Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
2Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, 70210 Kuopio, Finland
Email: jussi.sipila@uef.fi
ORCID: https://orcid.org/0000-0003-0183-9054
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
2Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, 70210 Kuopio, Finland
3Department of Neurology, Kuopio University Hospital, 70210 Kuopio, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
2Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, 70210 Kuopio, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
2Department of Neurology, Institute of Clinical Medicine, University of Eastern Finland, 70210 Kuopio, Finland
ORCID: https://orcid.org/0000-0001-9087-2408
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
1Department of Neurology, Siun Sote North Karelia Central Hospital, 80210 Joensuu, Finland
Affiliation:
3Department of Neurology, Kuopio University Hospital, 70210 Kuopio, Finland
ORCID: https://orcid.org/0009-0005-4646-9159
Explor Neurosci. 2025;4:100687 DOI: https://doi.org/10.37349/en.2025.100687
Received: March 22, 2025 Accepted: April 25, 2025 Published: May 08, 2025
Academic Editor: Ryszard Pluta, Medical University of Lublin, Poland
Aim: To report the incidence, characteristics, and prognosis of spontaneous intracerebral hemorrhage (ICH) in North Karelia Central Hospital Primary Stroke Center (PSC).
Methods: All patients admitted with ICH to North Karelia Central Hospital between January 1, 2021, and August 8, 2023, were identified from the center’s prospectively updated stroke care database. Post-hospital care data on outcomes were retrospectively updated between May 27 and June 5, 2024.
Results: During the entire study period, we identified altogether 56 ICH patients, of whom two thirds were men. The mean annual incidence of 2021–2022 was 12.3/100,000, and in the population 19–49 years of age, 1.8/100,000. Three months after the stroke, 50% of the patients were functionally independent. In-hospital mortality was 5%, and altogether 11 patients (20%) died during the follow-up (mean 1.72 years). In multivariate analyses, diabetes was associated with mortality [hazard ratio: 3.50, 95% confidence interval (CI): 1.02–11.95], and age was associated with functional outcome in three-month follow-up (odds ratio: 1.060, 95% CI: 1.015–1.107). New-onset epilepsy was diagnosed in three patients (6%) during the follow-up (mean: 1.84 years).
Conclusions: Short-term functional outcome of ICH was mostly favorable, continuing long-term trends of improving outcomes after stroke. Efficacy of multiple interventions care bundle in the treatment of ICH in a PSC-level hospital, a shift of using direct oral anticoagulants (DOACs) instead of warfarin, and improved health status of elderly citizens could be contributing to the better outcome.
Spontaneous intracerebral hemorrhage (ICH) is known as the deadliest form of stroke and the major cause of disability [1]. Acute spontaneous intracranial hemorrhage represents 14% of all strokes, and the in-hospital mortality was higher in cases of intraparenchymatous hemorrhage (31%) in a large classical stroke data bank [2]. The incidence rates of ICH also vary considerably between countries and continents, with the lowest rates reported for high-income Western Countries, including Finland, where case-fatality has declined continuously since the 1980s [1, 3–11].
Rescue therapies for achieving better outcomes for ICH are limited compared to acute ischemic stroke (AIS), and several individual therapies have failed to define a specific beneficial treatment. Multiple therapies better known from AIS have been the standard treatment choice for ICH patients as well: Management of high blood pressure, high body temperature, and control of glucose are widely used. In addition, ICH patients have also been treated with anticoagulant reversal therapy, and the evaluation for surgical evacuation of hematoma has been a possibility in centers with neurosurgical resources. The use of direct oral anticoagulants (DOACs) instead of warfarin (VKA) for non-valvular atrial fibrillation should have an impact on the incidence of ICH in the population [12].
However, Finnish functional outcomes are unknown, and studies with validated patient-level data are needed to investigate the burden and functional disability after ICH. Therefore, we investigated a cohort of consecutive patients admitted to North Karelia Central Hospital (NKCH) in easternmost Finland.
