacteria, and LPS in Gram-negative bacteria, gives cationic AMPs a greater affinity for microbial pathogens. AMPs can permeate the membrane to perform intracellular functions or produce membrane permeability or perforation to cause intracellular contents to seep out. The development of bacterial resistance to AMPs is prevented by the AMPs’ quick killing time, broad membrane and intracellular effects, and lack of targeting of particular molecules or pathways. For this reason, the use of AMPs in managing antimicrobial resistance is appealing. A broad class of cationic small molecules (AMPs) with between 30 and 60 amino acids that are extracted from bacteria are called bacteriocins. They were divided into two groups based on peptide synthesis mechanisms. Peptides produced by non-ribosomes with broad antibacterial action and peptides synthesized by ribosomes with relatively narrow antibacterial activity against bacteria and fungus make up the first group [108]. In the culture broth of Streptomyces albofaciens, Wang et al. [109] identified a novel short non-ribosomal AMP called albopeptide 6, which exhibited a narrow spectrum action against vancomycin-resistant E. faecalis. Belonging to the bacteriocins family, nisin exhibits strong antibacterial properties against a diverse array of Gram-positive and even Gram-negative bacteria [110]. Penicillin or chloramphenicol coupled with nisin enhanced the antibacterial impact against E. faecalis, although the single antibiotic did not significantly affect the bacterium, according to Tong et al. [111]. AMPs, therefore, serve as a novel therapeutic alternative for treating antibiotic-resistant bacteria, either in isolation or in combination with other agents. According to research, the forkhead box transcription factor O (FoxO) directly controls the production of AMPs in shrimp, including CrusI-3 and Alf-E1, but it is independent of the Imd signaling pathway [112]. Notably, resistance can be induced by prolonged exposure to low concentrations of AMPs. Therefore, it is advised to use a high concentration of AMPs to sustain the bactericidal activity [113].
Antiviral activity: AMPs exhibit broad-spectrum antiviral activity against enveloped viruses in addition to their antibacterial activity. For instance, in transmissible gastroenteritis viruses, bovine antimicrobial peptide-13 (APB-13) efficiently suppresses viral multiplication by interfering with the synthesis of viral proteins and the expression of viral genes [114]. Targeting the viral membrane glycoprotein, prointegrin, and indolicidin’s anti-herpes simplex virus (HSV) action has been linked to preventing the virus’s adherence and entrance [115, 116]. LL-37 inhibits a variety of enveloped viruses, including the dengue virus (DENV), zika virus (ZIKV), influenza A virus (IAV), vaccinia virus (VV), and HIV [117–122]. This is accomplished by damaging the viral membrane and preventing DNA replication. Furthermore, non-enveloped enterovirus 71 replication is significantly suppressed by LL-37 and mouse CRAMP via regulating the antiviral response and blocking viral binding [123]. According to LeMessurier et al. [124], AMPs change the immune system’s reaction to IAV infection, improving the host’s defense against the virus. By preventing the virus from attaching, pa-MAP and temporin B, both lessen HSV1 infection [125, 126]. Furthermore, temporin B has the ability to demolish the viral envelope and impact the post-infection phase that follows. The influenza virus’s hemagglutinin protein can be interacted with by temporin G, an analog of temporin B. This interaction prevents the HA2 subunit’s conformational rearrangements, which is necessary for the viral envelope to fuse with intracellular endocytic vesicles and allow the virus to enter host cells [127]. ZIKV is inhibited by cathelicidin-derived AMP GF-17 and BMAP-18, which interfere with the interferon (IFN) pathway and directly inactivate the virus [128]. Additionally, some AMPs exhibit antiviral properties against pseudorabies and DENV [129, 130]. Additionally, it has been observed that AMPs combat non-enveloped viruses. It has been demonstrated, for example, that LL-37 is effective against non-enveloped viruses, including adenovirus, rhinovirus, and Aichi virus [125, 131, 132]. As will be detailed below, AMPs not only directly suppress the growth of the virus by disrupting its replication cycle and viral particle, but they also indirectly do so by controlling the host immunological response [133, 134]. According to recent research, vitamin D can cause the body to produce cathelicidins and defensins, which slow down the pace at which viruses replicate and lower the risk of contracting influenza and coronavirus disease as well as the chance of dying from it [135].
A potential therapy strategy for COVID-19: Defensins and cathelicidin LL-37 are examples of human-derived AMPs, which belong to the innate immune system and are essential for early lung defense against viruses. These AMPs inhibit the growth of viruses by a number of different mechanisms, such as direct binding to virions, binding to and altering host cell-surface receptors, preventing viral replication, preventing the aggregation of viral particles, and indirectly acting as chemokines to boost or suppress adaptive immune responses. Four structural proteins called the S (spike), E (envelope), M (membrane), and N (nucleocapsid) proteins are present in all coronaviruses, including SARS-CoV-2 [136]. The S protein is the most relevant of these for research on AMP-related effects against coronaviruses. It is composed of two functional subunits, S1 and S2 [137]. S1 attaches to the angiotensin-converting enzyme 2 (ACE2) receptor on host cells, and S2 then facilitates the fusion of the viral and cellular membranes [138]. At sensitive mucosal regions in our bodies, β-defensins and LL-37 act as natural antimicrobials and are predisposed to act as “disruptors” of viral attachment, entrance, and infection. These AMPs are prime candidates to investigate as potential anti-SARS-CoV-2 medicines due to their various modes of action that have been established against a variety of viruses, including respiratory viruses. LL-37 [139] and hBD-2 [140] were predicted by recent in silico molecular docking studies to bind strongly with the receptor-binding domain (RBD) of SARS-CoV-2, indicating that these two peptides may be able to block the RBD. For LL-37 [141] and hBD-2 [140], biophysical experiments employing bio-layer interferometry (BLI) and microscale thermophoresis (MST) corroborated the in-silico reports.
