4 results in Exploration of Cardiology
Latest
Sort by :
- Latest
- Most Viewed
- Most Downloaded
- Most Cited
Open Access
Review
Impact of iodinated contrast media on X-ray-induced DNA damage: a comprehensive review
Chiara Iacconi ... Enrica Ciofini
Published: April 19, 2024 Explor Cardiol. 2024;2:79–87

Open Access
Original Article
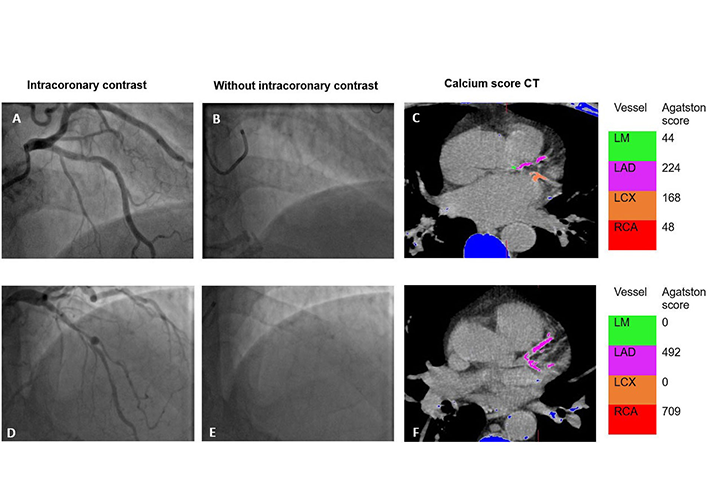
Coronary calcification on invasive angiography and the Agatston score—a single-center experience
Zafraan Zathar ... Vinoda Sharma
Published: April 15, 2024 Explor Cardiol. 2024;2:67–78
Open Access
Original Article
Nanoparticles loaded with the DNA methyltransferase inhibitor SGI-1027 decrease murine atherosclerosis and inflammation in cultured human macrophages
Ana Cristina Márquez-Sánchez ... Silvio Zaina
Published: April 10, 2024 Explor Cardiol. 2024;2:49–66
Open Access
Original Article
Role of cardio-ankle vascular index as a predictor of left ventricular hypertrophy in the evaluation of pediatric hypertension
Evan Harvey ... Ranjit Philip
Published: April 06, 2024 Explor Cardiol. 2024;2:40–48
This article belongs to the special issue Environmental Cardiology

Journal Information