Affiliation:
Department of Cardiac Surgery, Harefield Hospital, UB9 6JH London, United Kingdom
Email: drrajashahzad@hotmail.com
ORCID: https://orcid.org/0000-0003-1325-0490
Explor Med. 2025;6:1001361 DOI: https://doi.org/10.37349/emed.2025.1001361
Received: June 30, 2025 Accepted: August 27, 2025 Published: September 25, 2025
Academic Editor: Zhong Wang, University of Michigan, USA
Coronary artery bypass grafting (CABG) remains a cornerstone in the management of complex coronary artery disease, particularly in patients with multivessel involvement, diabetes, or left main disease. As surgical practice enters a new era of precision medicine and digital innovation, the need to reimagine CABG—beyond its traditional framework—has never been more pressing. This review explores the future of CABG across three central themes: innovation, individualization, and integration. Technological advancements such as robotic-assisted procedures, hybrid revascularization strategies, and artificial intelligence-driven decision support are reshaping operative planning and execution. Concurrently, biological innovations—including regenerative therapies and tissue-engineered grafts—are expanding the therapeutic envelope, offering potential solutions for anatomically complex or high-risk patients. Personalized medicine is gaining traction through genomic profiling, biomarker-guided risk stratification, and machine learning-based outcome prediction. Enhanced recovery after surgery (ERAS) protocols and telemedicine-enabled follow-up are redefining postoperative care, emphasizing early mobilization, opioid minimization, and remote monitoring. Ethical and economic considerations remain pivotal as these innovations transition into practice. Issues of equitable access, algorithmic transparency, and cost-effectiveness must be addressed to ensure responsible integration. In parallel, the professional development landscape for cardiac surgeons is evolving, with calls for structured training in advanced techniques and interdisciplinary collaboration. Future research priorities include validation of regenerative adjuncts, predictive analytics, and advanced conduit strategies, alongside investigations into health equity and subspecialization. Ultimately, achieving durable, patient-centered outcomes in the next phase of CABG requires a system-level shift that embraces innovation while safeguarding safety, accessibility, and sustainability. This article provides a comprehensive, forward-facing overview of these themes, identifying key directions for clinical practice, research, and education in the evolving world of coronary revascularization.
Coronary artery bypass grafting (CABG) has long served as the gold standard for revascularization in patients with complex coronary artery disease (CAD), particularly those with multivessel disease, diabetes mellitus, or left main stenosis [1]. Since its formal inception in the late 1960s (Figure 1), CABG has demonstrated robust survival benefits, improved quality of life, and durable symptom relief in a wide range of patient populations [2]. Even with the advancements in percutaneous coronary intervention (PCI), CABG retains its vital role—especially in anatomically and physiologically complex cases [3].
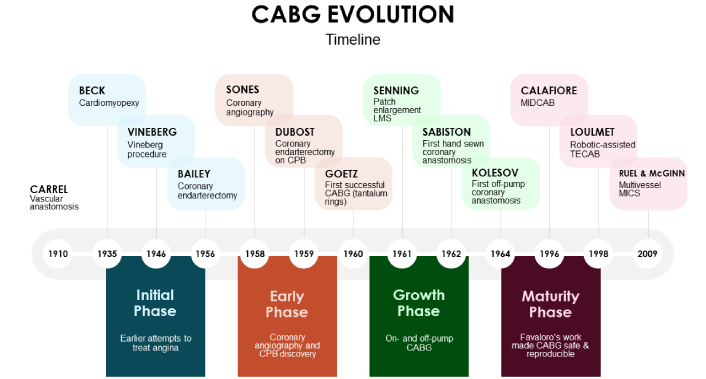
Coronary artery bypass grafting (CABG) evolution timeline. CPB: cardiopulmonary bypass; LMS: left main stem; MIDCAB: minimally invasive direct coronary artery bypass; TECAB: totally endoscopic coronary artery bypass; MICS: minimally invasive coronary surgery.
However, as the cardiovascular landscape evolves, so too must the approach to surgical revascularization. Increasingly complex comorbidities, the advent of personalized care paradigms, and the integration of digital technologies are compelling a re-examination of how CABG is delivered, evaluated, and taught [4–6]. This momentum is further driven by disparities in procedural access and outcomes, alongside mounting interest in redefining CABG as a subspecialty with focused training and credentialing [7, 8].
The aim of this review is to explore ten critical domains that are shaping the future of CABG. These include technological advancements, biological innovations, personalized medicine, enhanced recovery after surgery (ERAS) protocols, telemedicine and remote monitoring, ethical and economic considerations, evolving training requirements, and emerging research priorities. The overarching framework centers around three core principles: innovation, referring to the advancement of tools and techniques; individualization, highlighting patient-specific care models; and integration, emphasizing the need for cross-disciplinary and systemic coordination.
