Affiliation:
1Department of Internal Medicine, Division of Gastroenterology, Eastern Virginia Medical School, Norfolk, VA 23501, USA
Email: dajevms@aol.com
ORCID: https://orcid.org/0000-0002-8737-0711
Affiliation:
2Medical & Clinical Affairs, Johnson & Johnson Consumer Inc., Fort Washington, PA 19034, USA
Affiliation:
2Medical & Clinical Affairs, Johnson & Johnson Consumer Inc., Fort Washington, PA 19034, USA
ORCID: https://orcid.org/0009-0006-3831-7910
Affiliation:
6Department of Neurology, University of Washington School of Medicine, Seattle, WA 98104, USA
ORCID: https://orcid.org/0000-0003-4870-080X
Explor Med. 2023;4:1014–1025 DOI: https://doi.org/10.37349/emed.2023.00191
Received: July 01, 2023 Accepted: August 26, 2023 Published: December 25, 2023
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
Nocturnal heartburn (NHB) is a symptom that affects up to 25% of the general population and has been shown to cause sleep disruption that adversely affects quality of life and psychomotor performance. Few studies have evaluated the association between occasional NHB and sleep disturbances; therefore, this connection may be underappreciated and left untreated by the primary care provider and patient, with potentially significant negative clinical consequences and effects on quality of life. This review sought to describe what is currently known about the interplay between occasional NHB and sleep disruption, and identify whether acid suppression therapy can improve symptoms of occasional NHB and associated sleep disruptions. The pathophysiology of heartburn-induced sleep disruption appears to follow a bidirectional cycle due to the normal physiologic changes that occur in the upper gastrointestinal tract during sleep and due to the potential for heartburn symptoms to cause sleep arousal. The majority of the identified studies suggested that pharmacologic interventions for acid reduction, including proton pump inhibitors or histamine type-2 receptor antagonists (H2RAs), improved objective and/or subjective sleep outcomes among individuals with gastroesophageal reflux disease (GERD) and NHB. Several studies specific to famotidine demonstrated that treatment with 10 mg or 20 mg reduced nighttime awakenings due to NHB. In conclusion, NHB symptoms can cause sleep dysfunction that can have a profound adverse downstream effect on quality of life, next-day functioning, and health-related outcomes. The current approach to managing occasional NHB is similar to that associated with GERD, highlighting the need for studies specific to the occasional heartburn population. Health care providers should investigate NHB as one of the potential causes of sleep complaints, and patients with heartburn should be questioned about sleep quality, recalled arousals, next-day vitality, early fatigue, and next-day functioning.
Heartburn is a symptom caused by the reflux of gastrointestinal (GI) contents into the esophagus. The chronic occurrence of heartburn can be indicative of gastroesophageal reflux disease (GERD), which is a condition characterized by troublesome symptoms, such as frequent heartburn or regurgitation, and, in more serious cases, complications such as esophageal stricture or ulceration [1]. However, occasional heartburn that occurs once per week or less that is not associated with GERD can also occur. In many cases, occasional heartburn may be provoked by a trigger, such as certain foods or beverages.
Heartburn that occurs during nighttime sleep, called nocturnal heartburn (NHB), is a symptom that affects up to 25% of the general population and has been reported in over 70% of patients with frequent or persistent gastroesophageal reflux (GER) [2, 3]. Importantly, NHB has been shown to cause sleep disruption (also known as disturbed nocturnal sleep) that adversely affects quality of life and psychomotor performance, such as work productivity and driving [3–5]. The effect of NHB is of particular growing interest within the field of sleep medicine because of the negative clinical implications of chronic sleep disruption, including associated cognitive [6, 7] and non-cognitive effects [8–10].
