Affiliation:
1Department of Microbiology and Molecular Biology, ICMR-National Institute of Traditional Medicine, Belagavi 590010, Karnataka, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-0172-7423
Affiliation:
2Department of Pharmacy, Faculty of Pharmacy, Mansoura University, Mansoura 35516, Egypt
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-3826-9702
Affiliation:
3Department of Microbiology, All India Institute of Medical Sciences, Gorakhpur 273008, India
ORCID: https://orcid.org/0000-0001-5634-5014
Affiliation:
4Facultad de Medicina Humana, Universidad de San Martín de Porres, Chiclayo 15011, Peru
ORCID: https://orcid.org/0000-0001-7267-0204
Affiliation:
5Vicerrectorado de Investigación, Universidad Norbert Wiener, Lima 13001, Peru
ORCID: https://orcid.org/0000-0002-2896-1407
Affiliation:
6Department of Medicine, Institute of Global Health, Heidelberg University, 69115 Heidelberg, Germany
ORCID: https://orcid.org/0000-0001-6693-4525
Affiliation:
7Institute of Medicine, Tribhuvan University Teaching Hospital, Kathmandu 44600, Nepal
8Department of Microbiology, Dr. D.Y. Patil Medical College, Dr. D.Y. Patil Vidyapeeth, Pune 411018, Maharashtra, India
9Department of Public Health Dentistry, Dr. D.Y. Patil Dental College and Hospital, Dr. D.Y. Patil Vidyapeeth, Pune 411018, Maharashtra, India
Email: ranjitsah57@gmail.com; ranjitsah@iom.edu.np
ORCID: https://orcid.org/0000-0002-2695-8714
Explor Med. 2023;4:1026–1032 DOI: https://doi.org/10.37349/emed.2023.00192
Received: April 03, 2023 Accepted: June 13, 2023 Published: December 28, 2023
Academic Editor: Marcos Roberto Tovani-Palone, Saveetha Institute of Medical and Technical Sciences (SIMATS), India
The article belongs to the special issue Emerging Infectious Diseases
Within group A Streptococcus (GAS), only Streptococcus pyogenes exhibits clinical significance. GAS is typed serologically based on unique surface proteins and critical virulence factors, such as a hyaluronic acid capsule that shields GAS from phagocytosis. The burden of GAS was estimated in the last five years as 14,000 to 25,000 cases of the invasive group A streptococcal disease in the USA with an estimated death from 1,500 to 2,300 cases per year. Early in the summer of 2022 in England, there was more scarlet fever than was anticipated. Early in the current season, the number of notifications rose to unusual heights. The analysis of invasive GAS (iGAS) isolate typing data shows that this season has seen a wide variety of encoding mature M protein (emm) gene sequence types found. Therefore, public health authorities should think about initiatives to increase clinicians’ and the general public’s awareness of GAS infections and to promote their quick diagnosis, molecular testing and antibiotic susceptibility testing, and standard treatment.
Group A Streptococcus (GAS) are gram-positive cocci that produce an obvious zone of hemolysis (β) on blood agar, distinguishing them from other streptococci that produce partial hemolysis (α) and non-hemolytic (γ) streptococci [1]. Within GAS, only Streptococcus pyogenes exhibits clinical significance. GAS is typed serologically based on unique surface proteins and critical virulence factors, such as a hyaluronic acid capsule that shields GAS from phagocytosis [2].
GAS can colonize the anogenital tract, skin, and throat. GAS can be transmitted through direct skin contact, respiratory droplets, and close personal touch. Additionally, it can spread through touch with infected things like towels or bedding as well as eating the food that has been infected by a carrier. While some people who carry the bacterium in their bodies may not feel well or exhibit any signs of infection but are still capable of transmitting it to others, the risk of dissemination is significantly higher when a person is ill [1, 2].
