Affiliation:
2Department of Clinical Physiology, Danderyd Hospital, 18288 Stockholm, Sweden
3Department of Clinical Sciences, Danderyd Hospital, Karolinska Institutet, 18288 Stockholm, Sweden
ORCID: https://orcid.org/0000-0001-9677-8252
Affiliation:
4Department of Cardiology, Karolinska University Hospital, 17176 Stockholm, Sweden
5Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-9032-9031
Affiliation:
6Department of Anaesthesia and Intensive Care, Institution for Clinical Science, Karolinska Institutet, Danderyds University Hospital, 18288 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-5829-1803
Affiliation:
4Department of Cardiology, Karolinska University Hospital, 17176 Stockholm, Sweden
5Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-8690-7994
Affiliation:
7Division of Physiotherapy, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, 14186 Stockholm, Sweden
8Division of Physiotherapy, School of Health, Care and Social Welfare, Mälardalen University, 72123 Västerås, Sweden
ORCID: https://orcid.org/0000-0002-6989-719X
Affiliation:
7Division of Physiotherapy, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, 14186 Stockholm, Sweden
9Women’s Health and Allied Health Professionals Theme, Medical Unit Occupational Therapy and Physiotherapy, Karolinska University Hospital, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0001-8903-1273
Affiliation:
4Department of Cardiology, Karolinska University Hospital, 17176 Stockholm, Sweden
5Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-0676-0604
Affiliation:
7Division of Physiotherapy, Department of Neurobiology, Care Sciences and Society, Karolinska Institutet, 14186 Stockholm, Sweden
ORCID: https://orcid.org/0000-0001-6738-4879
Affiliation:
10Division of Rheumatology, Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-6068-9212
Affiliation:
4Department of Cardiology, Karolinska University Hospital, 17176 Stockholm, Sweden
5Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
ORCID: https://orcid.org/0000-0002-6617-2070
Affiliation:
4Department of Cardiology, Karolinska University Hospital, 17176 Stockholm, Sweden
5Department of Medicine Solna, Karolinska Institutet, 17176 Stockholm, Sweden
Email: Magnus.Back@ki.se
ORCID: https://orcid.org/0000-0003-0853-5141
Explor Cardiol. 2026;4:101288 DOI: https://doi.org/10.37349/ec.2026.101288
Received: September 21, 2025 Accepted: November 20, 2025 Published: January 18, 2026
Academic Editor: Karina Wierzbowska-Drabik, Medical University of Lodz, Poland
The article belongs to the special issue Exploring Exercise Cardiology: from Molecules to Humans
Rheumatoid arthritis (RA) confers an elevated cardiovascular disease (CVD) risk through systemic inflammation, arterial stiffness, and myocardial dysfunction. The ratio of carotid-femoral pulse wave velocity (PWV) to left ventricular global longitudinal strain (LV-GLS) has been proposed as a novel index of ventricular-arterial coupling. This study investigated whether a one-year physical activity intervention improves the PWV/LV-GLS ratio in RA patients without known CVD. Eighteen participants with RA from the prospective PARA 2010 study underwent baseline and one-year assessments. PWV was measured by oscillometry, and LV-GLS by speckle-tracking echocardiography. Physical activity was promoted through supervised circuit training and additional moderate-to-vigorous activity. At one year, very active participants [≥ 1,000 metabolic equivalent of task (MET)-minutes/week, n = 10] showed a relative reduction in PWV/LV-GLS ratio compared with baseline, while no change was observed in less active participants (< 1,000 MET-minutes/week, n = 8). Between-group comparison at follow-up demonstrated significantly lower PWV/LV-GLS ratio in very active versus less active patients (p < 0.05). No significant between-group differences were observed when PWV and LV-GLS were analyzed separately. In conclusion, a high level of physical activity was associated with improved ventricular-arterial coupling, reflected by a lower PWV/LV-GLS ratio, in RA patients. These findings support a potential dose-dependent effect of physical activity on subclinical cardiovascular function in RA. The clinical trial was registered at http://www.isrctn.com, unique identifier: ISRCTN88886304.