Antiparasitic activity: Although AMPs’ function in antibacterial and antiviral activity has been well documented in the literature, there are still few findings about their antiparasitic efficacy, especially in vivo and clinical settings. The variety of parasites is enormous, encompassing anything from worms to protozoa. An enormous global health burden is caused by parasites, which are a significant source of diseases affecting people globally [142, 143]. The World Health Organization (WHO) has classified eleven parasitic infections as neglected tropical illnesses because they disproportionately affect people with low incomes and endanger the health of millions of people [143]. The most significant parasite illnesses include schistosomiasis, trypanosomiasis, leishmaniasis, and malaria [144, 145]. Antiparasitic treatment approaches based on AMPs have attracted much attention lately. Many different organisms have been discovered to contain leishmanicidal amino acid polymers (AMPs). As an example, consider the following: (1) dragomide E, a linear lipopeptide isolated from the cyanobacteria Lyngbya majuscula, demonstrated anti-leishmanial activity against Leishmania donovani promas tigotes; (2) halictine-2, derived from the venom of eusocial honey bees, demonstrated notable anti-leishmanial activity without hemolytic activity for mouse macrophages and human erythrocytes [146]. Furthermore, by explicitly stopping the parasite-infected erythrocytes from synthesizing adenosine triphosphate (ATP), the peptide LZ1, which is derived from snake cathelicidin, showed strong suppression of blood-stage Plasmodium falciparum [147]. Owing to its distinct chemical structure, phylloseptin-1, which is derived from Phyllomedusa azurea’s skin secretions, exhibited intense antiparasitic activity and inhibited the emergence of cross-resistance [148].
Antifungal activity: According to the AMP database (https://aps.unmc.edu/database/anti), 1,549 AMPs have antifungal properties to date. The majority of these AMPs are against Candida species, including C. glabrata, C. krusei, C. albicans, and C. parapsilosis. These peptides are derived from plants, insects, arachnids, humans, etc. Plant-derived peptides are HsAFP1, Nad1, Psd1, and RsAFP2; insect and arachnids derived are heliomicin, Jelleine-I, Jelleine-II, comes in, and human-derived ones are CAG-N46, psoriasin, β-defensin-2, β-defensin-3 [149, 150].
Humans include three main AMP groups: histatins, cathelicidins, and defensins. Defensins are categorized as either β- or α-defensins according to the type of disulfide bond arrangement. Neutrophils, lymphocytes, and epithelial cells of the skin and mucous membranes produce α- and β-defensins. Epithelial cells, neutrophils, and lymphocytes present in mucosal membranes and the skin are the primary producers of α- and β-defensins [151]. Since neutrophils produce human α-defensins 1−4, also known as HNPs 1−4, these proteins can make up as much as 50% of the total protein content in these cells [152]. These peptides help break down bacterial pathogens in conjunction with other proteins, including lysozyme, proteases, and RNases. This makes them crucial for both local and systemic innate immunity [153]. For HNPs 1−3, the normal concentration in human blood plasma is 254.8 ± 7.1 pg/μL; in bacterial sickness, this can increase by 4.2 times, and in non-bacterial infection, by 3.2 times.
AMPs initiate an immune response specific to an antigen, encourage intercellular cooperation, and boost the generation of antibodies as components of innate immunity. A study revealed that when mice were given ovalbumin (OVA) and defensins HNPs 1−3 intraperitoneally, their immune system produced more of certain IgG and IgM but not IgA. The study’s authors found that while defensins improve the systemic immune response, they do not improve the mucosal immune response [154]. After 14 days of immunization, animals are given defensins HNPs 1−3 intraperitoneally produced far more KLH (keyhole limpet hemocyanin)-specific antibodies, including IgG1, IgG2a, and IgG2b. Additionally, defensins greatly enhanced the animals’ resistance to a transplantable tumor and the generation of antibodies against the antigen of a syngeneic tumor [155]. According to these findings, defensins function as potent immunological adjuvants that boost the synthesis of immunoglobulins that are specific to particular antigens. Defensins’ adjuvant properties are employed in the creation of vaccines to prevent bacterial and viral illnesses. Specifically, individuals with soft tissue infections often harbor the human pathogen Mycobacteroides abscessus (formerly Mycobacterium abscessus), which often results in postoperative infectious problems and is highly resistant to traditional antimicrobial medications. The efficiency of therapy has increased with the inclusion of hBD-2 as an adjuvant to the Mycobacteroides vaccination [156].
