Affiliation:
1Internal Medicine Department, Medicine and Clinical Studies Research Institute, National Research Center, Cairo 12622, Egypt
ORCID: https://orcid.org/0000-0003-1661-3891
Affiliation:
2Endemic Medicine Department, Faculty of Medicine, Helwan University, Cairo 11795, Egypt
ORCID: https://orcid.org/0000-0002-9020-8870
Affiliation:
3Faculty of Medicine, Helwan University, Cairo 11795, Egypt
ORCID: https://orcid.org/0009-0000-5479-4078
Affiliation:
2Endemic Medicine Department, Faculty of Medicine, Helwan University, Cairo 11795, Egypt
Email: ahmed.tawhid@med.helwan.edu.eg
ORCID: https://orcid.org/0000-0002-9382-8733
Affiliation:
2Endemic Medicine Department, Faculty of Medicine, Helwan University, Cairo 11795, Egypt
ORCID: https://orcid.org/0000-0002-3396-6894
Explor Med. 2025;6:1001344 DOI: https://doi.org/10.37349/emed.2025.1001344
Received: December 02, 2024 Accepted: May 24, 2025 Published: July 10, 2025
Academic Editor: Marcos Roberto Tovani-Palone, Saveetha Institute of Medical and Technical Sciences (SIMATS), India
The article belongs to the special issue Emerging Infectious Diseases
Hepatitis B virus (HBV) infection is an important global public issue with several routes of transmission. HBV infection among healthcare workers (HCWs) is of particular concern due to the high transmissibility of the virus compared with other bloodborne viruses, putting infected HCWs at risk of transmitting HBV to patients. These considerations necessitate the implementation of standard recommendations for preventing and managing HBV in HCWs. Over the past four decades, the incidence of HBV transmission from HCWs to patients has significantly decreased in frequency, and this could be due to enhanced screening, routine vaccination of HCWs, and following general precautions such as double-gloving during exposure-prone procedures. The protocols for monitoring infected HCWs differ based on the guidelines provided by health authorities.
The prevalence of hepatitis B virus (HBV) infection presents a considerable public health challenge on a global scale, ranking as the tenth leading cause of mortality. HBV, a DNA virus, is typically transmitted through parenteral or mucosal exposure to infected blood or secretions [1, 2].
HBV is a highly infectious bloodborne virus that affects the liver. Bloodborne viruses have been known to be transmitted between patients and healthcare workers (HCWs) during healthcare service delivery [3]. However, the transmission frequency has decreased after introducing standard (universal) precautions, adopting improved percutaneous injury precautions in surgery, and routine HBV vaccination [4].
HCWs have a four times higher risk of contracting HBV than the general population, so the possibility of viral hepatitis transmission among HCWs is a serious concern [5, 6]. High morbidity and mortality are caused by chronic hepatitis, cirrhosis, and hepatocellular carcinoma, most frequently caused by HBV and hepatitis C virus (HCV) [7, 8]. While HCV is the leading cause of chronic hepatitis, HBV is more infectious [2] and more common [9]. HBV can lead to a long-term liver infection that poses a high risk of death from cirrhosis and liver cancer for those affected. The frequency of HBV infection in HCWs varies with the frequency in the general population in different parts of the world and with the relative risk in HCWs vs. the general population, depending on preventive precautions in each country. In Western countries, the frequency is the same in HCWs and in the population [10].
This review explores the global burden of HBV infection, especially on HCWs in Africa, where occupational exposure poses a considerable risk. We discuss the various risks and transmission routes of HBV, focusing on how HCWs are particularly vulnerable to infection. The review also covers the diagnosis of HBV and emphasizes the essential knowledge HCWs should have to protect themselves from infection. Preventive strategies, including pre-exposure management through HBV vaccination and post-vaccination anti-HBs testing. Additionally, we outline the appropriate post-exposure management steps to mitigate infection risks. Finally, we address the critical question of when HCWs should consider pausing or discontinuing their professional practice due to HBV infection, ensuring both their well-being and patient safety.