Tonsillitis, pharyngitis, scarlet fever, impetigo, erysipelas, cellulitis, and pneumonia are the non-invasive diseases it can cause. Rheumatic fever and glomerulonephritis are two unusual post-streptococcal sequelae that individuals may experience [3, 4]. The invasion ability of the bacterium comes from multiple virulence factors such as the attachment to the epithelium with the aid of the surface proteins like M and M like proteins which play a role in the escape from the immune system by interfering with the complement system with different mechanisms and enzymes such as streptokinase, hyaluronidase, and DNases which are secreted from this type of bacterium. An infection known as invasive GAS (iGAS) occurs when bacteria are isolated from a typically sterile bodily location, like the blood [5, 6]. The diagnosis of the iGAS is mainly based on the isolation of the bacterium from a sterile site, and by the results, the clinicians can confirm that the causative agent of the presenting symptoms is the GAS and one of the severe manifestations of the GAS is the streptococcal toxic shock syndrome (STSS), which requires diagnostic criteria such as the criterion that was developed by Breiman et al. (Figure 1) [7].
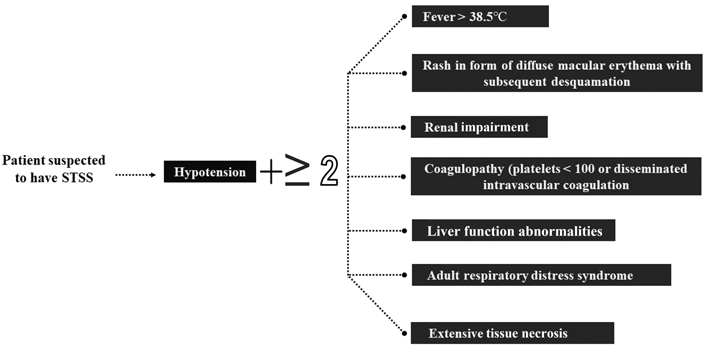
The diagnostic criterion that can be used for the diagnosis of STSS. There must be hypotension with two or more of the other findings
The burden of GAS was estimated in the last five years as 14,000 to 25,000 cases of the invasive group A streptococcal disease in the USA with an estimated death from 1,500 to 2,300 cases per year due to iGAS, and as per the World Health Organization (WHO), the disease is considered to be more apparent in the winter and early spring with an estimated death of 50,000 cases annually in the world [8–10]. According to reports, the greatest burden of GAS disease is placed on children between the ages of 0 and 4 [8].
The incidence of iGAS is recorded by the active bacterial core surveillance system (ABCs) tracked and generated by the Centers for Disease Control and Prevention (CDC), which shows a change in the ratio between survived and death cases between 1997 and 2020 [11]. As per the WHO report in mid-December, it was reported that there is an increase in the incidence of iGAS and scarlet fever, especially among children who are younger than 10 years old in France, Ireland, Netherlands, Sweden, and the United Kingdom (UK) [8–10]. The different syndromes that are caused by iGAS are shown in Figure 2, in the form of the percentage of cases that are infected with each type which shows that cellulitis is at the top percentage among the other diseases.
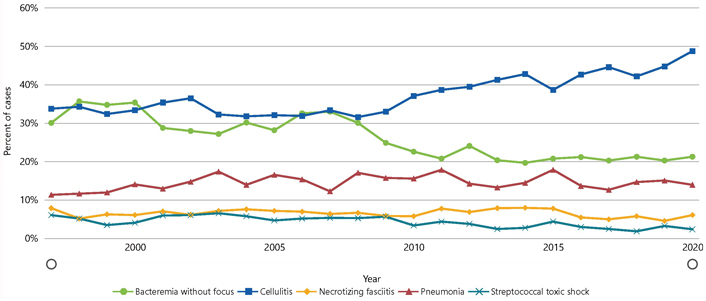
Percentage of cases that occurred with each of the five disease types caused by the iGAS as per ABCs bact facts interactive data dashboard [11]
Note. Reprinted from “ABCs Bact Facts Interactive Data Dashboard” by CDC (https://www.cdc.gov/abcs/bact-facts-interactive-dashboard.html). CC0. The use of the material on the CDC websites does not imply endorsement by CDC or the United States Government.