This narrative is intended for cardiovascular surgeons, interventionalists, researchers, and healthcare leaders seeking a forward-looking perspective on CABG’s evolution in the era of precision medicine.
Technological innovation has long been a driver of progress in surgical disciplines, and CABG is no exception. In the coming decades, advances in robotic systems, hybrid revascularization strategies, and artificial intelligence (AI) stand to substantially refine surgical precision, minimize morbidity, and individualize care delivery.
Robotic-assisted CABG is one modality within the broader spectrum of minimally invasive coronary surgery, which also includes thoracoscopic and mini-thoracotomy approaches. It constitutes a growing frontier in minimally invasive cardiac surgery, offering enhanced dexterity, motion scaling, and three-dimensional visualization. Through enhanced dexterity, motion scaling, and three-dimensional visualization, robotic platforms allow surgeons to perform precise anastomoses via thoracoscopic or mini-thoracotomy approaches, reducing the need for sternotomy [9]. Studies have demonstrated that robotic-assisted CABG is associated with lower transfusion requirements, reduced wound complications, and quicker return to daily activity—although long-term graft patency and cost-effectiveness remain under investigation (Table 1) [10–27]. As robotic technology matures and becomes more accessible, its role in standard CABG may expand beyond niche centers.
Key studies reporting outcomes of TECAB.
| Study (year) | Country | Design | Study period | TECAB (n) | Early complications | Late complications | Conversion to open surgery (%) |
|---|---|---|---|---|---|---|---|
| Mohr et al. [10] (2001) | Germany | Retrospective cohort | 2000 | 27 | NR | Graft failure (3.7%), respiratory failure (3.7%) | 6 (22.2%) |
| Dogan et al. [11] (2002) | Germany | Retrospective cohort | 1999–2002 | 62 | Bleeding (6.4%), graft failure (3.2%), CVA (1.6%), AF (4.8%) | NR | 16 (25.8%) |
| de Cannière et al. [12] (2007) | Belgium, Germany | Retrospective cohort | 1998–2002 | 228 | MI (0.9%) | NR | 64 (28.1%) |
| Kappert et al. [13] (2008) | Germany | Retrospective cohort | 1999–2001 | 41 | NR | MI (4.9%) | 0 (0.0%) |
| Argenziano et al. [14] (2006) | USA, Austria | RCT | 2002–2004 | 85 | Graft failure (1.2%) | NR | 5 (5.9%) |
| Mishra et al. [15] (2006) | India | Retrospective cohort | 2002–2005 | 13 | Bleeding (7.7%) | Graft failure (7.7%) | 0 (0.0%) |
| Srivastava et al. [16] (2010) | USA | Retrospective cohort | 2004–2007 | 214 | NR | NR | 17 (7.9%) |
| Balkhy et al. [17] (2011) | USA | Retrospective cohort | 2008–2010 | 120 | MI (0.8%), CVA (0.8%), bleeding (1.6%), pericardial effusion (0.8%) and pleural effusion (1.6%) | Graft failure (4.1%) | 3 (2.5%) |
| Jegaden et al. [18] (2011) | France | Retrospective cohort | 1998–2008 | 59 | Bleeding (8.5%), MI (3.3%) | Recurrent angina (13.5%), graft failure (3.3%) | 0 (0.0%) |
| Dhawan et al. [19] (2012) | USA | Retrospective cohort | 2007–2009 | 106 | AKI (7.5%), CVA (1.9%) | NR | 12 (11.3%) |
| Srivastava et al. [20] (2012) | USA | Retrospective cohort | 2008–2009 | 164 | CVA (0.6%), cardiac death (0.6%) | RCA dissection (0.6%), graft failure (1.8%) | 0 (0.0%) |
| Wiedemann et al. [21] (2013) | USA, Austria | Retrospective cohort | 2001–2011 | 500 | NR | NR | 63 (12.6%) |
| Zaouter et al. [22] (2015) | France | Retrospective cohort | 2011–2014 | 38 | AF (18.4%) | NR | 1 (2.6%) |
| Efendiev et al. [23] (2015) | Russia | Prospective cohort | 2012–2015 | 50 | NR | NR | 0 (0.0%) |
| Pasrija et al. [24] (2018) | USA | Retrospective cohort | 2011–2014 | 50 | Respiratory failure (2%) | NR | 2 (4.0%) |
| Cheng et al. [25] (2021) | China | Retrospective cohort | 2007–2017 | 126 | Graft failure (0.7%), bleeding (0.7%) | CVA (2.3%), graft failure (2.3%) | 1 (0.8%) |
| Balkhy et al. [26] (2022) | USA | Retrospective cohort | 2013–2020 | 544 | AF (13%), AKI (2.9%), CVA (0.2%), MI (0.2%), bleeding (1.1%) | MI (2.2%) | 1 (0.2%) |
| Claessens et al. [27] (2022) | Belgium | Retrospective cohort | 2016–2018 | 244 | Cardiac death (1.4%), MI (0.6%), CVA (0.8%) | Cardiac death (3.4%), MI (2.6%), CVA (0.0%) | 5 (2.0%) |
TECAB: totally endoscopic coronary artery bypass; CVA: cerebrovascular accident; AF: atrial fibrillation; NR: not reported; MI: myocardial infarction; RCT: randomized controlled trial; AKI: acute kidney injury; RCA: right coronary artery.