The novelty of this literature review is that we tried to update and summarize the current evidence regarding HBV in HCWs’ situations.
In 2019, the WHO estimated that 296 million individuals had chronic HBV infection, with 1.5 million new infections occurring per year [11]. Nearly 90% of all hepatitis infections worldwide occur in Africa and Asia, where the situation needs attention [12, 13]. According to estimates from the WHO, there are 116 million and 81 million chronically infected individuals in the Western Pacific and the WHO African regions, respectively, with high prevalence rates and negative effects in some African nations [14–16]. The WHO Eastern Mediterranean Region has 60 million infected people, the WHO Southeast Asia Region has 18 million, the WHO European Region has 14 million, and the WHO Region of the Americas has 5 million. With approximately 66,000 cases and 261 deaths reported annually in low-income countries among HCWs. They face a significant risk of HBV infection due to exposure to contaminated sharp injuries, which serve as a major transmission route [17, 18]. Viral hepatitis infection was the seventh leading cause of death worldwide in 2013 [19]. An estimated 820,000 people died from cirrhosis and hepatocellular carcinoma (primary liver cancer) in 2019 due to HBV infection [11].
Although different reports have addressed HBV prevalence in Africa among HCWs, there are no clear data on the pooled prevalence. The research that is now available indicates that many HCWs in Africa are more likely to become infected with HBV [16]. For instance, 1.4% of Egyptians in 2015 [20], 2.4% of Ethiopians in 2011 [21], 17.8% of Senegalese in 1987 [22], and 25.7% of Nigerians in 2007 [23] had HBV infection. Additionally, roughly half of African HCWs are exposed to blood and other fluids on the job [14, 15]. These figures suggest significant variability across regions and time periods, likely reflecting differences in local vaccination strategies, access to healthcare, and occupational safety protocols. The lack of pooled data limits the ability to generalize findings across the continent, but the consistently high risk among HCWs underscores the urgent need for comprehensive screening, immunization, and post-exposure management programs tailored to regional contexts.
Recent data from a subgroup analysis indicate a variation in the prevalence of HBV infection among HCWs across African regions. Despite the small sample size, the western and northern regions of Africa, respectively, had the most remarkable and lowest prevalence of HBV infection among HCWs. The region’s HCWs’ level of immunization may explain this variation [24]. This variation may be attributed to differences in vaccination coverage, healthcare infrastructure, and adherence to standard precautions. A deeper regional analysis could help identify successful strategies that might be replicated in high-burden settings.
All paid and unpaid staff members working in healthcare facilities who may come into contact with patients or infectious materials, such as bodily fluids, contaminated medical supplies, devices, and equipment, contaminated environmental surfaces, or contaminated air, are referred to as “HCWs” or “healthcare personnel”. Suggestions to address occupational infection prevention and control for HCWs are available elsewhere [19].
HCWs are the participating clinicians, surgeons, staff members, trainees, subcontractors, public safety personnel, and volunteers [11].
In addition, “healthcare settings” refers to locations where healthcare is provided. These locations may include, but are not limited to, acute care facilities, long-term acute care facilities, inpatient rehabilitation facilities, nursing homes and assisted living facilities, home healthcare, vehicles where healthcare is provided (such as mobile clinics), and outpatient facilities like dialysis centers, doctor’s offices, and others [19]. Understanding this broad definition is crucial when assessing occupational risk and designing infection prevention strategies. The inclusion of unpaid staff and volunteers highlights the need for universal precautions and consistent policy enforcement across all healthcare roles, regardless of formal employment status. Moreover, the wide range of healthcare settings emphasizes that HBV exposure is not limited to hospitals but extends to outpatient and community-based environments, requiring tailored control measures for each type of setting.
The parenteral and mucosal exposure to possibly infected blood or blood-contaminated material can be regarded as a risk of HBV transmission to nonimmune/noninfected subjects because HBV is known to circulate in the peripheral blood of infected patients [25].