Multiple studies have demonstrated that NHB associated with GERD negatively affects sleep. Although GERD and associated NHB is well-studied in the clinical literature, there is limited literature on occasional heartburn. In addition, the current literature is complicated by the lack of a standard threshold for heartburn symptom severity or frequency that constitutes GERD, which varies between studies from 1 to 2 or more episodes of heartburn symptoms per week [1, 11–13]. Given this, the connection between occasional heartburn and nighttime symptoms may be underappreciated and left untreated by the primary care provider and patient, with potentially significant negative clinical consequences and effects on quality of life. This review sought to describe what is currently known about the interplay between occasional NHB and sleep disruption, and identify whether acid suppression therapy can improve symptoms of occasional NHB and associated sleep disruptions.
The standard treatment of classic GERD is once daily proton pump inhibitor (PPI) therapy, taken 30–60 min before breakfast [14]. This has been shown to also improve NHB symptoms [15–19]. However, occasional NHB is underrecognized and undertreated by clinicians and patients; therefore, these patients are at risk of experiencing sleep disruption and the associated negative health effects. This study sought to understand whether acid suppression therapy can improve nighttime symptoms and sleep quality among patients suffering from occasional NHB. To this end, the answers to three main questions were sought:
What impact does occasional heartburn have on sleep; and what impact does sleep have on heartburn?
What are the implications of occasional NHB on sleep and next-day functioning?
Do acid suppression therapies improve sleep parameters among patients with occasional or frequent NHB?
In an attempt to answer the three main questions outlined in the Introduction, a literature search was conducted and internal studies of acid suppression therapy were reviewed to determine whether acid suppression can effectively improve symptoms of NHB and thereby improve sleep.
To further understand the potential clinical implications of NHB experienced by occasional (≤ 1 time per week) or frequent (≥ 2 times per week) heartburn sufferers, a non-systematic review of the published literature was conducted for studies that evaluated clinical outcomes of NHB and the effect of interventions targeting acid reduction. ProQuest and PubMed were searched on June 16, 2022. In ProQuest, the following search terms yielded 2,554 returns: the terms heartburn, pyrosis, or gastroesophageal reflux disease each combined with the term sleep in humans, including only publications in the English language and published beginning in 1980. In PubMed, the search terms included heartburn or gastroesophageal reflux disease, and the filters of clinical trial, meta-analysis, randomized controlled trial, humans, and English, from 1980–2022 were applied, resulting in 129 returns. After reviewing the 2,683 total publications returned, 83 articles published within the last 15 years were found to be relevant (Table S1). There were no studies of NHB among occasional heartburn sufferers reported.
To potentially add to the published data about the prevention of NHB with acid suppression therapy, an internal review was conducted to determine if any histamine type-2 receptor antagonist (H2RA) studies reported the prevention of NHB and corresponding sleep outcomes. There were 3 unpublished studies and 1 published study identified that evaluated famotidine for NHB prevention (Table S2–S4).
The symptoms of NHB during sleep and resulting sleep disturbances are common manifestations of GERD. Indeed, approximately 80% to 90% of GERD patients have experienced at least one conscious awakening during sleep [10, 20, 21]. The available data suggest that occasional heartburn sufferers without GERD also experience sleep disturbances as a result of their symptoms. In a prospective cohort study, 25% of 15,314 adults reported that they had experienced heartburn during sleep [2]. In a population-based study that conducted a telephone survey of 1,000 adults who experienced at least 1 heartburn episode per week, 43% of individuals reported 1 to 2 episodes of heartburn per week, 45% reported more than 2 episodes per week, and 13% could not quantify the number of episodes they experienced [21]. Overall, 13% of individuals reported heartburn only during nighttime, and 65% experienced heartburn both day and night. NHB was reported by a total of 79% of heartburn sufferers [21]. When considering individuals who most closely resembled occasional heartburn sufferers, 44% of individuals who experienced less than one episode of heartburn per week reported that they were kept awake and 41% reported that they woke up during the night due to heartburn symptoms. For individuals who experienced heartburn 1–2 times per week, 53% and 54% reported that they were kept awake or woke up during the night, respectively, due to heartburn. Furthermore, NHB may induce arousals that are amnestic, but cause a fragmented sleep pattern, increased daytime sleepiness, and decreased daytime functioning [10, 20, 22].