All stroke patients treated by neurologists, regardless of the specific treatment unit, at NKCH have been included in an internal quality of stroke care database of the neurology clinic. The database was inducted on January 1, 2021, and updated until August 2023, when prospective recording was suspended due to technical reasons. The neurologists enter a predefined set of basic demographic and clinical data of every stroke patient into the database when the patient leaves the hospital. These data were used in this study, and outcome data were retrospectively obtained from electronic health and social care records between May 27 and June 5, 2024.
NKCH is the only acute care hospital and Primary Stroke Center (PSC) in North Karelia (adult population c. 136,000, surface area c. 21,500 km2 with many lakes and rivers). Stroke patients from the northern and westernmost parts of the province are often taken initially to the nearest Comprehensive Stroke Center (CSC), Kuopio University Hospital (KUH), which is as close as NKCH in those parts of the province. These patients are usually transferred to NKCH after initial care at KUH.
Shapiro-Wilk and Kolmogorov-Smirnov tests were used to assess the distribution of continuous variables, and subsequently, results were presented as medians and interquartile ranges as the distributions were invariably skewed. The Mann-Whitney U test, independent samples of the Kruskall-Wallis test, and Fisher’s exact test were used to analyze patient characteristics. Linear and logistic regression were used to analyze possible correlations between variables and outcomes, with Cox regression utilized for multivariate analyses. 95% confidence intervals (95% CI) for case proportions were calculated using the binomial function. Statistical significance was inferred at a p-value < 0.05. Analyses were conducted using IBM SPSS Statistics for Windows, Version 29.0 (Armonk, NY: IBM Corp).
We identified 56 ICH patients, of whom two thirds were men (Table 1). Apart from women being older than men and more often having lobar hemorrhage, there were no differences between genders. Two patients (4%) were < 50 years of age (both male), and all were at least 30 years old. Of the 16 anticoagulated patients (29% of all patients), five (31%) had used VKA while the rest had been treated with DOACs. Diabetes was newly diagnosed during the hospitalization in two patients (one male, one female), and hypertension in seven patients (three men, four women). The most common (64%) location for hemorrhage was in deep brain structures basal ganglia and thalamus. Patient age and preceding modified Rankin Scale (mRS) status were correlated (p = 0.0012), and the latter was therefore excluded from further regression analyses.
Patient characteristics and outcomes according to sex
| Characteristic | Men (N = 37) | Women (N = 19) | p |
|---|---|---|---|
| Age (mean, std.) | 69.8 (11.2) | 77.0 (15.2) | 0.0299 |
| Preceding mRS (median) | 0 | 0 | 0.15 |
| Deep hemorrhage (basal ganglia, thalamus) (N = 36) | 27 (75%) | 9 (25%) | 0.056 |
| Lobar (N = 12) | 3 (25%) | 9 (75%) | 0.002 |
| Infratentorial hemorrhage (N = 8) | 7 (87.5%) | 1 (12.5%) | 0.17 |
| Neurosurgical evacuation (N = 3) | 2 (66.7%) | 1 (33.3%) | 0.74 |
| Smokers | 7 (19%) | 1 (5%) | 0.24 |
| Diabetes | 9 (24%) | 6 (32%) | 0.75 |
| Hypertension | 34 (92%) | 17 (89%) | 1.00 |
| Anticoagulation | 8 (22%) | 8 (42%) | 0.13 |
| Pre-existing epilepsy | 3 (8%) | 0 | 0.28 |
| Length of stay (median, days) | 6 | 6 | 0.88 |
| mRS at discharge (median, IQR) | 4 (2) | 4 (2) | 0.65 |
| mRS at three months (median, IQR) | 3 (3) | 2 (3) | 0.73 |
| Mean follow-up (years, std.) | 1.8 (1.0) | 1.6 (1.0) | 0.38 |
| Developed new epilepsy | 2 (5%) | 1 (5%) | 1.00 |
| Mean time to epilepsy (years, std.) | 0.25 (0.14) | 0.80 (0) | 1.00 |
| Died | 6 (16%) | 5 (26%) | 0.48 |
| Mean survival time (years, standard error) | 2.8 (0.19) | 2.3 (0.30) | 0.38 |
IQR: interquartile range; mRS: modified Rankin Scale; std.: standard deviation
Every seventh (14%) had been functionally non-independent before the stroke, whereas over two thirds (68%) had been healthy and had no discernible signs or symptoms (mRS 0) (Figure 1). In-hospital mortality was 5%, and only 20% were functionally independent (mRS 0–2) at hospital discharge, whereas this proportion had more than doubled to 50% at 3 months after the ICH. In regression analyses, only patient age was associated with functional status at three months (Table 2). Of the patients with preceding VKA therapy, 40% were functionally independent at three months, compared to 60% of those with DOAC-associated ICH (p = 0.61). Three patients had neurosurgical evacuation of the hematoma: two of them were functionally independent (mRS 2) and one of them was functionally dependent (mRS 4).