Increasing physical inactivity and chronic inflammation are emerging risk factors for cardiovascular disease (CVD), while physical activity is a strong protective factor. Rheumatoid arthritis (RA), the most common inflammatory joint disease, promotes systemic inflammation, leading to arterial stiffness and endothelial dysfunction, contributing to atherosclerosis [1]. RA patients have a higher risk of cardiovascular mortality, with the incidence of acute myocardial infarction (AMI) being twice as high in RA patients compared to non-RA individuals, even surpassing type 2 diabetes [2]. Increased vascular stiffness in RA without known CVD is another indicator of higher cardiovascular risk [3]. International guidelines suggest that physical activity is safe and beneficial for people with RA [4].
Early detection of subclinical atherosclerosis may help to assess CVD risk. Carotid-femoral pulse wave velocity (PWV), measuring arterial stiffness, is an independent marker of cardiovascular mortality but is not yet included in guidelines [5]. A study suggested that every 1 m/s increase in PWV signifies a 14% higher risk of cardiovascular events [6]. Additionally, cardiac dysfunction in RA patients, assessed via left ventricular global longitudinal strain (LV-GLS), has been linked to future cardiovascular hospitalizations [7]. The combination of vascular and cardiac performance parameters through the ratio between PWV and LV-GLS has been suggested as a novel measure of ventricular-arterial coupling [8]. The PWV/LV-GLS ratio has been shown to allow an early detection of altered ventricular-arterial coupling in hypertensive patients [8], but studies in other populations are currently scarce. The reversibility of altered ventricular-arterial coupling measured through PWV/LV-GLS ratio has not previously been studied.
The aim of the present study was therefore to establish if physical activity intervention improved ventricular-arterial coupling, determined as the PWV/LV-GLS ratio in subjects with RA. To this end, the PARA 2010 study of early markers of cardiovascular function in RA was explored for PWV/LV-GLS.
In the prospective PARA 2010 study of a physical activity intervention in persons with RA according to the American College of Rheumatology criteria, without a known history of CVD and not reaching the recommended levels of health-enhancing physical activity at study baseline [9–11], 29 participants underwent cardiovascular explorations [3]. At baseline and after year 1, echocardiography, exercise test, and vascular stiffness measurements were performed as previously described [12], and participants had no clinical signs of atrial fibrillation or depressed LV function. In brief, PWV was measured using an oscillometric Arteriograph (TensioMed, Budapest, Hungary), and LV-GLS was measured on transthoracic echocardiography examinations using speckle tracking as previously described [3]. LV-GLS was expressed as the mean of the peak systolic strain across all segments. The newly proposed index, PWV/LV-GLS ratio, was calculated as the ratio of PWV and the myocardial performance estimated with LV-GLS.
The physical activity detailed program has been previously described [9]. In brief, during one year, participants took part in moderate-to-vigorous physical activity (MVPA) with a guided circuit training including aerobic exercise and muscle strength exercises, and in addition least 30 minutes on most days of the week [9, 10, 12]. The International Physical Activity Questionnaire (IPAQ) was used to measure the level of MVPA of participants [9] as expenditure of energy measured as metabolic equivalent of task (MET minutes per week) [13, 14]. In the present report, we defined 1,000 MET minutes per week as a cut-off to distinguish participants reaching an activity level exceeding EULAR recommendations for MVPA [14]. A paired t-test was used for comparisons of PWV/LV-GLS ratio at baseline vs. year 1 in both physical activity subgroups, and a t-test was used to study the differences in PWV/LV-GLS ratio in group 1 vs. group 2 at year 1. Fold changes were presented with 95% confidence intervals.
The clinical trial was registered at http://www.isrctn.com, unique identifier: ISRCTN88886304.
Of the n = 29 from PARA 2010 who underwent cardiovascular explorations, n = 18 participants with available data for physical activity and PWV/LV-GLS ratio obtained at baseline and year one were included in the present analysis (Figure 1). Among these participants, group 1 (n = 10) was very active (≥ 1,000 MET minutes per week), and group 2 (n = 8) was less active (< 1,000 MET minutes per week). Pertinent clinical and biochemical data for the physical activity subgroups are shown in Table 1.