Hybrid coronary revascularization (HCR) offers a synergistic model that combines the durability of surgical left internal thoracic artery (LITA) to left anterior descending (LAD) grafting with the flexibility of PCI for non-LAD lesions [28]. This approach allows for revascularization customization based on coronary anatomy, lesion complexity, and patient comorbidity. Published studies report comparable outcomes of HCR and CABG (Table 2) [29–35]. Future developments in HCR may include real-time multimodal imaging integration and robotic-PCI coordination platforms to enhance workflow and decision-making.
Comparative outcomes of HCR vs. CABG.
| Study (year, country) | Study design | Patient population & follow-up | Key findings | Summary insight |
|---|---|---|---|---|
| Basman et al. [29] (2020, USA) | Retrospective, propensity-matched | 200 patients with triple-vessel CAD; ~7.1 years |
| Notable follow-up duration, though significant disparities in CAD severity between groups impede interpretation |
| Esteves et al. [30] (2021, Brazil) | RCT | 60 patients with complex triple-vessel CAD; ~2.2 years |
| Small sample with underpowered statistics; outcome disparities noted but not statistically significant |
| Ganyukov et al. [31] (2020, Russia) | RCT | 98 patients with multivessel CAD; 12 ± 1 months |
| Demonstrates comparable ischaemic burden; however, outcomes are observational in nature and the trial was descriptively interpreted |
| Hage et al. [32] (2019, Canada) | Observational cohort | 363 patients with 2-vessel CAD; follow-up: 96 months (HCR) vs. 81 months (CABG) |
| Suggests enhanced symptom control with HCR. Differences in urgency and selection may confound survival analysis |
| Qiu et al. [33] (2019, China) | Propensity-matched retrospective study | 94 matched patients with 2-vessel CAD; ~5-year follow-up |
| Reasonably matched population; however, small sample size hinders generalizability |
| Tajstra et al. [34] (2018, Poland) | RCT | 191 multivessel CAD patients; ~5.9-year median follow-up |
| Strongest RCT evidence to date; trends favor HCR, though results not statistically definitive |
| Giambruno et al. [35] (2018, Canada) | Retrospective cohort | 690 2-vessel CAD patients; median follow-up ~70 months |
| Provides meaningful follow-up; absence of full MACCE evaluation limits completeness |
CABG: coronary artery bypass grafting; CAD: coronary artery disease; HCR: hybrid coronary revascularization; MI: myocardial infarction; TVR: target vessel revascularization; MACE: major adverse cardiac events; MACCE: major adverse cardiac and cerebrovascular events; NS: not significant; RCT: randomized controlled trial.
Perhaps the most disruptive innovation lies in the application of AI and machine learning to CABG. Predictive algorithms trained on large datasets—such as the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database—have demonstrated superior risk stratification compared to conventional scoring systems [36]. These tools can incorporate preoperative and intraoperative variables to assist in perioperative decision-making, predict complications such as stroke or prolonged ventilation, and refine resource allocation (Table 3) [36–44]. Further integration of AI into intraoperative imaging, graft flow assessment, and even robotic suturing may eventually lead to real-time, AI-augmented surgical execution.
Key ML-based risk prediction studies in CABG.