HBV can survive for at least a week outside the body. During this time, the virus can still infect if it gets into the body. People are susceptible to contaminated sharp injuries with infected blood and bodily fluids, such as saliva and menstrual, vaginal, and seminal fluids, as well as needlestick injuries, piercing, and these injuries can result in HBV infection. Reusing contaminated needles, syringes, or sharp items in healthcare facilities among the general public or among drug injectors is another way the virus can spread (drug addicts).
One thing that has been noted is how significantly the effects of the various transmission routes vary from country to country [26]. In countries where HBV infection is highly endemic, the infection is typically contracted from an infected mother [hepatitis B e antigen (HbeAg)-positive] at birth, in this instance around 90% of instances progress to a chronic illness, or through transmission horizontally during early childhood through household interaction, which develops into a chronic disease in 10–40% of cases [26].
Additionally, in regions with low HBV prevalence, like the United States, unprotected sexual activity and intravenous drug use are the main risk factors for HBV infection [27]. The HBV incubation period lasts between 60 and 180 days, and it can also be found within 30 to 60 days of infection. On the other hand, the potential for HBV transmission from blood and blood product transfusions has been significantly reduced because blood donors are screened for HBV infection indicators. In low-prevalent areas, the risk was estimated to be 1–4 cases per million blood products transfused [28] and roughly 1 case per 20,000 blood transfusions in high-prevalent areas [29]. Professional hazards were to blame for 40% to 60% of HBV infections among HCWs [30]. This underscores the disproportionate occupational risk faced by HCWs, especially in under-resourced settings. Effective vaccination, proper use of personal protective equipment (PPE), and continuous training in infection control are fundamental to reducing these avoidable infections.
HCWs are more frequently exposed than the general population to human blood and other potentially infectious biological materials, which put them at risk of contracting bloodborne virus (HBV) infections through mucosal-cutaneous exposure (the blood of the patient, blood derivatives, or other possibly infectious biological substance accidentally coming into contact with the skin). The entire occupational exposure comprises around 75% percutaneous and 25% mucosal-cutaneous exposure [31]. Infection rarely results from simple contact with the skin, but the risk increases if the skin is damaged by an injury with contaminated needles or sharp instruments or dermatological disease. The injury with a contaminated instrument can occur during the medical procedure or when handling these instruments, so the risk increases if procedures for disposing of contaminated instruments are not adequate.
According to estimates from other studies, the annual frequency of injuries to HCWs from sharp objects ranges from 1.4 to 9.5 per 100 HCWs, with 0.42 HBV infections per 100 sharp object injuries [32]. The level of infectiousness of the contaminated biological material to which the HCW has been exposed mainly affects the transmission of infection [33]. It is generally known that exposure to blood or its products is followed by the highest rates of HBV infection transmission; nevertheless, exposure to ascites, cerebrospinal fluid, or cell culture solutions can also result in a lower rate of transmission [32]. The size and depth of the cutaneous or mucosal wound and the amount of blood that has been transmitted are additional critical parameters that impact the transmission of infectious pathogens [33].
Most infections and conversions to positive HBV serum indicators worldwide are directly caused by the medical instruments used to access blood vessels. Due to the lower amount of organic material on their surfaces, such conversions are less common after using lancets for capillary blood collection or hollow needles intramuscularly or subcutaneously. These clinical procedures are typically carried out by nurses, who also happen to be the profession with the highest risk of needlestick injuries [31–33]. The surgeons, physicians, and dentists are also at high risk among HCWs.
The source patient’s HBV burden has an impact on infection transmission. If the source patient is HBeAg-positive and/or has an HBV load of > 106 international units (IU)/mL, the chance of HBV transmission has been estimated to be 19–30%, whereas it is only 5% if the source patient has negative HBeAg and/or has an HBV load of < 106 IU/mL [32].
Hepatitis B surface antigen (HBsAg) is the first HBV infection marker found in the serum. Clinical symptoms and an increase in serum aminotransferase levels can occur before. HBV has various serum markers that have different explanations when they appear in the serum, as shown in Table 1 [11].