Characteristics at baseline for patients undergoing physical activity equivalent to ≥ 1,000 MET minutes per week (group 1) or < 1,000 MET minutes per week (group 2).
| Variables | Group 1 (n = 10) | Group 2 (n = 8) |
|---|---|---|
| Clinical characteristics | ||
| Age (years) | 60 (43–72) | 60 (50–71) |
| Females | 9 (90%) | 7 (88%) |
| BMI (kg/m2) | 25 (20–31) | 25 (21–32) |
| Resting systolic blood pressure (mmHg) | 123 (108–140) | 129 (105–146) |
| Resting diastolic blood pressure (mmHg) | 76 (63–82) | 83 (70–100) |
| Pharmacological treatment | ||
| NSAID | 4 (40%) | 2 (25%) |
| Biological treatment | 6 (60%) | 7 (88%) |
| Biochemical characteristics | ||
| CRP | 3 (1–9) | 3 (0–7) |
| Echocardiographic parameters | ||
| LV-GLS (%) | 21 (15–26) | 20 (15–26) |
| LA-GLS (%) | 34 (18–55) | 31 (24–39) |
| PWV (m/s) | 10 (7–15) | 12 (10–15) |
Data presented as median and interquartile range (IQR) for continuous variables and as number (%) for dichotomous variables. MET: metabolic equivalent of task; BMI: body mass index; NSAID: nonsteroidal anti-inflammatory drugs; CRP: C-reactive protein; LV-GLS: Left ventricular global longitudinal strain; LA-GLS: Left atrial global longitudinal strain; PWV: pulse wave velocity.
In the present study, we found a trend towards a relative reduction of the PWV/LV-GLS ratio after 1 year in participants performing more intense MVPA, whereas no changes were observed for participants performing less MVPA (Figure 2), suggesting a possible dose-dependent effect of MVPA on ventricular-arterial coupling. When considering the attained PVW/LV-GLS at year one, depending on the level of performed physical activity, the present study revealed a significantly higher PWV/LV-GLS ratio in active compared with very active participants (Figure 2A). When comparing PWV and LV-GLS at year one separately, there were no significant differences between group 1 and group 2 (data not shown). Figure 2B displays the fold changes in PWV/LV-GLS ratio at year 1 of physical activity compared with baseline, with a trend towards a decrease in group 1, and no significant changes in group 2.
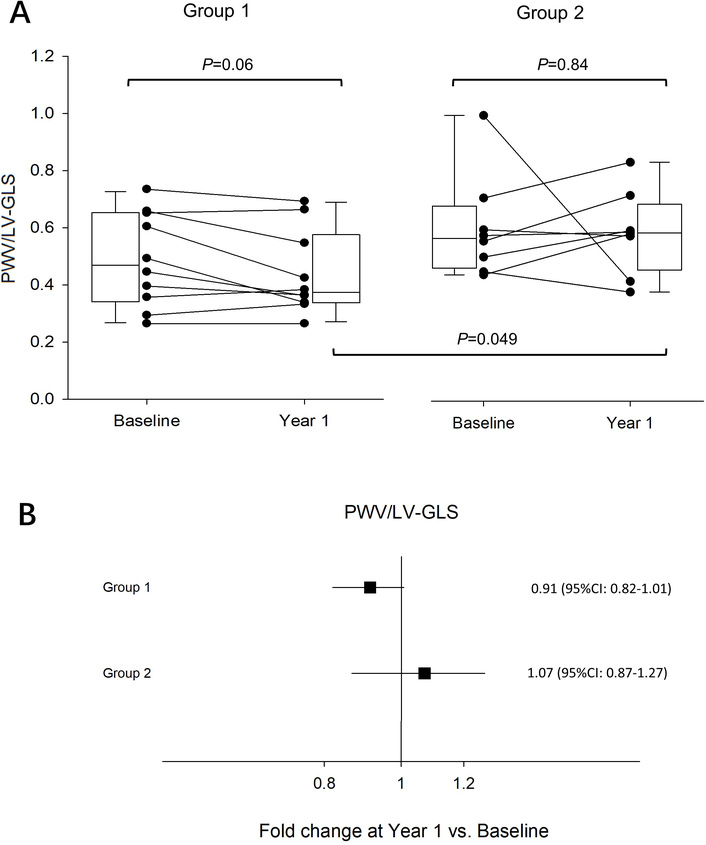
Pulse wave velocity (PWV) to left ventricular global longitudinal strain (LV-GLS) before (Baseline) and after (Year 1) physical activity. (A) Differences in PWV/LV-GLS ratio at baseline and year 1 in group 1 performing moderate-to-vigorous physical activity (MVPA) at ≥ 1,000 metabolic equivalent of task (MET) minutes per week, and in group 2 with MVPA at < 1,000 MET minutes per week. The bottom of each box represents the 25th percentile, the top the 75th percentile, and the line in the middle the median, with the corresponding value; whiskers are the minimum and maximum values. P values are displayed for paired comparisons between baseline and year 1 within the groups and differences between groups at year 1. (B) Fold change in PVW/LV-GLS ratio at year 1 compared with baseline in Group 1 (n = 10) and 2 (n = 8). Results are displayed as mean fold change ± 95% confidence intervals (CI).