| Study (year) | Population & setting | AI model(s) | Prediction target(s) | Validation | Performance metrics | Key predictors/features | Comparators | Key takeaways |
|---|---|---|---|---|---|---|---|---|
| Ma et al. [36] (2025)NOAF prediction | 2,994 CABG patients (2 centers, China) | Stacked ensemble (11 learners) | NOAF | Internal + external | AUC: 0.931F1: 0.797 | BNP, LVEDD, EF, BMI, NAR, LA diameter | CHA2DS2-VASc, HATCH, POAF | SHAP-informed bedside tool with superior accuracy |
| Akbulut et al. [37] (2025)POAF biomarkers (pilot) | 100 CABG patients (Turkey) | Not specified | POAF | Internal | Thresholds only | Mg 442 mmol/L, albumin < 29 g/L | None | First use of ML to identify lab cutoffs for POAF |
| Li et al. [38] (2025)AKI in elderly patients | 2,155 elderly CABG patients (China) | RF, XGBoost, LightGBM, etc. | AKI | Internal | RF AUC: 0.737 | Age, eGFR, UA, BNP, ALT, IABP use | None | Strong RF performance with explainability via SHAP |
| Jafarkhani et al. [39] (2025)ICU LOS (general) | 605 CABG patients (Iran) | RF + others | ICU LOS | Internal | R2: 0.28MSE: 1.64 | Intubation time, BMI, age, PRBCs, surgery time | None | Identifies modifiable contributors to ICU burden |
| Dong et al. [40] 2025GI bleeding (GIBCG) | 16,440 patients (4 centers + MIMIC-IV) (China) | Top model of 30 tested | GI bleeding | External | AUC: 0.848–0.851MIMIC: 0.781 | DAPT, PPI, anticoagulants, albumin | None | Web-based tool enables personalized prophylaxis |
| Yang et al. [41] 2025Prolonged ICU stay (IABP) | 236 IABP CABG patients (China) | 7 models (XGBoost best) | ICU stay > 14 days | Internal (train/val) | AUC: 0.92 (train); 0.73 (val) | Tracheotomy, albumin, Sv1, troponin T | None | Targeted model for IABP cohort; SHAP interpretation |
| Chen et al. [42] 2024Post-op stroke risk | 1,200 CABG patients (China) | RF (best of 6), LASSO | Stroke after CABG | Internal (70:30 split) | AUC: 0.901F1: 0.721 | Cr, IABP, ventilation, clamp time, COPD | None | Online tool; ranked features via SHAP |
| Xu et al. [43] 2024XCL ensemble mortality model | 4,764 patients (3 Chinese centers) | XGBoost + CatBoost + LightGBM | In-hospital mortality | Internal + external | AUC: 0.9145 | Composite model features | EuroSCORE II | Outperformed EuroSCORE II in all performance domains |
| Couto et al. [44] 2024Hospital LOS (Brazil) | 9,584 CABG patients (133 centers)Val: 2,627 | RF (best), XGBoost, Neural Net | Hospital stay duration | External | RMSLE: 0.412 (train); 0.454 (val) | Public hospital, emergency, HF, age | Poisson, NB, linear regression | National tool for capacity planning and benchmarking |
F1: F1 score (harmonic mean of precision and recall). CABG: coronary artery bypass grafting; AI: artificial intelligence; NOAF: new-onset atrial fibrillation; AUC: area under the receiver operating characteristic curve; BNP: B-type natriuretic peptide; LVEDD: left ventricular end-diastolic diameter; EF: ejection fraction; BMI: body mass index; NAR: neutrophil-to-albumin ratio; LA: left atrium; CHA2DS2-VASc: congestive heart failure, hypertension, age ≥ 75 (doubled), diabetes, stroke/transient ischemic attack (TIA)/thromboembolism (doubled), vascular disease, age 65–74, sex category; HATCH: hypertension, age ≥ 75, TIA or stroke, chronic obstructive pulmonary disease, heart failure (stroke risk score); POAF: postoperative atrial fibrillation; SHAP: SHapley Additive exPlanations; Mg: magnesium; ML: machine learning; AKI: acute kidney injury; RF: random forest; XGBoost: eXtreme Gradient Boosting; LightGBM: light gradient boosting machine; eGFR: estimated glomerular filtration rate; UA: uric acid; ALT: alanine aminotransferase; IABP: intra-aortic balloon pump; ICU: intensive care unit; LOS: length of stay; MSE: mean squared error; R2: coefficient of determination; PRBCs: packed red blood cells; GI: gastrointestinal; GIBCG: GI bleeding in CABG cohort; MIMIC-IV: Medical Information Mart for Intensive Care IV; DAPT: dual antiplatelet therapy; PPI: proton pump inhibitor; val: validation set; Sv1: S wave in lead V1; LASSO: least absolute shrinkage and selection operator; Cr: creatinine; COPD: chronic obstructive pulmonary disease; XCL: eXplainable Composite Learner; CatBoost: categorical boosting algorithm; EuroSCORE II: European System for Cardiac Operative Risk Evaluation II; Neural Net: neural network; RMSLE: root mean squared logarithmic error; HF: heart failure; NB: negative binomial.
Technological advancements are not merely tools for refinement; they are potential levers for systemic transformation in how CABG is conceptualized, performed, and evaluated. Their implementation, however, must be accompanied by structured validation and equitable access to ensure broad patient benefit.
In parallel with technological progress, advances in biological science are unlocking new pathways for enhancing the efficacy and durability of CABG. Central to this evolution are regenerative strategies—including stem cell therapy—and the engineering of next-generation graft materials aimed at overcoming limitations of autologous conduits.