The management of iGAS patients mainly focuses on the supportive management of the case by preventing dehydration, especially in the case of toxic shock syndrome when the patient is experiencing life-threatening hypotension with sufficient monitoring for the kidney markers as they may develop renal failure [7]. The antibacterial agents are considered the main core in the management of the cases as the first line drug of penicillin in the initial period. Therefore the patient should take a broad-spectrum antibiotic that covers the Staphylococcus aureus till the culture results become available. If there is a suspected infection with methicillin-resistant Staphylococcus aureus (MRSA), the patient should take vancomycin to cover it so the type of administered antibacterial differs according to the presenting case [12].
Clindamycin is considered to be an adjunctive antibiotic to penicillin, especially in cases of toxic shock syndrome and necrotizing fasciitis [12]. The management of the disease also includes the early removal of the infected tissues, especially in severe cases such as necrotizing fasciitis as this is correlated with a lower mortality rate, besides the other management tools such as the intravenous immunoglobulin in which the clinician must weigh between the benefits and the risks, and it is advisable to avoid the use of non-steroidal anti-inflammatory drugs (NSAIDs) in case of any presenting fever without a known cause as it may mask the initial manifestations which will be associated with a delayed identification of the case [3].
In the UK, Scarlet fever and iGAS infection are both notifiable diseases. Medical professionals are required to alert the local health system if any suspected cases are documented and follow the defined policies for managing scarlet fever outbreaks in educational settings as well as iGAS cases in the community and clinical settings [13].
Global GAS outbreaks cause major illness, necessitating continuous monitoring and continuing attempts to define pathogenic GAS populations’ evolutionary trajectories. Based on the gene sequence of the amino terminus of the surface-exposed M protein, GAS may be epidemiologically divided into more than 220 encoding mature M protein gene (emm) type 2. Multiclonal outbreaks of scarlet fever have been found in Asia and the UK, with the UK epidemic coinciding with a rise in invasive infections. The development of novel GAS clones, typically with new virulence features or antimicrobial characteristics that better fit the infection niche or avoid human immunity, characterizes worldwide GAS epidemiology. Clinical GAS isolates with first-step PBP2x mutations and rising macrolide resistance endanger the frontline and role of penicillin-adjunctive antibiotic treatment. WHO has come up with a GAS research and technology roadmap and has emphasized the vaccine qualities taking into account safe and effective GAS vaccinations [14, 15].
In the UK, seasons are regarded as beginning in week 37 (mid-September) of one year and ending in week 36 of the following for notification. An upsurge in the cases was noticed and medical professionals were alerted to an early increase in the incidence of scarlet fever and iGAS infection among toddlers on December 2nd, 2022 [16, 17].
Early in the summer of 2022 in England, there was more scarlet fever than anticipated. Early in the current season, the number of notifications rose to unusual heights. iGAS notification levels are still higher than what is usual for this season of the year. From week 37 of 2022 to week 12 of 2023 in England, a total of 52,183 notifications of scarlet fever have been reported, with a pre-Christmas peak of 10,068 notifications in week 49. These figures were far more significant than the last peak season for scarlet fever alerts between 2017 and 2018, during which 30,768 reports were received overall. The rise in reports is likely the result of people becoming more health-conscious as a consequence of national alerts. Since week 8 of 2023, notifications remain within the usual range for this time of year and are still lower than those documented in December 2022. In the first 12 weeks of 2023, there have been 15,767 reports of scarlet fever, more than typical at this stage of the year (5,612, ranging from 340 to 13,262 between 2018 and 2022). The number of scarlet fever reports recorded thus far this season in England varied widely, from 73.2 (West Midlands) to 132.7 (East Midlands), per 100,000 individuals [16].
This season’s laboratory notifications of iGAS infection (week 37 of 2022 to week 12 of 2023) have been significantly greater than anticipated. iGAS disease notifications numbered 2,651 in England, exceeding the average of the previous five seasons for the same weeks (1,030 notifications on average) with a weekly maximum of 212 notifications in week 52 (26 December 2022 to 1 January 2023). In the first 12 weeks of 2023, there have been more iGAS laboratory reports (1,244) than which was typical at this time of the year (an average of 535 cases between 2018 and 2022). Yorkshire and Humber region documented the highest rates during the current season so far (6.3 per 100,000 residents), followed by the North East (5.9 per 100,000) and the South East region (5.1 per 100,000) [16].