Interpretation of various hepatitis B markers in different clinical situations
| HBsAg | HBeAg | Anti-HBc | Anti-HBe IgM | Anti-HBe IgG | Anti-HBs | Interpretation |
|---|---|---|---|---|---|---|
| + | − | + | − | − | − | HBV infection, but in the incubation period |
| + | − | + | + | + | − | Acute HBV or persistent HBV carrier state |
| + | − | + | − | + | − | Persistent HBV carrier state |
| − | + | + | ± | + | − | Persistent HBV carrier state |
| − | − | − | ± | + | − | HBV convalescent state |
| − | − | − | − | + | + | HBV in recovery state |
| − | − | − | + | − | + | HBV infection without detectable HBsAg |
| − | − | − | − | + | − | HBV recovery with loss of detectable anti-HBs |
| − | − | − | − | − | + | HBV immunization without infection (repeated exposure to antigen without infection) |
HBsAg: hepatitis B surface antigen; HBeAg: hepatitis B e antigen; anti-HBc: hepatitis B core antibody; anti-HBe: hepatitis B e antibody; IgM: immunoglobulin M; IgG: immunoglobulin G; anti-HBs: hepatitis B surface antibody; (+): indicates a positive result, suggesting the presence of the corresponding marker; (−): indicates a negative result, suggesting the absence of the corresponding marker
Note. Adapted with permission from [11], © 2009 Journal of Laboratory Physicians
The first and primary strategy to decrease the risk of occupational infections by bloodborne pathogens is to prevent exposure by educating the HCWs on infection control and by vaccinating them. Therefore, a high level of knowledge, a positive attitude and strong HBV infection prevention practice are necessary for developing a practical HBV infection prevention approach [34].
Implementing HBV infection prevention practices like hepatitis B screening, hepatitis B vaccination, post-hepatitis B vaccination antibody testing, changing gloves for each client, not recapping needles after use, preventing needlestick injuries, and avoiding blood splashes on the body are examples of good practices [35]. HCWs need to be taught how to take appropriate precautions to prevent occupational exposure to blood and blood products, including avoiding unnecessary injections, using all necessary safety measures, HCWs should dispose sharp objects immediately after use. Recapping needles should be avoided, and using safer tools like needles that sheath or retract [36]. These preventive behaviors reflect both individual and institutional responsibilities. Sustained training, proper resource allocation, and regulatory enforcement are key to reducing occupational HBV exposure risks among HCWs.
Self-reported hepatitis B vaccination coverage among adults at risk for HBV infection in the United States rose from 30% in 1981 to 45% between 2000 and 2004 [37]. This increase in vaccination coverage was likely responsible for the 35% drop in acute hepatitis B incidence during that time (from 3.7 to 2.4 per 100,000 people) [37]. As a result, it is well known that the HBV vaccination offers excellent protection and that anyone who works in a position where they may come into contact with blood, bodily fluids tainted with blood, or sharp objects ought to get vaccinated against hepatitis B [38]. A 1- to 1.5-inch-long needle should ideally be used to inject the three standard doses of recombinant HBV vaccination intramuscularly in the deltoid region on a 0-, 1-, and 6-month schedule.
In order to prevent infection and the emergence of the carrier status, all HCWs should get vaccinated before the beginning of their career. They should follow a regular vaccination schedule, and 1–2 months after finishing the three-dose vaccine series, the blood levels of anti-HBs should be checked [39]. HCWs who engage in exposure-prone procedures (EPPs) are more likely to contract an infection. As a result, postvaccination testing is suggested to determine the situation of vaccinated HCWs. Ensuring early vaccination and timely antibody titer testing is not only beneficial to the HCW but also represents a critical public health measure that reduces patient risk in clinical practice.