Stem cell and regenerative therapies hold particular promise in cases of ischemic cardiomyopathy and diffuse coronary disease, where viable targets for bypass may be limited. Preclinical and early-phase clinical studies suggest that mesenchymal stem cells (MSCs), cardiac progenitor cells, and pluripotent-derived cardiomyocytes can promote neovascularization and improve myocardial function when delivered adjunctively with CABG [45, 46]. While long-term efficacy and delivery mechanisms remain subjects of ongoing investigation, future iterations of CABG may include cell-based myocardial enhancement as a routine component in select patient cohorts.
Meanwhile, advances in graft technology are addressing the inherent limitations of saphenous vein grafts and the scarcity of arterial conduits. Tissue-engineered vascular grafts (TEVGs)—constructed using biodegradable scaffolds seeded with endothelial or stem cells—have shown early promise in resisting thrombosis and intimal hyperplasia [47]. Ongoing refinement in scaffold composition, surface coating, and biointegration could allow TEVGs to eventually mimic the vasoprotective effects of native arteries. Despite encouraging preclinical data, TEVGs are not yet part of routine clinical CABG practice. Their translational trajectory is still evolving, and further validation through large-scale clinical trials is needed before widespread adoption. Simultaneously, interest persists in xenograft-derived or decellularized allogenic conduits, though concerns about immunogenicity and structural degradation remain [48].
Further biological modulation may come from local drug delivery platforms, such as anti-inflammatory or antiproliferative agents embedded within graft matrices to reduce early vein graft failure. Experimental studies are also exploring the potential for nitric oxide-releasing materials and endothelial glycocalyx mimetics to enhance antithrombotic properties [49].
Critically, arterial conduits themselves may confer biological benefit beyond flow delivery. Internal thoracic arteries have been shown to produce antithrombotic and antiatherogenic substances, such as nitric oxide and prostacyclin, potentially exerting a protective effect on distal coronary vasculature [50]. Such findings reinforce the value of multiarterial grafting strategies and fuel interest in bioactive conduit engineering.
Biological innovations are positioned to extend CABG’s therapeutic envelope—transforming it from a purely mechanical revascularization technique into a synergistic intervention encompassing vascular regeneration, myocardial support, and cellular protection.
As cardiovascular disease management increasingly embraces precision medicine principles, CABG too is poised to evolve from a standardized intervention into a patient-specific therapeutic modality. Personalized approaches—incorporating genomic data, biomarker profiling, and AI-driven analytics—can improve risk stratification, optimize perioperative planning, and enhance long-term outcomes.
Genomic and transcriptomic profiling offer novel opportunities to tailor surgical revascularization strategies. Specific polymorphisms in inflammatory and thrombosis-related genes—such as interleukin-6 (IL-6), tumor necrosis factor-α (TNF-α), and cytochrome P450 2C19 (CYP2C19)—have been linked to postoperative outcomes, including graft patency, myocardial infarction, and bleeding risk [51, 52]. Integrating this data into preoperative evaluation may soon allow for individualized conduit selection, antiplatelet therapy adjustment, or targeted perioperative anti-inflammatory interventions. Moreover, research into epigenetic modifiers and RNA expression signatures holds promise for predicting response to myocardial ischemia or left ventricular remodeling post-CABG [53].
Circulating biomarkers are also emerging as valuable adjuncts to conventional risk scores. Beyond troponins and natriuretic peptides, novel markers such as soluble suppression of tumorigenicity-2 (ST2), growth differentiation factor-15 (GDF-15), galectin-3, and high-sensitivity C-reactive protein (hs-CRP) have demonstrated associations with surgical risk, perioperative complications, and long-term mortality [54, 55]. Biomarker-guided algorithms may facilitate early identification of high-risk patients and guide the timing of surgery, graft strategy, or need for adjunctive support such as mechanical circulatory devices.
Machine learning models and AI, when combined with multi-omic datasets and imaging inputs, offer a transformative capability for personalized operative planning. Algorithms trained on historical CABG cohorts have demonstrated superior calibration in predicting complications like atrial fibrillation, renal dysfunction, or stroke compared to traditional scores such as European System for Cardiac Operative Risk Evaluation (EuroSCORE) or STS Predicted Risk of Mortality (STS-PROM) [56]. Importantly, these models can also be retrained with local data, making them adaptable to institutional practice patterns and patient populations.
Looking ahead, personalized CABG may encompass genomic-tailored pharmacotherapy, conduit optimization based on predicted long-term endothelial response, and intraoperative alerts triggered by patient-specific risk profiles. Such individualization may extend postoperatively, with biomarker-guided rehabilitation protocols and tailored follow-up strategies.