The median age of iGAS-infected patients this season is 52 years old (aged 1 to 102), which is a little lower than the range seen at this time in the last five seasons (aged 52 to 58). The rates are most significant among people aged 75 and older (12.4 per 100,000), then children aged 1 to 4 (12.1 per 100,000), and infants under 1 year old (10.2 per 100,000). Children (aged 15 and under) account for 21% of iGAS infections recorded so far this season, which is higher than the 4% to 15% range seen over the past 5 seasons. In the first seven days following the diagnosis of an iGAS infection this season, 355 deaths (from any cause) have been reported (176 in 2023), with 65% (n = 230) of the deaths recorded being in people aged 65 and older and 9% (n = 32) in children under the age of ten. Forty deaths have been reported in people aged under 18 years old [case fatality rate (CFR) of 6.7%], of the 635 iGAS reported cases [16].
Tetracycline resistance has been detected in 12% of GAS sterile site isolates so far this season, which is lower than at the same stage last season (39%), according to antimicrobial susceptibility findings acquired from routine laboratory surveillance. A total of 4% of iGAS isolates tested resistant to erythromycin (down from 15% last season) and 4% of iGAS isolates tested resistant to clindamycin at this stage in the season (down from 13% last season). Penicillin continued to be completely effective against isolates [16].
The analysis of iGAS isolate typing data shows that this season has seen a wide variety of emm gene sequence types found. In comparison to 23%, 7%, and 10% at the same point in the 2017 to 2018 season, respectively, the findings show that emm 1.0 continues to be the most prevalent (54% of referrals), followed by emm 12.0 (11%) and emm 89.0 (4%). This season, emm 1.0 and emm 12.0 have dominated among children (under the age of 15), accounting for 65% and 16% of the total, respectively (compared to 28% and 8% at this time in the 2017–2018 season) [16, 17].
The upsurge of cases shall indeed create an increased demand for antibiotics in the health systems. Similarly, increased demand for amoxicillin and penicillin, the main antibiotic treatments, over the past week has been noticed in the UK [16]. Drugstores claim that products are “flying off the shelves” and that some are already distributing medicine at a loss with escalating wholesale prices, which have risen. The UK National Health Service or pharmacies have claimed to meet up the requirements to control fatalities which are on the upsurge. The UK Government has dismissed the fears of the national shortage of antibiotics this season [18, 19].
Given the recent increase in the spread of respiratory viruses across Europe, all of the member states of the European Union should be on the lookout for a similar increase in cases among children. The likelihood of developing a severe iGAS infection can be decreased by reducing the spread of GAS. Early diagnosis of iGAS illness and timely beginning of targeted, supportive therapy for patients may save lives. Children with severe respiratory syndromes, those who have had a previous viral illness (including chickenpox), and those who have had close contact with those who have scarlet fever should all have GAS infections considered in the differential diagnosis. Close contacts of iGAS cases should be located, evaluated, and treated as per national standards [20, 21].
Therefore, public health authorities should think about initiatives to increase clinicians’ and the general public’s awareness of GAS infections and to promote their quick diagnosis, molecular testing, antibiotic susceptibility testing, and standard treatment as per national recommendations. Awareness of immunization against COVID-19 and seasonal influenza (since viral illness prevention is anticipated to lower the chance of developing iGAS disease) to be spread through public health messages to guardians of young children and their parents [22, 23].
ABCs: active bacterial core surveillance system
emm: encoding mature M protein gene
GAS: Group A Streptococcus
iGAS: invasive Group A Streptococcus
WHO: World Health Organization
KZ, AS, and AM: Conceptualization, Data curation, Formal analysis, Methodology, Writing—original draft, Writing—review & editing. DALF: Data curation, Formal analysis, Writing—review & editing. JJB: Data curation, Formal analysis, Writing—review & editing. TAA: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Writing—review & editing. RS: Conceptualization, Funding acquisition, Methodology, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Reference cited.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4568
Download: 40
Times Cited: 0
Phey Liana ... Tungki Pratama Umar
Wael Abu Ruqa ... Antonio Minni
Anna Antipov, Nikolai Petrovsky
Oliver Meek ... Miles W. Carroll
Hend Radwan ... Mohamed El-Kassas