In addition to directly attacking the pathogen and eliminating it, constitutively produced AMPs also stimulate the release of cytokines and chemokines, which draw immune-competent cells to the pathogen invasion zone and preserve immunological homeostasis. As a result, the body is protected against infection on multiple levels. Defensins’ selective activity was demonstrated in an extensive investigation into how hBD-1, hBD-2, and hBD-3 affect peripheral blood mononuclear cells (PBMCs) cytokine production [157]. The synthesis of MCP-1 (monocyte chemoattractant protein 1, CCL2), EGF (epidermal growth factor), IL-6, IL-8, and IL-10 was increased by hBD-1 on the first day. During the first six days, insulin-like growth factor-binding protein 3 (IGFBP-3) increased before declining. hBD-1 significantly decreased IL-5, but had no effect on IL-1-β, IL-16, or MCP-2. The following cytokines were raised in a dose-dependent manner by hBD-2: HGF (hepatocyte growth factor), EGF, IGFBP-3, IL-10, IL-8, IL-1-β, and IL-6.
By preventing the early formation of C1q and MBL complexes from the classical and lectin routes of complement system activation, α-defensin HNP 1 shields the body from tissue injury. The human complement system can also be impacted by invertebrate AMPs, and the effects can be multidirectional depending on the concentration. Specifically, at relatively low doses (1–40 µg/mL), the marine polychaete Arenicola marina’s peptide arsenic in-1 increases complement activation and target erythrocyte lysis; at higher concentrations (80–160 µg/mL), arenicin functions as a complement inhibitor. The likelihood of a relationship between AMPs and complement proteins C1q and C3, as well as the control of their functional activity, are discussed by the study’s authors [158]. It has been investigated how structural alterations in arenicins affect their biological activity and how they interact with complement proteins [159].
Numerous insect AMPs have been found to have cytotoxic effects on various malignant cell lines, including breast, lung, melanoma, leukemia, and lymphoma [160]. Anticancer peptides (ACPs) are cationic low-molecular-weight AMPs that exhibit both antibacterial and anticancer properties. AMPs have several characteristics, such as amphipathic structure, high hydrophobicity, and cationic (positive net charge), which enhance their affinity for cell membranes. Because of these qualities, ACPs can be thought of as a valuable resource with a low tendency to develop cancer cell resistance. The outer membranes of cancer cells have more negatively charged molecules than those of normal cells. Through electrostatic interactions, this feature facilitates the ACPs’ adhesion to cancer cells, leading to selective membrane disruption in the cancer cells and necrosis or death [161]. A brief overview of AMPs in combatting cancer types is highlighted in Table 1.
Brief details of anti-microbial peptides highlighting the sequence, structure type, source, and targeting different cancer types
| S. NO. | Name of AMP | Amino acid sequence of AMP | Structure | Source | Target | Reference |
|---|---|---|---|---|---|---|
| 1 | Magainin 2 | GIGKFLHSAKKFGKAFVGEIMNS | α-helical | African clawed frog | Melanoma, breast and lung cancer, bladder | [161] |
| 2 | BmKn2 | FIGAIARLLSKIF | α-helical | Scorpion venom | Colorectal cancer | [161] |
| 3 | CT20p | VTIFVAGVLTASLTIWKKMG | α-helical | Synthetic (C-terminal) | Breast cancer | [162] |
| 4 | NRC-03 | GRRKRKWLRRIGKGVKIIGGAALDHL-NH2 | α-helical | Pleurocidin family (epithelial mucous cells of flounder skin) | Breast cancer | [162] |
| 5 | NRC-07 | RWGKWFKKATHVGKHVGKAALTAYL-NH2 | α-helical | Pleurocidin family (epithelial mucous cells of flounder skin) | Breast cancer | [162] |
| 6 | H-10 | CASSQDLGSNTGQLYFGE | Cyclic | Mouse malignant melanoma | Melanoma | [1] |
| 7 | Buforin IIb | TRSSRAGLQFPVGRVHRLLRK | α-helical | Stomach tissue of Asian toad | Breast, colon, prostate, lung cancer, leukemia | [36] |
| 8 | BMAP-28 | GGLRSLGRKILRAWKKYGPIIVPIIRI | α-helix | Cattle-bostaurus | Colon cancer | [163] |
| 9 | RT-2 | NGVQPKYRWWRWWRRWW-NH | - | Crocodylus siamensis | Colorectal cancer | [164] |
| 10 | Temporin A | FLPLIGRVLSGIL | α-helical and β-sheet | Rana temporaria | Lung cancer | [165] |
| 11 | Bombinin H4 | IIGPVLGLVGSALGGLLKKI | α-helical and β-sheet | Bombina variegata | Lung cancer | [165] |
AMP: antimicrobial peptide; S.No.: serial number
Colorectal cancer is considered the third most common type of cancer in adults, affecting both genders equally. It accounts for 6% of all cancer-related deaths worldwide and is the fourth most prevalent disease [166]. Colorectal carcinomas exhibit heterogeneity in their morphology due to a variety of gene changes, such as oncogenes, DNA repair, and tumor suppressor genes, which pose therapeutic challenges. There has been much research on AMPs’ capacity to eradicate colorectal cancer cells. AMP is thought to affect colorectal cancer through a variety of pathways, including cytotoxicity, metabolic profile changes, cell cycle regulatory proteins, and microRNA activity. It is known that human colon cancer cells are cytotoxically affected by the scorpion peptide BmKn2 [161].
According to Das et al.’s [163] study from 2023, the BMAP-27 peptide can decrease colon cancer cell proliferation and induce apoptosis by speeding up cell death, decreasing cell proliferation, increasing tumor suppression, and controlling genes related to Wnt signaling.