However, successful integration of personalized medicine into CABG will require standardization of genomic and biomarker panels, robust prospective validation, and equitable access. Only then can personalized CABG fulfill its potential as a cornerstone of precision cardiovascular surgery.
ERAS protocols, initially pioneered in colorectal surgery, are increasingly being adopted in cardiac surgical practice to reduce complications, hasten recovery, and improve overall patient experience. Their implementation in CABG marks a shift from traditional postoperative management toward a more structured, evidence-based, and multidisciplinary model of care [57].
Core components of ERAS in cardiac surgery include preoperative education, carbohydrate loading, opioid-sparing analgesia, early extubation, normothermia maintenance, minimal fluid overload, and prompt mobilization [58]. When tailored to CABG patients, these protocols have demonstrated reductions in length of stay, surgical site infections, atrial fibrillation, and opioid consumption—without increasing readmission risk [59].
Recent efforts are expanding ERAS paradigms to accommodate high-risk CABG populations, such as those with diabetes, frailty, or reduced left ventricular function. Prehabilitation strategies—including supervised exercise, nutrition optimization, and mental resilience training—are showing promise in reducing postoperative delirium and intensive care unit (ICU) stays in elderly patients undergoing CABG [60]. Additionally, intraoperative modifications such as goal-directed hemodynamic therapy and lung-protective ventilation are being examined for their role in minimizing end-organ dysfunction [61].
In-hospital optimization strategies form the backbone of ERAS implementation in CABG and are increasingly supported by evidence-based protocols. Goal-directed fluid therapy, for instance, has demonstrated superiority over conventional volume loading by maintaining hemodynamic stability and reducing postoperative complications such as acute kidney injury and pulmonary edema [61]. Similarly, lung-protective ventilation strategies—employing lower tidal volumes and optimal positive end-expiratory settings—are being adopted to minimize ventilator-associated lung injury and facilitate early extubation. These intraoperative and immediate postoperative interventions are particularly impactful in patients with reduced ventricular function or pulmonary comorbidity [57].
Postoperative opioid minimization is another critical pillar of ERAS in CABG. Multimodal analgesia regimens incorporating acetaminophen, non-steroidal anti-inflammatory drugs, gabapentinoids, and regional techniques (e.g., paravertebral blocks) have shown efficacy in reducing opioid consumption, enhancing mobilization, and lowering delirium risk [62]. Structured early mobilization protocols—initiated within 24 h of surgery—are now standard in many ERAS programs and have been linked to reduced ICU and hospital length of stay. Multidisciplinary rounding, involving surgeons, anesthesiologists, nurses, physiotherapists, and pharmacists, ensures coordinated care and timely intervention, further reinforcing the ERAS framework [57].
Importantly, ERAS protocols are also a platform for cultural transformation within surgical units, promoting standardized communication, goal alignment across teams, and data-driven quality monitoring. Institutional adoption often hinges on leadership buy-in, clinician education, and availability of audit tools to track compliance and outcomes [62]. Future enhancements may include AI-powered dashboards that integrate patient-specific risk models to guide protocol adjustments in real time.
Emerging data further support the integration of ERAS with telemedicine, allowing for early discharge with structured remote follow-up and digital symptom tracking [63]. This model not only improves patient satisfaction but may also reduce healthcare costs—a crucial consideration as CABG evolves within value-based care frameworks.
Ultimately, ERAS represents more than a checklist—it is a philosophy of patient-centered care grounded in perioperative science. Its evolution in CABG holds potential to redefine surgical recovery, enabling faster, safer, and more personalized outcomes across patient populations.
As healthcare delivery shifts toward virtual and decentralized models, telemedicine has emerged as a pivotal tool for enhancing continuity of care following CABG. Remote monitoring technologies enable early detection of complications, structured rehabilitation, and improved patient engagement—especially in geographically dispersed or resource-constrained populations [64].
Teleconsultation platforms, including video and app-based systems, have demonstrated feasibility and acceptability in early follow-up after cardiac surgery. These tools allow clinicians to assess wound healing, manage medications, and provide reassurance, all without requiring physical clinic visits. A randomized trial evaluating video-enabled follow-up found significant reductions in unplanned readmissions and higher patient satisfaction compared to standard care [65].
Wearable devices—such as telemetry patches, smartwatches, and Bluetooth-enabled oximeters—are enabling real-time data collection on heart rate variability, arrhythmia detection, respiratory parameters, and activity levels [66]. Integration of these metrics into clinician dashboards or patient apps allows for trend recognition and timely interventions. Emerging applications also include home-based cardiac rehabilitation programs that combine telemonitoring with interactive coaching to improve adherence and outcomes [67].