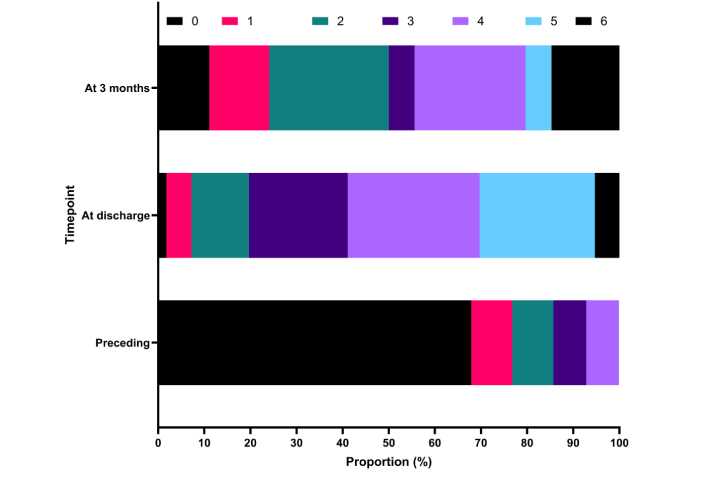
Patients’ functional status at different time points as modified Rankin scale scores. Data missing for two patients at three months
Regression analyses for predictors of functional status at three months
| Characteristic | p univariate regression | Odds ratio | 95% Confidence interval |
|---|---|---|---|
| Sex | 0.73 | ||
| Lobar | 0.83 | ||
| Smoking | 0.78 | ||
| Diabetes | 0.22 | ||
| Hypertension | 0.057 | ||
| Anticoagulation | 0.86 | ||
| Age | 0.008 | 1.060 | 1.015–1.107 |
Mean follow-up duration was 1.72 years, during which 11 patients (20%) died, with 10 of these deaths (91%) occurring within four months of the ICH (Figure 2). Of the three patients who died during the index hospitalization, two perished the same day the ICH occurred. In nine cases (all deceased within 4 months), the cause of death was the ICH, followed by an infection in some cases. In one case (who died within 3 months), it was cancer. In one case, the cause of death was unavailable. In univariate analyses, diabetes, hypertension, and patient age were correlated with mortality, but in multivariate analyses, only diabetes was associated with mortality during follow-up (Table 3 and Figure 3). One patient with VKA-associated ICH (20%) and two patients with DOAC-associated ICHs (18%) had died (p = 1.0).
Regression analyses for predictors of mortality
| Characteristic | p univariate regression | p Cox regression | HR Cox regression | HR 95% CI |
|---|---|---|---|---|
| Sex | 0.37 | |||
| ICH location | 0.82 | |||
| Smoking | 1.00 | |||
| Diabetes | 0.030 | 0.046 | 3.50 | 1.02–11.95 |
| Hypertension | 0.035 | 0.95 | ||
| Anticoagulation | 0.92 | |||
| Age | 0.005 | 0.091 |
CI: confidence interval; HR: hazard ratio; ICH: intracerebral hemorrhage
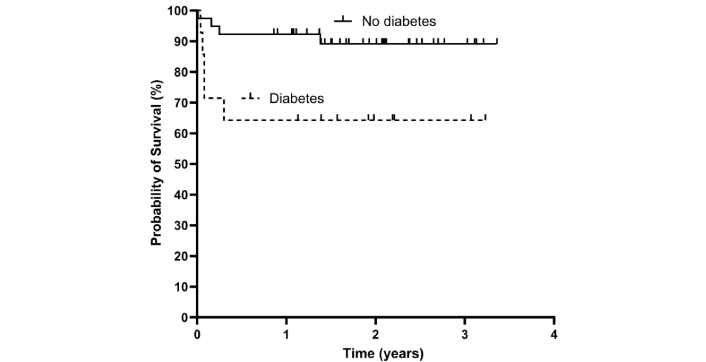
Survival after spontaneous intracranial haemorrhage in patients with (N = 15) and without (N = 41) diabetes
Of the 50 patients who did not have a pre-existing epilepsy and did not die within a week of the ICH, three (6%) developed epilepsy during the follow-up (mean: 1.84 years). Epilepsy diagnoses were made 0.24, 0.26, and 0.80 years after the ICH. No patient characteristic was associated with this outcome in the regression analyses (Table 4).