Breast cancer is the most common malignancy in women that ends in death. Metastatic cancer cells that do not respond well to therapy typically feature negatively charged phosphatidylserine or anionic structures on their outer membrane, in contrast to healthy cells, which are frequently zwitterionic. This characteristic allows some selective cytotoxic medications, including cationic AMPs, to target cancer cells through electrostatic interactions. AMPs induce antineoplastic features in breast cancer cells by a variety of mechanisms, including disruption of the cell membrane, mitochondrial-dependent apoptosis, activation of specific intracellular pathways, and disruption of the nucleus. Research has shown that mammalian-derived AMPs can be highly deadly for breast cancer. Peptides from bovine lactoferricin (LfcinB), a peptide fragment of bovine lactoferrin, for instance, were most effective at killing breast cancer cells at a dose-dependent manner when applied at a concentration of 22 µM [68].
AMPs have demonstrated potential anti-cancer properties, including cytotoxicity, disruption of tubulin function, reduction of cell adhesion, inhibition of cell proliferation, and anti-angiogenesis, in preclinical trials for the treatment of lung cancer. Temporin A and bombinin H4 interacted more successfully with non-small cell lung cancer (NSCLC) cell lines than they did with healthy lung cell lines, as shown by [165]. Temporin A holds the most promise as an anticancer medication because it can kill a large number of cells in both NSCLC cell lines at the same concentration without harming the Beas-2B cell line. It is interesting to observe that A549 cells were the only ones destroyed by bombinin H4, not Calu-3 cells.
As AMPs are thought to be promising antibacterial agents, their mechanisms of action have been extensively studied. AMPs have both bacteriostatic and bactericidal properties, and they cause less germ resistance than traditional antibiotics. Electrostatic interaction between the positively charged cationic peptides and the negatively charged cell membranes causes membrane adsorption and conformational change. Peptides can connect to the cell membrane in a variety of ways, including the ones listed below.
The positively charged AMPs, when interacting with bacterial membrane (negatively charged), lead to an increase in membrane permeability, which causes cell membrane lysis and cell content release. As AMPs approach the cytoplasmic membrane through electrostatic interaction with the microbial membrane, they bind with the microbial membrane and interact with the anionic components of the plasma membrane. AMPs must first pass through the capsular polysaccharide and other components of the cell wall, such as LPS in Gram-negative bacteria and lipoteichoic acid and peptidoglycan in Gram-positive bacteria. Interaction is influenced by two primary elements in this step: i) conformational change and ii) peptide-lipid ratio. Numerous studies have demonstrated that AMPs having α-helical structures bind to anionic lipid membranes and change their disordered structure in an aqueous solution to facilitate their interaction with the membrane. According to research, as the peptide-lipid ratio rises, AMPs are oriented vertically and inset into the hydrophobic center of the membrane, resulting in the discharge of intracellular ions, metabolites, biosynthesis, and cell death. Barrell-stave, toroidal-pore, carpet, and aggregate models are a few speculative explanations for how AMPs work on membranes (Figure 1).
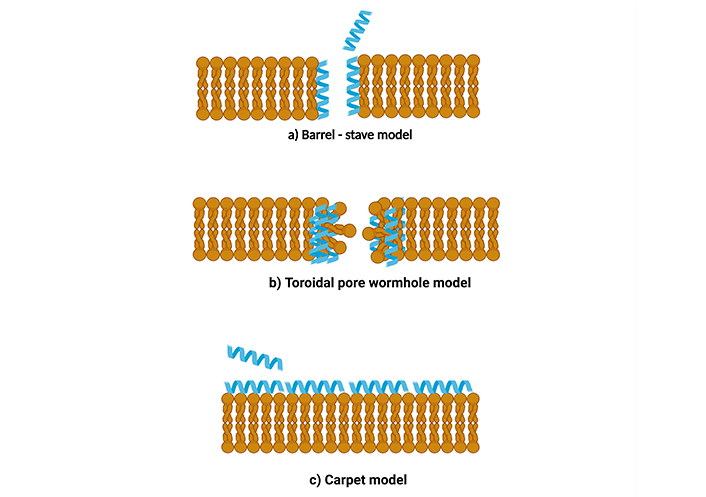
Different types of models for action of AMP on membrane. (a) Barrel-stave model, (b) toroidal pore wormhole model, (c) carpet model. AMP: antimicrobial peptide. Created in BioRender. Dilawari, R. (2025) https://BioRender.com/iw6qstv
Barrel-stave model: Aggregation and conformational modification occur with enhanced peptide binding to the membrane in the barrel-stave model, resulting in localized phospholipid head group shifting and membrane thinning. The hydrophilic areas of the peptide helixes face inwards during penetration into the phospholipid bilayer. In contrast, the hydrophobic sections of the -helical and -sheet peptides are close to the hydrophobic regions of the membrane phospholipid. Several helical molecules subsequently form the core lumen placed parallel to one another. This newly developed structure is constructed of many peptides introduced into the membrane to form a ring resembling a “barrel” pore. Here, “stave” refers to the spokes housed inside the barrel [167] (Figure 1a).