In most countries, the anti-HBV vaccination of HCWs has been in place since the 1980s. All HCWs, including trainees, who have a high risk of occupational percutaneous or mucosal exposure to blood or bodily fluids should undergo postvaccination testing for antibodies to the anti-HBs. Testing should be done between one and two months following the last dosage of the vaccine. Postvaccination testing is probably not cost-effective for people who are at low risk of mucosal or percutaneous exposure to blood or bodily fluids (such as public safety personnel and HCWs who do not have direct patient contact); however, those who do not receive postvaccination testing should be advised to get tested right away if exposed [40].
Even though the vaccine has excellent immunogenicity, anti-HBs titers were less than 10 IU/mL in 20% of vaccination recipients. However, those who have been vaccinated against HBV and they have an anti-HBs titer of less than 10 IU/mL, or have developed an anti-HBs negative status can be regarded as immune to HBV infection because they may still have immunological memory for HBsAg. It would guarantee that, in the case of an HBV attack, protective antibody levels would quickly increase. This is for people who develop significant anti-HBs titer immediately after vaccination, but with anti-HBs titer that became negative or < 10 IU/mL thereafter. For people who never develop anti-HBs after vaccination the immune status is undetermined and probably less favorable [41]. However, low antibody titers against an HCW’s HBsAg titer may be overcome by a highly infectious HBV inoculum. Giving an additional dose of the HBV vaccine may be an option in those circumstances [42]. This emphasizes the importance of individualized follow-up strategies in occupational health, especially for HCWs with suboptimal vaccine response, to ensure continued protection and reduce nosocomial transmission risks.
Those who are at occupational risk of exposure to blood or body fluids might undergo anti-HBs testing upon recruitment. Figure 1 shows an algorithm for managing HCWs using documentation that revealed a completed HBV vaccination series with no documentation of anti-HBs titers of ≥ 10 mIU/mL [43, 44].
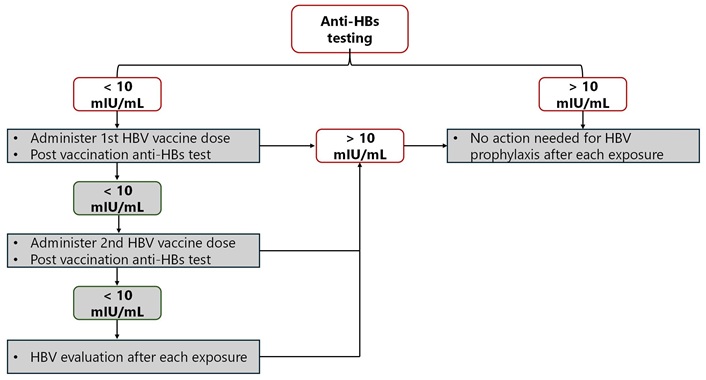
All HCWs should have an HBV vaccination, according to the 2012 CDC guidelines, and then have their levels of hepatitis B surface antibody (anti-HBs) checked. Additionally, revaccination is taken into consideration in cases of vaccine nonresponse. If the HCWs do not achieve protective levels of anti-HBs following a second three-dose series of the HBV vaccination, they should be tested for HBsAg and hepatitis B core antibody (anti-HBc) to find out whether they have ever been infected or have a chronic infection. Pre-vaccination serology is advised for HCWs who are sexually active men, who have sex with men, who conduct EPPs, and/or whose mothers are from endemic countries [45]. The United Kingdom Department of Health (UKDH) further suggests that all HCWs who do EPPs have an HBV vaccination and undergo postvaccination testing for an anti-HBs response. If the HCW does not respond to one series of vaccinations, they need to be checked for HBsAg, and if the result is negative, the HCW should also be tested for anti-HBc to ascertain whether he or she had previously been infected or is an actual vaccine nonresponder [46]. However, the 2003 European Consensus group recommendations urge all HCWs who come into contact with patients, blood, or other body fluids to receive an HBV vaccination, including a one-month post-immunization vaccine response test [47]. Nonresponders should have additional vaccinations, and if they still do not show any improvement, their HBV status should be looked into based on their line of work. These recommendations underline the need for tailored vaccination and testing strategies based on occupational exposure risk and local epidemiology. They also highlight the importance of establishing clear institutional policies for managing nonresponders.