Analyses for predictors of a new diagnosis of epilepsy during follow-up
| Characteristic | Epilepsy (N = 3) | No epilepsy (N = 47) | p comparing groups | p univariate regression |
|---|---|---|---|---|
| Sex (male) | 67% | 64% | 1.00 | 0.92 |
| Lobar ICH | 33% | 19% | 0.50 | 0.56 |
| Smoking | 33% | 15% | 0.41 | 0.42 |
| Diabetes | 33% | 28% | 1.00 | 0.85 |
| Hypertension | 100% | 92% | 1.00 | 1.00 |
| Anticoagulation | 0% | 28% | 0.36 | 1.00 |
| mRS at discharge (median, IQR) | 0 (0) | 0 (1) | 0.64 | |
| Age, years (median, IQR) | 58.3 (0) | 73.0 (12.3) | 0.047 | 0.058 |
ICH: intracerebral hemorrhage; IQR: interquartile range; mRS: modified Rankin Scale
In 2021–2022 combined 41 new ICH occurred, indicating a mean incidence of 12.3/100,000 person-years at risk in the total population, 15.1/100,000 in the population > 18 years of age, and 1.8/100,000 in the population 19–49 years of age. ICH occurrence was not more common in the warmer half of the year (April–September, 23 cases, 95% CI: 17–29 cases) compared to the colder half of the year (October–March, 18 cases, 95% CI: 12–24 cases) (Figure 4).
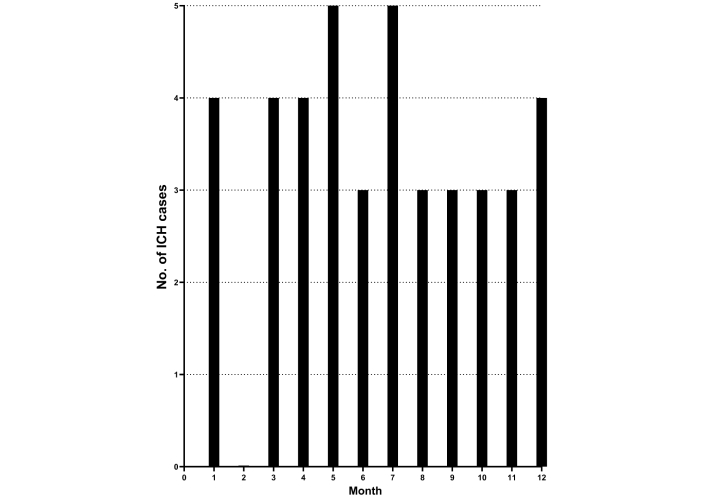
Monthly intracerebral hemorrhage occurrence (ICH) (No. of cases) in 2021–2022 (N = 41)
This single-center study showed a relatively low incidence of ICH compared to previous reports, being 12.3/100,000 person-years at risk in the total population. The short-term prognosis after ICH was mostly favorable in this cohort. Half of all patients were functionally independent three months after the stroke, and 20% overall mortality during follow-up (mean: 1.7 years). Independent prognostic clinical characteristics were limited to diabetes for mortality and patient age for functional outcome. New-onset epilepsy was rare and only occurred within the first year of follow-up.