Toroidal pore wormhole model: This model functions similarly to the “barrel stave” method. In this scenario, AMPs gather vertically and are buried in the cell membrane, eventually bending to create a ring hole that is 1–2 nm in diameter. In this arrangement, the aqueous phase is external to the membrane, and the hydrophobic portion is located within the hydrophobic core of the membrane. The hydrophilic head of the peptides confronts the hydrophilic section of the lipids (Figure 1b). The peptide’s hydrophobic regions bind to and move the phospholipid head regions. This results in a strain and a rupture of the hydrophobic region of the membrane. By destroying the membrane’s composition, strain, and membrane thinning make the surface of the bilayer vulnerable to AMPs. The theory states that this arrangement allows peptides to arrange themselves perpendicularly within the bilayer membrane and does not necessitate particular peptide-peptide interactions. Instead, the peptides cause the local curvature of the membrane, which is partly produced by the phospholipid head regions, to develop pores. The entire configuration of the lipid membrane distinguishes the toroidal-pore model from the barrel-stave model [168]. The barrel-stave mechanism maintains the bilayer’s hydrophobic and hydrophilic configuration, but in toroidal pores, the lipids are broken, resulting in lipid head and tail groups interacting with each other. AMPs such as magainin 2, lacticin Q, and melittin act via toroidal pore models.
Carpet model: This carpet model, for AMPs acting on the membrane, is studied for destabilization. This model acts without forming pores in the membrane. The mechanism is similar to the other two hypotheses in that cationic strong electrostatic interaction causes AMPs to be first drawn to a negatively charged phospholipid membrane. AMPs are arranged parallel to the lipid bilayer membrane’s surface. When peptides build up to a sufficient concentration, they form a “carpet” on the membrane, producing unwanted binding interactions on its outer surface. This causes the membrane to rupture, producing micelle [169] (Figure 1c). There are a few indistinguishable models. A detergent-like model is one in which the membrane bilayer is broken down into micelles. The carpet method does not require peptide-peptide interactions between the peptide individuals bound to the membrane or for the peptide to embed itself in the hydrophobic region in order to build transmembrane channels. Cecropin and aurein are two AMPs that work through the carpet model mechanism.
It was found that the HNP 1 peptide could enter the inner and outer membranes of Escherichia coli (E. coli) and reduce the production of bacterial protein, RNA, and DNA. Notably, the deadly event appears to involve inner membrane permeabilization. By preventing the production of D-Ala-D-Ala dipeptide of lipid II of the peptidoglycan precursor and alanine racemase from working, cycloserine’s antibacterial impact can be reduced. HNP 1 appears to interact with lipid II to kill bacteria by adhering to a highly conserved motif in lipids II and III, which are the components of teichoic acid in cell walls; teixobactin prevents the synthesis of cell walls [170].
It is known that AMPs can move through the lipid membrane without significantly disrupting the bilayer and then engage in interactions with biological elements, including RNA and DNA. Additionally, there are several findings in the literature of peptides from the Trp-rich family of AMPs that are active antimicrobials but do not significantly cause membrane permeabilization. According to the current theory, transcription or translation is disrupted because aromatic Trp residues encourage contact with the nucleotide bases of RNA or DNA. Buforin II, a 21-amino acid-long AMP, shows antibacterial action against a wide variety of microorganisms. It shares a sequence with the protein histone H2A, which directly engages nucleic acids [171]. Buforin II has previously been proven to permeate lipid vesicles in vitro without changing membrane permeability and to bind to nucleic acids (DNA & RNA) [172].
An AMP isolated from a pig’s small intestine was reported to rapidly penetrate the outer membrane of E. coli, which is PR-39 (rich in proline and arginine). Whenever PR-39 enters the cytoplasm, it inhibits protein synthesis while promoting the degradation of proteins required for DNA synthesis, causing DNA synthesis to be disrupted. Proline-enriched AMPs often inhibit protein synthesis by attaching to ribosomes. As a result of the phosphorylation of TSC2 and the regulatory-associated protein of mTOR (raptor), AMPK inhibits the growth regulator mammalian target of rapamycin, or mTOR complex 1 (mTORC1). As a result of P70-S6 kinase inhibition and the activation of the multifunctional protein eukaryotic translation initiation factor 4E-binding protein 1, also known as 4E-BP1, mTORC1 inhibition prevents cap-dependent translation from occurring during the initiation phase in ribosomal proteins [173]. Another study found that nonlytic proline-rich AMP (PrAMP) Bac5’s N-terminal fragments (1–25) and (1–31) bind to the ribosome tunnel and block the progression of translation from the initial stage to the elongation stage, hence inhibiting the production of bacterial proteins [174].
According to studies, AMPs are likely to limit the function of bacterial intercellular enzymes. Microcin J25, a peptide synthesized by ribosomes and changed post-translationally, was found to bind to the secondary channel of the RNA polymerase and prevent trigger-loop folding, which is required for the RNA polymerase to catalyze RNA efficiently. As a result, it suppresses the activity of RNA polymerase by blocking the entry of substrates through this channel. According to [175], the AMP NP-6 from Sichuan pepper seeds significantly and dose-dependently reduced the β-galactosidase activity of E. coli. It was discovered that LL-37 inhibited the activity of the palmitoyl transferase PagP, which is located in the outer membrane of Gram-negative bacteria and restores membrane permeability by activating lipid transporters—a type of acylation.