When exposed percutaneously, the area must be cleaned with soap and water. Only water should be used to flush when the mucous membranes are contaminated. Eyes should be cleaned with saline or clean water. Although it has not been demonstrated that using antiseptics (such as 2–4% chlorhexidine) for wound care or further compressing the wound to express fluid reduces the risk of HBV transmission, doing so is not against the rules. It is not advised to apply caustic agents (like bleach) or inject antiseptics or disinfectants into the wound [48].
The treatment of exposed HCWs is based on their anti-HBs status and the source patient’s HBsAg status. As soon as feasible after the exposure, the HCW must be tested for anti-HBs, and the source patient (if known) must be tested for HBsAg [40].
The risk assessment and exposure score will determine, depending on the presence or not of anti-HBs titer > 10 mIU/mL post vaccination, if post-exposure prophylaxis is necessary or not, and whether to employ only active vaccination (HBV vaccine) or both active and passive immunization [hepatitis B immunoglobulin (HBIG)] [11]. Postexposure prophylaxis can be carried out with the HBIG, the HBV vaccination, or both. It must begin as soon as feasible, ideally 24 hours after exposure and no later than one week [48]. Management recommendations based on the testing results are shown in Table 2 [43].
Postexposure management of HCWs after occupational exposure according to the previous hepatitis B vaccination and response status
| HCW status | Postexposure serological test | Postexposure prophylaxis | Postvaccination serological (anti-HBs) test | ||
|---|---|---|---|---|---|
| Source patientHBsAg | HCW testingAnti-HBs | HBIG | HBV vaccine | ||
| Confirmed response after complete HBV vaccine series | No action needed | ||||
| Confirmed nonresponder after two complete HBV vaccine series | Positive or unknown | Not indicated | HBIG × 2 separated by 1 month | Not indicated | No |
| Negative | No action needed | ||||
| Unknown response after complete HBV vaccine series | Positive or unknown | < 10 mIU/mL | HBIG × 1 | Initiate revaccination | Yes |
| Negative | < 10 mIU/mL | No | |||
| Any results | ≥ 10 mIU/mL | No action needed | |||
| Unvaccinated or incompletely vaccinated | Positive or unknown | Not indicated | HBIG × 1 | Complete vaccination | Yes |
| Negative | Not indicated | No | Complete vaccination | Yes | |
HCW: healthcare worker; HBsAg: hepatitis B surface antigen; anti-HBs: hepatitis B surface antibody; HBIG: hepatitis B immunoglobulin; HBV: hepatitis B virus
Note. Adapted with permission from [43], © 2018 Morbidity and Mortality Weekly Report (MMWR)
Most of the management recommendations for HBV-infected HCWs are based on data coming from transmission of the HBV from HCW to patient, and they try ethically to strike a balance between the patient’s danger of becoming infected and the infected HCW’s right to perform their job safely without losing their confidentiality [4]. We should know that transmission of blood from HCWs to patients involves a double injury simultaneously in the patient and HCW and is therefore mostly observed in procedures with limited visibility. The proportion of patients who were infected after surgery with an infected surgeon [infected HCW (IHCW)] is between 0.5% and 13.1% [49].
The Society for Healthcare Epidemiology of America, in 2010, updated its recommendations and suggested that HCWs who have a positive HBeAg result and circulating HBV burden of 104 genome equivalents (GE) per mL (roughly comparable to 8,000 IU/mL) of blood use double-gloving for all invasive procedures, for all contact with mucous membranes or nonintact skin, and for all patient care situations where gloving is advised. In contrast, for HCWs with a circulating HBV burden of < 104 GE/mL of blood, it is recommended that providers perform all Category I, II, and III (minimal, low-risk procedures and high-risk procedures) provided they [50]:
(1) Did not infect patients with the HBV virus.
(2) Consult an expert review panel for guidance.
(3) Be subjected to regular occupational medicine follow-up, including testing for viruses twice a year, to ensure the viral load stays below 104 GE/mL.