Compared to previous Finnish ICH studies, our data suggest that the age of ICH patients has continued to increase, and their mortality has continued to decrease [7, 9, 11]. Declining mortality has also been observed internationally [3]. Nevertheless, the 5% in-hospital mortality and 20% overall mortality in an almost 2-year follow-up are remarkably low. For example, previous data from Northern Finland following ICH patients diagnosed between 1993 and 2008 reported cumulative mortality rates of 41.7% at 3 months and 47.9% at one year after the ICH. Furthermore, the recent national Finnish ICH study, which utilized registry data up until 2018, reported a 30-day mortality rate of 28.4%, with half of these patients dying during the first three days following the ICH [12]. A recent review also observed a 50% ICH survival rate at one year in high-income countries using study periods between 1996 and 2015 [13]. On the other hand, recent Japanese data have shown 5.2–11.9% in-hospital mortality in ICH patients who had not been on VKA prior to the ICH. Indeed, > 90% of patients in the current data were in this category [14].
Considering that VKA has been associated with worse ICH outcomes compared to DOACs and patients with no anticoagulation [15], it seems that abandoning VKA, also evident in Finnish drug consumption data, has led to better ICH outcomes [16]. Nevertheless, the 33–43% mortality at 30 days of patients not taking VKA in the recent Swiss and Norwegian data is considerably higher compared to the follow-up mortality in the current data [15]. This might partly result from lower proportion of VKA use in our data, but also from differences in cohort compositions since, for example, the proportion of women was markedly lower in our data and female sex has been associated with a worse survival prognosis after ICH in a recent European stroke registry study [17]. On the other hand, the proportion of patients with diabetes was markedly higher in the current data, in which it also independently predicted higher mortality, which also occurred early after ICH. Diabetes has been identified as a predictor of both early and later death after ICH in earlier Finnish studies, but this association has been less clear or missing in other populations [18–22]. Its influence may therefore be population-specific and potentially related to, e.g., characteristics of diabetes cohorts and treatment regimens.
The functional outcome at three months was surprisingly good in the current study compared to previous data, where only 1/6 to 1/4 achieved functional independence [3]. Current study did not show any difference in functional outcome between genders compared to the recent meta-analysis of sex modifying severity and outcome of ICH [23]. The overall good functional outcome in the study population might be associated with abandoning VKA for DOACs since ICHs associated with the latter have more often been followed by functional independence and less often by poor outcome in recent ICH cohort studies [14]. This is unsurprising since VKA, but not DOACs, has been associated with a devastating effect on hematoma expansion and bleeding volumes [24]. The current data, along with previous studies, suggests that this difference can also be seen as better outcomes in real-world clinical data. Acute care bundles including rapid imaging with stroke CT and when diagnosed, rapid management of high blood pressure under 180 mmHg systolic and management of high body temperature (if over 38℃) and control of glucose (over 8 mmol/L) are used as part of our stroke protocol for ICH as well and may have a positive impact on outcome and prognosis. In addition, ICH patients have also been treated with anticoagulant reversal therapy (PCC for VKA and selectively for DOACs andexanet-alfa). When needed, our patients are also referred to KUH Department of Neurosurgery for hematoma evacuation [10]. Finnish cohort of 75- and 80-year-old men and women has also shown better maximal physical performance in comparison of the cohort 28 years ago which might decrease the risk of mortality and morbidity after a stroke the elderly [25, 26].
The low number of lobar hematomas (12/56) and patients with VKA might explain the comparably low frequency of new-onset epilepsy during the follow-up (6% during 1.8 years) compared to previous Finnish data (10% at 2 years or 13.5% in a mean follow-up of 6.4 years) [27, 28]. Interestingly, a recent Danish registry study also reported a lower cumulative epilepsy incidence for all strokes (8.6% at 2 years) compared to previous data but also a higher epilepsy risk in patients with ICH after 2011 compared to the period before 2011 which was suggested to be a consequence of improved survival [29]. Medication data were not reported.