In a large cohort including 569 patients with AMI, PWV/LV-GLS ratio was worse in patients with AMI at higher risk for cardiovascular complications and independently associated with a composite endpoint of all‐cause mortality, stroke, or myocardial infarction [15]. Whereas the ratio hence may be a predictor of cardiovascular prognosis, it is not known if improved PWV/LV-GLS correlates with a reduced CVD risk. An explanation for the improved PWV/LV-GLS ratio among patients performing MVPA in the first year of follow-up in the present study may be that the physical activity was supervised and coached carefully, which increased the adherence and led to a more intense MVPA.
Combining arterial stiffness with myocardial deformation provides a measure of ventricular-arterial interaction that can be obtained using standard non-invasive and reproducible measures. However, PWV and LV-GLS may be influenced by sex, age, and blood pressure, and reference values across different populations remain to be established. The need for complete data on PWV, GLS, and physical activity led to the exclusion of n = 11 participants (Figure 1), which means that selection bias for these parameters cannot be excluded. The observations’ borderline statistical significance is also acknowledged as a limitation of the present study, which may be the result of the limited sample size with insufficient power. The results should hence be interpreted with care. Previous results from PARA 2010 have shown that physical activity provides interrelated improvements in vascular stiffness and diastolic function [3]. Further larger studies are needed to determine if physical activity can improve the arterial-ventricular coupling as measured by PWV/LV-GLS and to determine the relative changes in PWV vs. LV-GLS.
In summary, the present study reports a trend towards improved PWV/LV-GLS ratio in participants performing a high MVPA, by being the most adherent to the program, compared to the group performing less MVPA (and being less adherent to the program) after one year of coached physical activity. Nevertheless, the attained PWV/LV-GLS ratio at year 1 was significantly lower after high compared with low MVPA. In conclusion, these results suggest that the PWV/LV-GLS ratio reflects improved ventricular-arterial coupling by physical activity in RA patients.
AMI: acute myocardial infarction
CVD: cardiovascular disease
GLS: global longitudinal strain
LV: left ventricular
MET: metabolic equivalent of task
MVPA: moderate-to-vigorous physical activity
PWV: pulse wave velocity
RA: rheumatoid arthritis
SM: Writing—original draft, Writing—review & editing, Data curation, Formal analysis. KS and MB: Conceptualization, Investigation, Writing—review & editing, Supervision, Data curation, Formal analysis. DH and AM: Writing—review & editing, Supervision. JGJ, PS, and AV: Writing—review & editing, Formal analysis. CF, BN, CHO, and IEL: Conceptualization, Investigation, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
The PARA study complied with the Declaration of Helsinki. The original study and the extended follow-up protocol were approved by the Stockholm regional ethics committee (2012/769-32). Clinical trial registration: http://www.isrctn.com. Unique identifier: ISRCTN88886304.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
The data that support the findings of this study are not publicly available. The study presented here has been subject to an application to an ethical board and approved for publication related to the specific aim of our research project. With reference to the European General Data Protection Regulation (GDPR), the data are personal data and thereby protected by secrecy.
PARA 2010 is supported by grants from AFA Insurance (grant number [100172]), the Swedish Research Council, Combine Sweden, the Swedish Rheumatism Foundation, and the KI National Postgraduate School of Health Care Sciences. M.B. is supported by grants from the Swedish Research Council (grant number [2023-02652]), Heart-Lung Foundation (grant number [20240697]), and Region Stockholm (grant number [RS2023-0859]). The funding sources had no role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; and decision to submit the manuscript for publication.
© The Author(s) 2026.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 552
Download: 67
Times Cited: 0
Wollner Materko ... Carlos Alberto Machado de Oliveira Figueira
Toru Maruyama ... Michinari Hieda