(4) Follow-up with a personal doctor with experience in treating HBV infections and who is permitted to consult with the expert review panel regarding the clinical condition of the HCW.
(5) Strictly follow recommended infection control techniques after consulting an expert.
(6) Accept and sign the expert review panel’s contract or letter outlining their obligations.
The CDC advised that HCWs with HBV infections should still be able to practice medicine, dentistry, surgery, and other allied healthcare professions [45]. If they have a low (1,000 IU/mL or 5,000 GE/mL) or undetectable HBV viral load and have been monitored every six months, HBV-infected HCWs who perform EPPs should continue to do so. The performance of EPPs should be limited if the viral load is higher than what is advised until further testing is done [45]. EPP stands for exposure-prone procedures. These are medical procedures that carry a risk of transmission of bloodborne pathogens from the HCW to the patient, especially when the HCW is infected with HBV. The distinction between EPPs and non-EPPs is shown in Table 3 [45].
Examples of exposure-prone and non-exposure-prone procedures
| Exposure-prone procedures | Non-exposure-prone procedures |
|---|---|
| Open abdominal or thoracic surgery | Taking medical history and physical examination |
| Surgery involving hand insertion into closed cavities (e.g., oral or pelvic surgery) | Administering subcutaneous or intravenous injections |
| Vaginal or cesarean delivery | Superficial skin suturing |
| Deep oral or maxillofacial surgical procedures | Routine dental checkups and cleanings |
| Surgical endoscopy with sharp instruments inside non-visible cavities | Blood sampling |
| Procedures using sharp instruments in areas where hands are not visible | Using a stethoscope, taking blood pressure, and inserting a urinary catheter |
| Organ or vascular transplant surgeries | Vaccination or care of minor wounds |
The CDC also advises that organizations have established HBV-infected HCW management policies and procedures in place, including the capability to create an expert review panel to help with this management [45]. According to the UKDH, all HCWs who have a positive HBsAg test should also undergo an HBeAg test. HCWs with an HBV viral load greater than 10³ GE/mL, regardless of their HBeAg status (positive or negative), should not perform EPPs. Annual monitoring is recommended, and practice should not be limited if the HBeAg test is negative and the viral load is < 103 GE/mL without antiviral therapy [51]. HBeAg-negative HCWs should be allowed to perform EPPs while on antiviral therapy as long as their viral load is at or below 103 GE/mL on three-monthly monitoring and before treatment, viral load is at or below 105 GE/mL, according to guidelines shared by the UKDH in 2007. Although the UKDH recommendations do not suggest creating an expert panel to direct the management of infected HCWs with HBV, they do suggest an occupational health physician’s involvement [51]. However, the 2003 European Consensus group recommendations said that HCWs should only be permitted to proceed with EPPs if their HBsAg test results are negative. HCWs who are HBsAg-positive should have their HBeAg levels tested; if the test is negative, they can do EPPs if their HBV DNA level is < 104 GE/mL. Every year, the HBV DNA level should be checked. Unless their viral load is below the authorized cut-off and they are examined by an expert panel that has approved the performance of EPPs [52]. It should be specified that HCWs with a high viral load are authorized to engage in non-EPPs without inappropriate restrictions. Non-EPPs are procedures that do not pose a significant risk of transmitting infectious agents from the HCW to the patient. As long as appropriate infection control measures are followed. In fact, imposing unnecessary limitations on HCWs could lead to workforce shortages and may contribute to unnecessary stigmatization, especially when there is no clear evidence that such restrictions improve patient safety [45].
The 2017 European Association for the Study of the Liver (EASL) guidelines recommend that HBV infection alone should not disqualify infected persons from the practice or study of surgery, dentistry, medicine, or allied health fields [53]. Regular monitoring of HBV DNA levels is advised. If viral load is low (e.g., < 200 IU/mL according to some criteria), performing EPPs may be allowed under specific conditions. HCWs performing EPPs with serum HBV DNA > 200 IU/mL may be treated with NA to reduce transmission risk [53].