Sleep deficiency may also be an underlying mechanism that can promote abnormal esophageal acid exposure [23]. The pathophysiology of heartburn-induced sleep disruption appears to follow a bidirectional cycle due to the normal physiologic changes that occur in the upper GI tract during sleep and due to the potential for heartburn symptoms to cause sleep arousal (Table 1, Figure 1) [10, 24, 25]. Although this cycle has been proposed for patients who suffer from GERD, by extension, it likely also applies to patients who have occasional NHB. In this cycle, the supine sleep position results in decreased swallowing frequency, which impairs acid clearance [2, 26–28]. Other sleep-related physiologic changes increase esophageal hypersensitivity, which reduces the threshold for symptom perception [23, 26, 29, 30]. A recent case-control study suggests that the transient receptor potential vanilloid type 1 receptor (TRPV1), which is expressed by the esophageal mucosa, may be involved in this perception [22]. Increased esophageal acid exposure also occurs due to the effect of sleep physiology on satiety hormones and increased inflammatory cytokines [23, 31].
Normal physiologic changes in the upper GI tract during sleep [10]
| Location | Physiologic changes |
|---|---|
| Oropharynx | Decreased:
|
| Esophagus | Decreased:
|
| Stomach | Increased:
Decreased:
|
Note. Adapted with permission from “Sleep and gastrointestinal health,” by D’Souza SM, Fass R, Shibli F, Johnson DA. In: Kryger MH, Roth T, Goldstein CA, editors. Principles and practice of sleep medicine. 7th ed. Amsterdam: Elsevier; 2022. pp. 1557–64 (https://www.us.elsevierhealth.com/principles-and-practice-of-sleep-medicine-2-volume-set-9780323661898.html). © 2022 Elsevier.
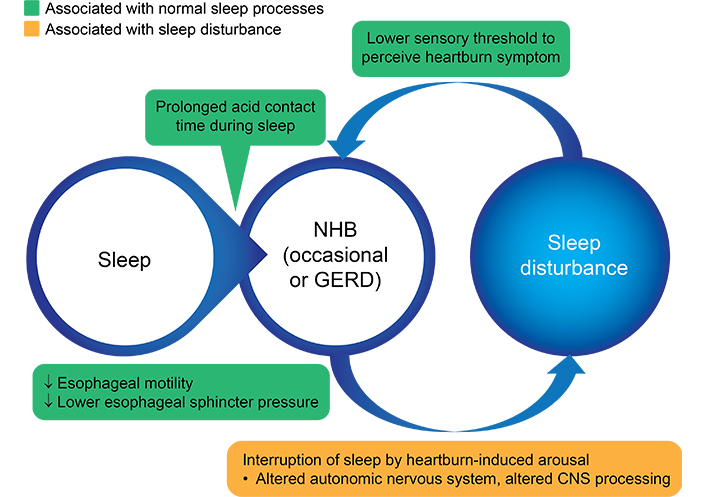
Bidirectional cycle of NHB and sleep disturbance. CNS: central nervous system. Black arrows: decreased
Note. Adapted from “Gastroesophageal reflux disease and sleep disorders: evidence for a causal link and therapeutic implications,” by Jung HK, Choung RS, Talley NJ. J Neurogastroenterol Motil. 2010;16:22–9 (https://www.jnmjournal.org/journal/view.html?doi=10.5056/jnm.2010.16.1.22). CC BY-NC.
Supportive evidence for this bidirectional cycle continues to increase, as data suggest that sleep disturbances can cause occasional heartburn symptoms in otherwise healthy individuals [23]. In one study, nearly 50% of healthy controls developed an abnormal esophageal acid exposure time after sleep deprivation, whereas all control subjects had normal esophageal acid exposure time after normal sleep [23].
Sleep disruption caused by GERD symptoms has also been shown to negatively affect next-day functioning and work loss [19, 32–34]. However, the data of sleep disruption caused by occasional NHB are sparse. Because occasional NHB can cause sleep disturbance, the current best available data are that which are extrapolated from studies of NHB symptoms associated with GERD.