The short AMPs like pyrrhocoricin, drosocin, and apidaecin interact with the bacterial DnaK heat shock protein to have an antibacterial action. Drosocin and pyrrhocoricin prevent the folding of proteins with the help of chaperones by binding to DnaK. The bacterial membrane is affected by these AMPs. As cell wall lyase is released, both the sugar chain and peptide bridge of the murein are further hydrolyzed, which ultimately leads to staphylococci lysis. For instance, Mel4 caused the release of bacterial autolysin, which led to the death of S. aureus (Staphylococcus aureus) cells. Additionally, AMP PFR causes endoplasmic reticulum (ER) stress, higher levels of cytoplasmic calcium, and mitochondrial ROS to trigger necroptosis [176].
Since many fungi, yeasts, and other pathogenic micro-organisms are to blame for the ongoing spread of illnesses and the development of medication resistance against bacteria, microbial infections continue to rank among the leading public health concerns. The growth of antibiotic resistance is making it more important to create novel defenses against superbugs that are resistant to drugs. Small peptide-based compounds with a length of 5–100 amino acids that possess strong and all-around antibacterial capabilities are known as antimicrobial peptides or AMPs for short. Easy access to cutting-edge carriers made possible by nanomedicine eventually makes it possible to design and build focused delivery systems for the most effective medications, ones with lower toxicity and greater efficacy.
Due to gold nanoparticles’ (NPs’) many benefits, including their ease of synthesis and conjugation to biomolecules, their ability to maintain their own structure while in circulation, and their enhanced efficacy against bacteria—research on these particles is growing, indicating their great potential in the field of nanomedicine. There are currently two published studies looking into the application of Au nanostructures for AMP delivery. Chen et al. [177] developed photoluminescent Au nanodots (AuNDs). Hybridized ligands, 1-dodecanethiol (DT), and an AMP (surfactin; SFT) were used to functionalize these AuNDs on AuNPs. It was possible to create ultrasmall SFT/DT–AuNDs (size ≈ 2.5 nm), and these showed exceptionally effective antibacterial activity.
In order to provide a synergistic dose-dependent effect on bacterial cells, Geilich et al. [178] combined ampicillin with silver NPs to create innovative delivery methods. With a silver-to-ampicillin ratio of one to 0.64, the produced polymersomes were more successful in suppressing bacterial cells while being safe for human fibroblasts. An intriguing nanostructure known as nano-clays or layered silicates has been applied to the removal of pollutants from the environment, the delivery of medications and other active chemicals, and the improvement of the mechanical and barrier qualities of polymers in packaging films. Usually, they exhibit a nanometric thickness of silicate layers layered in an arrangement [179].
As AMP delivery systems, silicon, and its derivative nanostructures have also received recent attention. For mesoporous SiNPs to be effectively exploited, membrane interactions are necessary. In an effort to shed light on this, Braun et al. [180] have examined the effects of NP charge and porosity on AMP loading and release, as well as the implications for membrane interactions and antibacterial effects. Anionic mesoporous SiNPs were found to integrate the CAMP LL-37 at considerable quantities, but non-porous or positively charged SiNPs exhibited significantly reduced loading. The findings also showed that anionic mesoporous particles, but not the other particles, shield LL-37 from being broken down by infection-related proteases because of their preferred pore location [181]. Membrane rupture for anionic SiNPs is virtually entirely mediated by peptide release. On the other hand, because of their greater negative surface charge, non-porous SiNPs developed a robust LL-37 surface coating and primarily exhibit particle-mediated membrane contacts and antibacterial activities. The insertion of LL-37 into positively charged mesoporous SiNPs resulted in toxicity against human erythrocytes while simultaneously promoting the membrane binding and disruption that the particles exhibited when the peptide was absent [180].
In multicellular organisms, AMPs, commonly called HDPs, are significant effector immune defense molecules. AMPs use various methods to carry out their antimicrobial actions; it has been thought improbable that AMPs might induce drug resistance. They, therefore, have much potential as antibacterial agents of the next generation. Currently, many medications related to AMP are undergoing clinical trials [182]. Recent research indicates that certain AMPs and traditional antibiotics work well together. Drug-resistant infections can be eliminated, drug resistance can be avoided, and the therapeutic benefits of antibiotics can be significantly enhanced when AMPs and antibiotics are used together. Much research demonstrated the advantages of combining AMPs with traditional antibiotics, which frequently result in increased activity against strains resistant to drugs and an extended range of action for antibiotics [183]. Table 2 enumerates the peptide and antibiotic combinatorial approach in various research projects against microbial pathogens.