In high-risk post-CABG patients, mobile health ecosystems may be particularly valuable. Protocols integrating telephonic nurse visits, SMS-based reminders, and wearable sensors have been shown to reduce emergency department visits and improve medication compliance [68]. More sophisticated platforms employing AI algorithms can flag deviations from recovery baselines—such as escalating weight, oxygen desaturation, or arrhythmic trends—to trigger clinician alerts or automated guidance.
However, barriers persist. Variability in digital literacy, data integration across platforms, reimbursement ambiguity, and regulatory constraints continue to limit wide-scale implementation. Addressing these challenges requires collaborative efforts among healthcare systems, technology developers, and policymakers to ensure interoperability, data privacy, and equitable access [69].
Importantly, telehealth is not a replacement for in-person assessment but a complement—ideally as part of a hybrid model tailored to patient risk and preference. As CABG becomes increasingly personalized and outpatient-focused, telemedicine will play an integral role in shaping the postoperative experience, ensuring that the benefits of surgical innovation extend beyond the operating room and into the patient’s home.
The rapid evolution of CABG—spanning robotic techniques, AI analytics, regenerative grafts, and remote care platforms—promises significant clinical gains, yet also raises pressing ethical and economic questions. Ensuring that these advances do not exacerbate existing disparities or strain healthcare systems is paramount to their responsible integration.
One of the foremost challenges lies in equitable access. Robotic-assisted CABG, advanced genomic testing, and AI-powered risk stratification tools are predominantly available in high-resource settings. Studies have shown that patients from lower socioeconomic backgrounds and minority groups are less likely to receive guideline-directed surgical care, such as multiarterial grafting or LITA-to-LAD procedures [70]. Without deliberate policy design and funding mechanisms, innovative modalities risk deepening disparities rather than closing them.
There are also concerns around algorithmic bias. Machine learning models trained on skewed datasets may inadvertently reinforce racial, sex-based, or geographic inequities if not appropriately validated across diverse populations [71]. Transparent model development, frequent recalibration, and interdisciplinary oversight are essential to preserving clinical fairness.
From a financial perspective, the cost-effectiveness of advanced CABG technologies remains an open question. While some innovations—such as ERAS protocols or telemonitoring—have demonstrated favorable cost-utility profiles by reducing complications and length of stay, others (e.g., robotic surgery or genomic profiling) carry substantial upfront expenses [72]. Cost-benefit modeling will be crucial in informing reimbursement structures, particularly in systems transitioning toward value-based care.
Informed consent also acquires new dimensions. Surgeons must not only explain the conventional procedural risks but also communicate the uncertainties surrounding newer adjuncts—be it the longevity of engineered conduits or the interpretability of AI-derived recommendations. Shared decision-making frameworks will need updating to accommodate these novel variables.
Moreover, as CABG becomes more technologically dependent, ethical questions emerge regarding surgeon skill erosion, automation accountability, and data privacy. Who is responsible when an algorithm-guided decision leads to harm? How do we safeguard sensitive biometric data streamed via wearables? Answering these questions requires collaborative dialogue across ethics committees, regulatory bodies, and technology developers.
Ultimately, innovation in CABG must be tethered to ethical stewardship and economic prudence. Advances should be measured not only by survival curves or patency rates, but by their ability to deliver just, transparent, and sustainable cardiac surgical care across all patient populations.
The emergence of novel technologies in CABG—from robotic assistance to bioengineered grafts and AI decision-support—demands a parallel transformation in how cardiac surgeons are trained and upskilled. As procedures become more specialized, digitally integrated, and technique-sensitive, traditional apprenticeship models alone may no longer suffice.
Current curricula often lack structured exposure to robotic CABG, hybrid procedures, or off-pump techniques, especially in centers without high case volumes [73]. This variability in exposure translates to inconsistent proficiency and may limit the adoption of innovative modalities. To address this, dedicated fellowships in advanced coronary surgery are increasingly being proposed, emphasizing multiarterial grafting, total arterial revascularization, and device-enabled procedures [74].
Simulation-based education is also gaining traction. High-fidelity simulators and virtual reality platforms now offer immersive environments for anastomotic skill development, intraoperative decision-making, and familiarization with robotic systems—all without patient risk [75]. These tools not only shorten learning curves but may enable credentialing of specific skillsets in low-volume surgeons through validated competency benchmarks.
In parallel, the inclusion of AI and data literacy in surgical training is becoming essential. Surgeons will need to interpret predictive models, evaluate risk visualizations, and understand algorithm limitations—particularly as these tools become embedded in electronic health records and intraoperative platforms [76]. Cross-disciplinary training in informatics, bioethics, and data governance may thus become core components of future CABG curricula.
Moreover, lifelong learning will play a central role in maintaining proficiency. Platforms offering modular, on-demand content—such as online surgical libraries, case-based webinars, and mentorship communities—allow practicing surgeons to stay abreast of emerging techniques and guidelines [77].