According to the 2018 American Association for the Study of Liver Diseases (AASLD) guidelines, performing EPPs is not permitted for HBV-infected HCWs if their serum HBV DNA level exceeds 1,000 IU/mL. However, they may resume such procedures once their viral load is reduced and maintained below this threshold [54]. These decisions should be made in consultation with an expert review panel, and institutional policies should be based on up-to-date evidence and individualized assessment rather than on blanket restrictions [54]. Reflecting on the different guidelines and recommendations provided, we find the ethical considerations to be the most complex aspect of managing HBV-infected HCWs. From my perspective, the balance between patient safety and the rights of HCWs is delicate. On one hand, HCWs must be protected from potential discrimination or unnecessary restrictions if they follow recommended guidelines and maintain low or undetectable viral loads. On the other hand, healthcare institutions must prioritize the safety of their patients, especially in high-risk procedures that could expose them to infection.
In my opinion, it’s crucial that the policies are not just based on broad standards but that they take into account the individual circumstances of each HCW, such as their viral load, adherence to treatment, and whether they have been adequately monitored. This individualized approach allows for more humane treatment of the HCWs, avoiding unnecessary stigma while ensuring that patient safety remains intact. Moreover, we think one of the key aspects that needs more emphasis is the role of the expert review panels. These panels provide an essential check-and-balance system, ensuring that the decision-making process is evidence-based and appropriately tailored to the situation at hand. They allow for more flexibility and precision in decision-making, which is especially important considering the variation in HBV load and the potential for new, evolving treatment options.
Finally, it was suggested in the 2012 Australian guidelines that HCWs be prohibited from doing EPPs if HBV DNA could be detected by an authorized polymerase chain reaction assay. The HCW may perform EPPs even if they are HBsAg-positive and receive antiviral medication if HBV DNA is undetectable by testing every three months. The HCW may administer EPPs if the HBsAg result turns negative twice in a row, but they should test the HBV DNA level regularly every year [47].
HBV is one of the numerous bloodborne pathogens known to be transmissible in healthcare settings, with a well-documented risk of hepatitis B infection among HCWs. HBV infection in HCWs has decreased with the hepatitis B vaccine, but there is still substantial scope for acquiring HBV infection, as several HCWs remain unvaccinated.
Exposure prevention (all measures should be taken to prevent HCWs from infection) is the primary strategy to reduce the risk of occupational exposure to bloodborne pathogen infections in HCWs. Therefore, a clear, well-planned policy for vaccination, screening, diagnosing, and managing HCWs, especially those at a greater risk of exposure to blood or other potentially infectious products, should be implemented.
Moreover, HCWs should be aware of reporting any exposure and have access to expert consultants to receive the appropriate counseling, treatment, and follow-up. A well-trained infection control expert team should conduct follow-ups to ensure and maintain safety measures within the healthcare setting.
anti-HBc: hepatitis B core antibody
anti-HBs: hepatitis B surface antibody
EPPs: exposure-prone procedures
GE: genome equivalents
HBeAg: hepatitis B e antigen
HBIG: hepatitis B immunoglobulin
HBsAg: hepatitis B surface antigen
HBV: hepatitis B virus
HCV: hepatitis C virus
HCWs: healthcare workers
IU: international units
UKDH: United Kingdom Department of Health
HR and AT: Data curation, Writing—original draft, Writing—review & editing. ME: Supervision, Data curation, Writing—original draft. AGR: Data curation, Writing—original draft. MEK: Conceptualization, Data curation, Writing—original draft. All authors read and approved the submitted version.
The authors declare that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
All datasets generated or analyzed in the reviewed literature are included in the cited studies and referenced accordingly.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2760
Download: 21
Times Cited: 0
Phey Liana ... Tungki Pratama Umar
Kamran Zaman ... Ranjit Sah
Wael Abu Ruqa ... Antonio Minni
Anna Antipov, Nikolai Petrovsky
Oliver Meek ... Miles W. Carroll