Since DOACs have been consistently associated with a considerably lower risk of ICH [30, 31], the move away from VKA anticoagulation may also explain why ICH incidence in the current data was approximately half of that in previous European studies [32]. Indeed, meta-analyses have shown that with DOACs, the risk of ICH is approximately half of that when using VKA [31–33]. Moreover, Danish registry data from 2005–2018 showed that even as the use of oral anticoagulation became more common, the incidence of ICH decreased as the use shifted from VKA to DOACs [34]. Declining smoking rates have probably also contributed [35]. Previous Finnish studies have consistently shown a trend of decreasing stroke incidence over several decades [7, 31, 36–38]. In international comparisons, it should also be noted that epidemiological studies on ICH show very high data heterogeneity for which no obvious source can be identified [39]. The differences between the results may therefore reflect, e.g., true differences between populations, but any conclusions should remain cautious.
Future lines of research on spontaneous ICH (sICH) should focus on the identification of different blood biomarkers to differentiate ICH from AIS. This would help in optimizing the prehospital blood pressure management in patients with ICH and better imaging protocols to evaluate the best candidates for microsurgery. Registry data on the epidemiology of ICH is also needed to better understand the subgroups of sICH.
The current study relied on data generated prospectively by clinicians for the specific purpose of stroke monitoring. The data are therefore very likely more accurate than general administrative data, but interrater reliability is unknown. Moreover, as the data were generated while also attending to regular clinical duties, some patients may have been forgotten to add to the list in the everyday bustle. Patients taken initially to KUH with large stroke symptoms according to the transportation protocol could have been missed if they had died during the hospitalization or been referred to local health care center wards after diagnostics and treatment. However, most North Karelian stroke patients are routinely transferred to NKCH for further treatment and rehabilitation, and based on regular monitoring of administrative billing data, this is unlikely source of bias. The initial involvement of the university hospital also means that we did not have the original imaging data for all patients, which precluded some analyses, such as investigating the role of intraventricular extension, a known determinant of poor early outcome [40]. With a non-randomized observational study design, there is naturally always the risk of non-random (unmeasured) residual confounding, which should be considered when interpreting the results.
In conclusion, the prognosis of ICH was considerably better in these new data compared to previous results. This reflects not only continuing long-term trends of improving outcomes after stroke with different measures as a care bundle effect, but probably also shows one result of replacing VKA with DOACs.
AIS: acute ischemic stroke
DOACs: direct oral anticoagulants
ICH: intracerebral hemorrhage
KUH: Kuopio University Hospital
mRS: modified Rankin Scale
NKCH: North Karelia Central Hospital
sICH: spontaneous intracerebral hemorrhage
VKA: warfarin
JOTS: Conceptualization, Investigation, Data curation, Formal analysis, Methodology, Project administration, Supervision, Validation, Visualization, Writing—original draft, Writing—review & editing. JT: Investigation, Supervision, Writing—review & editing. JP, LB, M Hassan, M Heikkinen, AK, AM, AMS, and JW: Investigation, Writing—review & editing. AMK: Formal analysis, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
Jussi O.T. Sipilä, who is the Editorial Board Member of Exploration of Neuroscience, had no involvement in the decision-making or the review process of this manuscript. Jussi O.T. Sipilä: Honoraria (Novartis), Congress attendance support (Lundbeck), Advisory Board (Sandoz, Boehringer-Ingelheim), Shareholder (Orion). Ayla Mehdiyeva: Congress attendance support (Sanofi). Mohamed Hassan: Congress attendance support (Merck). Johanna Willman: Congress attendance support (Orion). Anne-Mari Kantanen: Advisory board (Boehringer-Ingelheim, Astra-Zeneca), Congress Attendance support Astra Zeneca. All other authors declare that they have no conflicts of interest.
This study involves no direct contact (outside of normal healthcare procedures) with the patients, and ethical approval is therefore not required according to Finnish law.
This study involves no direct contact (outside of normal healthcare procedures) with the patients or publishing identifiable individual level information, and patient consent is therefore not required according to Finnish law.
Not applicable.
These data are subject to restrictions by Finnish law. Access can be applied for from Siun Sote (https://www.siunsote.fi/web/english/contact-information).
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2871
Download: 13
Times Cited: 0