Ultimately, CABG education must align with the principle of specialized, structured, and scalable training. Recognizing coronary surgery as a distinct subspecialty with defined competencies, certification pathways, and academic identity could foster excellence, drive standardization, and enable surgeons to deliver the full spectrum of modern revascularization safely and confidently.
The future of CABG lies not only in technological adoption but in the robust validation of these innovations through focused research. As surgical revascularization evolves, systematic investigation is essential to ensure efficacy, scalability, and equity.
Several emerging innovations referenced in this manuscript—particularly regenerative therapies and TEVGs—remain far from widespread clinical use. Their inclusion reflects the field’s aspirational trajectory rather than current practice. These technologies face significant translational barriers, including immunogenicity, durability, delivery logistics, and regulatory approval. Accordingly, they are best positioned within the context of future research, where ongoing preclinical and early-phase clinical studies continue to shape their potential role in CABG.
A primary area ripe for research is the long-term outcomes of advanced conduit strategies, especially total arterial and tissue-engineered grafts. While multiarterial grafting has demonstrated survival advantages in select cohorts, its broader adoption remains limited due to concerns about technical complexity, conduit availability, and lack of randomized trial data across diverse populations [78]. Future multicenter trials should examine outcomes across age groups, comorbidities, and ethnic backgrounds to refine patient selection criteria.
Similarly, adjunctive regenerative therapies—such as cell-based myocardial augmentation and bioactive graft coatings—require larger phase II/III studies to assess safety, functional improvement, and integration with standard CABG workflows [79]. This includes optimal cell types, delivery mechanisms, and concurrent imaging strategies to assess viability.
Another key priority is the validation of AI and predictive analytics platforms. While early studies show promise, few have been prospectively tested in real-world surgical settings [80]. Future research should emphasize interpretability, patient engagement, and integration into multidisciplinary care models, alongside regulatory and ethical analysis.
Exploration of health equity in CABG outcomes is also critical. The links between socioeconomic status, race, sex, and procedural disparities are well-documented, yet mechanisms remain poorly understood. Prospective studies evaluating tailored interventions—such as community-driven care models, mobile health equity platforms, or targeted surgical outreach—could inform scalable solutions [81].
Finally, trials examining CABG subspecialization are needed to define metrics for credentialing, assess learning curves, and evaluate how focused training impacts outcomes. Registry-based studies and prospective cohort analyses can offer insights into volume-outcome relationships and help shape future training paradigms [82].
Collaborative networks, such as surgical trial platforms and real-world data consortia, will play a vital role in generating high-quality evidence. As CABG enters a new era, research must remain patient-centered, adaptable, and transparent, ensuring that innovation translates into meaningful, measurable benefit.
CABG is poised at a transformative juncture. No longer solely defined by its surgical mechanics, its future lies in embracing a multidimensional evolution—one shaped by technology, biology, and personalized care. As robotic platforms, hybrid techniques, and AI-enhanced decision-making integrate into clinical workflows, the precision and adaptability of CABG will only grow. Simultaneously, regenerative therapies and novel graft materials may expand their therapeutic reach, offering hope to high-risk and anatomically complex patients.
Personalized medicine, leveraging genomics, biomarkers, and predictive modeling, introduces new pathways for individualized risk stratification and perioperative optimization. ERAS protocols, telemonitoring tools, and hybrid models of follow-up care are reframing recovery as a proactive, data-informed, and patient-centered continuum.
However, progress must be pursued with ethical vigilance. Equitable access, algorithmic transparency, and cost-effectiveness must guide the translation of these innovations into practice. Structured training pathways, dedicated coronary subspecialization, and cross-disciplinary education will be vital to prepare the next generation of CABG surgeons.
Looking forward, the field must maintain momentum through high-quality research. Trials validating regenerative approaches, intelligent systems, and advanced conduits will solidify clinical adoption. Investigations into disparities and surgical specialization will further refine standards of care.
In conclusion, the future of CABG is not defined by any single advance but by the integration of innovation, individualization, and systemic improvement. By aligning emerging science with ethical imperatives and educational reform, CABG can continue to serve as a cornerstone of modern cardiovascular therapy—smarter, safer, and more patient-centered than ever before.
AI: artificial intelligence
CABG: coronary artery bypass grafting
ERAS: enhanced recovery after surgery
HCR: hybrid coronary revascularization
ICU: intensive care unit
LAD: left anterior descending
LITA: left internal thoracic artery
PCI: percutaneous coronary intervention
STS: Society of Thoracic Surgeons
TEVGs: tissue-engineered vascular grafts
SGR: Conceptualization, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
Shahzad G. Raja, who is the Editorial Board Member of Exploration of Medicine, had no involvement in the decision-making or the review process of this manuscript.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