List of few peptide and drug combinations found helpful in combatting microbial infections
| Peptide(s) | Combined compound(s) | Bacterial specie(s) | Testing models | Concentrations | Synergism | Reference |
|---|---|---|---|---|---|---|
| CRAMP | Vancomycin | Salmonella enterica serovar Typhimurium | Clinical (in-vitro) | - | Yes | [184] |
| DJK-5 and DJK-6 | Ciprofloxacin, ceftazidime, tobramycin | Acinetobacter baumannii, Klebsiella pneumoniae, Escherichia coli, Pseudomonas aeruginosa | Clinical (in-vitro) | 0.8 µg/mL of DJK-5 and DJK-6 | Yes | [185] |
| AMP38 | Imipenem | Pseudomonas aeruginosa | Clinical (in-vitro) | 62.5 µg/mL of AMP38 plus imipenem | Yes | [186] |
| HPMA | Ciprofloxacin | Acinetobacter baumannii | Clinical (in-vitro) | 25 µM of HPMA, 400 µM of ciprofloxacin | Yes | [187] |
| Lactoferrin | Ciprofloxacin | Porphyromonas gingivalis | In vivo | 10 μg/mL of ciprofloxacin, 8 mg/mL of lactoferrin | Yes | [188] |
| Nisin | Penicillin | Enterococcus faecalis | Clinical (in-vitro) | 31.25 U/mL of nicin, 0.0625 mg/L of penicillin | Yes | [111] |
| Nisin | DHBA | Staphylococcus aureus | Clinical (in-vitro) | 50 mg/mL of DHBA, 15 mg/mL of nisin | Yes | [189] |
| hBD-3 | DNase I | Haemophilus influenzae | In vivo (chinchillas) | 0.5 g (r)hBD-3/mL, 0.5 g enzyme/mL sBHI | Yes | [190] |
| KSL-W | Dispersin B | Acinetobacter baumanii, Klebsiella pneumoniae, Staphylococcus aureus, Staphylococcus epidermidis | Clinical (in-vitro) | 1 mg/mL of KSL-W, 0.2 mg/mL of dispersin B | Yes | [191] |
| Temporin 1Tb | EDTA | Staphylococcus epidermidis | Clinical (in-vitro) | 2 mg/mL of temporin 1Tb, 50 mM (18.6 mg/mL) of EDTA | Yes | [192] |
| hBD-2 | NO, colistin, chloramphenicol | Pseudomonas aeruginosa | Clinical (in-vitro) | 1.5 flux NO, 20 μg/mL AMP, 100 μg/mL antibiotics | Yes | [193] |
| DD13 | RNA III-inhibiting peptide | Staphylococcus aureus, Staphylococcus epidermidis | In vivo (rat) | 10 mg/L of DD13, RNA III-inhibiting peptide | Yes | [194] |
| Citropin 1.1, temporin A, an analog of tachyplesin I | Colistin | Pseudomonas aeruginosa, Staphylococcus aureus | Clinical (in-vitro) | 32 mg/L of colistin, 1 mg/mL of citropin 1.1, 4 mg/L of temporin A | Yes | [195] |
| Nisin | Colistin, polymyxin B | Pseudomonas aeruginosa | Clinical (in-vitro) | 100 µM of nisin, 128 or 256 µg/mL of colistin, polymyxin B | Yes | [196] |
AMP: antimicrobial peptide; hBD: human beta-defensin; NO: nitric oxide. Adapted with permission from [197], © 2017 the authors (CC BY)
Our skin is the first line of defense against different diseases. Since skin infections can be viruses, fungi, or protozoa, and different types of bacteria, AMPs with such a broad spectrum may be more effective than conventional antibiotics. Due to their low bloodstream permeability, AMP preparations also offer the benefit of high concentration at the target side for topical administration. The growth of infections and the development of biofilms in lesional skin are inhibited explicitly by AMPs in wounds, and they also speed up wound healing by controlling angiogenesis, cell migration, chemotaxis, and cytokine production. Therefore, these positive benefits point to AMPs as a possible therapy option for infectious and noninfectious wounds. According to [198], the majority of peptide-related antibiotics that the FDA has approved so far for treating infected skin infections are topically applied, such as tyrothricin, bacitracin, and gramicidin. Experiments reveal that LL37 peptide can boost cell growth factors, promote chondroitin and dermatan production, stimulate keratinocyte proliferation, and speed damaged skin’s sustained healing and regeneration. As a result, AMP speeds up wound healing not just by limiting bacterial development but also by stimulating the proliferation of normal cells. These peptide materials, which may stimulate wound healing from the inside and the exterior, exhibit excellent wound-healing performance and have a high application value.
Micrologix Biotech Inc. has entered clinical studies with three different AMPs related to indolicidin. MBI-226, the latest and most developed peptide, is now in phase III clinical studies to prevent catheter-related bloodstream infections. According to the company, preclinical studies showed MBI-226 is beneficial in animal models, reducing skin colonization by a range of bacteria causing catheter-related illnesses and demonstrating intense antifungal action against C. albicans in guinea pig skin [198]. According to Park and Lee’s 2009 study [199], the antibacterial peptide arenicin-1 (RWCVYAYVRVRGVLVRYRRCW), which has 21 residues, was discovered in the coelomocytes of the marine polychaete lugworm Arenicola marina. According to reports, it exhibits potent antibacterial activity against several bacterial strains. The MTT assay was used to evaluate the antifungal activity of arenicin-1. According to the findings, all of the investigated fungal strains had arenicin-1 MIC values that ranged from 4.5 to 9 μM [199]. Arenicin-1 significantly inhibited the growth of the Candida species, including C. albicans. The most common systemic fungal pathogen in humans, C. albicans, is linked to a variety of clinical disorders, from irritable vaginal and oral mucosal infections to fatal systemic diseases in immunocompromised people.
AMPs demonstrated a broad antibacterial range against both Gram-negative and positive bacteria. They displayed direct activity by disrupting the bacterium membrane or inhibiting intracellular processes. In more immense research, an AMP produced from chicken (CHAP) demonstrated substantial and quick efficacy against 19 different types of bacteria, including drug-resistant strains, with CHAP inhibiting almost all tested bacterium species within only 30 minutes. Two clinical trials employing indolicidin-like peptides have been approved by the FDA for the treatment of acute acne (in phase II trials) and the killing of MRSA (methicillin-resistant S. aureus) in the nose (in phase II trials) [200]. AMPs are effective in treating infections worldwide; AMPs, such as magainin, have already entered the worldwide market and are being used to treat viral and bacterial disorders. The RNA isolation technique was used to extract stomoxynZH1 from Hermetia illucens, and this compound exhibited inhibitory activity against S. aureus, E. coli, Rhizoctonia solani, and Sclero-tinia sclerotiorum. AMPs are crucial in the fight against bacteria that are resistant to antibiotics. Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii are all capable of having their growth inhibited by polymyxin B. 90% of Salmonella enterica, E. coli, Acinetobacter baumannii, and Enterobacter cloacae were shown to be inhibited by LS-sarcotoxin and LS-stomoxyn, which were isolated from Lucilia sericata.
Some cationic AMPs also demonstrated their antiviral properties. Studies have shown that AMPs have apparent inhibitory effects on a variety of DNA and RNA viruses, including the HIV, influenza, herpes, and hepatitis B viruses. Different ways for AMPs to act as antiviral agents have been discovered. One is how the peptides fight viruses by engaging with virions directly. In order to replicate the viral infectious mechanism, the peptides also prevent viral multiplication. For instance, peptides may cause viral harm by obstructing the assembly of the viruses. Those parasites that cause parasitic disorders in humans and animals, such as malaria, dysentery, and other protozoa, can also be successfully eliminated by these cationic peptides. Zhang et al. [140] tested DP7’s (VQWRIRVAVIRK) antiviral effectiveness towards the SARS coronavirus. He asserted that by observing the activity of the cell receptor ACE2, DP7 has the capacity to prevent the infection of SARS-CoV and SARS-CoV-2. He also stated that the SARS-CoV and SARS-CoV-2 pseudo viruses must be inhibited by DP7 at concentrations of 104 µg/mL and 73.625 µg/mL (50 percent inhibitory concentration), respectively.
Cationic AMPs serve critical roles in the host’s innate immunity. They not only eliminate pathogenic bacteria that infiltrate the human body, but they also demonstrate their numerous activities at various stages of normal immune responses. These cationic AMPs play a role in host defenses during acute inflammation. They can cause bacterial lysis, promote macrophage phagocytosis, inhibit infection spread, stimulate fibroblast and epithelial cell mitosis, and promote fibroblast development to improve wound healing. They can trigger human lymphocytes to remove virus and bacteria-infected cells, as well as cancer cells. These AMPs are also involved in chronic inflammation. They boost the amount of antibody IgG in the body, encourage macrophage death, and activate lymphocytes to destroy infected cells. Additionally, they encourage the development of chemokines and the growth of helper T cells.
Altogether, AMPs are candidates for broad-spectrum anti-microbial, immune-modulatory, and anti-cancerous properties. AMPs possess potent MDR bacterial and anticancer activity. Hereby, AMPs offer researchers and industries a promising target for addressing emerging antibiotic resistance of already available drugs and chemotherapy resistance of cancer cells. However, the short half-life of these peptides, their toxicity to some extent, and their few side effects may limit clinical and therapeutic development. There is a requirement for new technological interventions to transform the natural AMPs into efficacious, less toxic, and more potent in action modulation to make these amphiphilic and lipophilic with bare minimum side effects and prevent drug resistance. There is a long road ahead for the potential FDA-approved AMP candidates to enter clinical trials. We look forward to the development of AMP-based curing cancer and microbial infections in the human race with enhanced specificity, safety, and efficacy to the coming generations for a better life.
ACE2: angiotensin-converting enzyme 2
ACPs: anticancer peptides
AMPs: antimicrobial peptides
ATCUN: amino terminal copper and nickel
CHAP: antimicrobial peptide produced from chicken
DBAASP: Database of Antimicrobial Activity and Structure of Peptides
DENV: dengue virus
DT: 1-dodecanethiol
EGF: epidermal growth factor
hBD-3: human beta-defensin 3
HDPs: host defense peptides
HIV: human immunodeficiency virus
HLH: helix-loop-helix
HNP 1: human neutrophilic peptide 1
HSV: herpes simplex virus
IAV: influenza A virus
IGFBP-3: insulin-like growth factor-binding protein 3
MCP-1: monocyte chemoattractant protein 1
MIC: minimum inhibitory concentration
mTORC1: mammalian target of rapamycin complex 1
NDs: nanodots
NPs: nanoparticles
NSCLC: non-small cell lung cancer
RBD: receptor-binding domain
ROS: reactive oxygen species
S: spike
surfactin: SFT
ZIKV: zika virus
The supplementary Table for this article is available at: https://www.explorationpub.com/uploads/Article/file/1008110_sup_1.pdf.
The authors acknowledge Dr. Chandru Subramani, who provided valuable insights during the manuscript preparation.
RD and GKC: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. N Priyadarshi and MB: Validation, Writing—review & editing. N Parmar: Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not Applicable.
Not Applicable.
Not Applicable.
Not Applicable.
Not Applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