One study evaluated 1,002 patients with GERD, including 476 with frequent nocturnal symptoms and 513 healthy controls [35]. GERD was determined using a prespecified GERD symptom and medication questionnaire scored with > 9 and at least 1 episode of heartburn or regurgitation during the prior week. Frequent NHB was defined as 2 or more nocturnal symptom nights during the prior week. In this study, 34% of patients with frequent NHB were considered to have mild GERD severity, which may be most analogous to patients with occasional heartburn. Sleep parameters were measured subjectively using the Epworth Sleepiness Scale and a dichotomous and Likert scale for questions about the frequency and severity of daytime and nighttime symptoms and the frequency of sleep impairment. The occurrence of frequent nocturnal symptoms was significantly associated with more frequent sleep abnormalities (P < 0.0001), with 64% or more reporting that their symptoms prevented them from getting a good night’s sleep, feeling tired the next day from lack of sleep, waking up during the night, waking up during the night and having trouble falling back to sleep, and trouble getting to sleep. Furthermore, patients with NHB reported significantly more overall work productivity loss (P < 0.0001) and impairment while working (P < 0.0001). Although patients with more severe symptoms reported greater work loss, a large proportion of patients with mild symptoms reported work loss as well.
Another study evaluated the driving performance of 15 patients with 3 or more episodes per week of NHB or regurgitation with or without acid suppression therapy, 15 matched controls without GERD symptoms, 15 healthy elderly patients, and 15 patients with sleep apnea [5]. GERD was defined as patients with 3 or more episodes per week of typical heartburn. Sleep parameters were assessed by the modified Pittsburgh Sleep Quality Index (PSQI), Epworth Sleepiness Scale, and a driving simulator assessment. Frequent NHB was defined as a cumulative 2 nights of heartburn during the last 7 days. Patients with nocturnal GERD symptoms demonstrated significantly greater abnormal lane variability using a validated commercial driving simulator compared with the healthy controls and the healthy elderly cohorts (P = 0.004). Acid suppression therapy improved these measures to levels similar to the healthy and elderly cohorts. In addition, acid suppression therapy significantly decreased the average percentage of nights of GERD-induced sleep dysfunction from 63% to 10% (P < 0.001) and improved the Epworth Sleepiness Scale from 7.8 to 6.0 (P = 0.036).
Some patients may not perceive the symptoms of NHB, yet have reflux with acid exposure resulting in sleep disturbance. In one study, 51% to 60% of primary care patients with GERD-like symptoms or a GERD diagnosis who had reflux without recognizable heartburn reported a subjective sleep disturbance of any type [36]. The most common type of sleep disturbance reported for all groups was difficulty getting a good night’s sleep, feeling tired or worn out owing to lack of sleep, and having trouble getting to sleep.
Both daytime and NHB symptoms respond well to acid suppression, with related improvements in sleep [17–19, 33, 34, 37–39]. Lifestyle and behavioral modifications may help prevent NHB and thereby improve sleep, but can be less reliable than pharmacologic methods [11, 40, 41]. These behavioral interventions include weight loss, abstinence from eating at least 3–4 h before sleep, avoidance of triggering foods, and positional modification including inclined or left lateral recumbent sleep positions. Currently, approved pharmacologic treatments include antacids, H2RA, and PPIs.
Relief of nocturnal symptoms of GERD has been an important clinical endpoint in many clinical trials of anti-reflux medications. PPIs alone have consistently been shown to improve nocturnal GERD, subjective sleep quality, and overall quality of life [15–19]. The American Gastroenterological Association (AGA) 2022 clinical practice update on the personalized approach to the evaluation and management of GERD recommends nighttime H2RAs as a potential adjunctive agent to PPIs among patients with breakthrough nocturnal symptoms [37].
The majority of the identified studies suggested that pharmacologic interventions for acid reduction, including PPIs or H2RAs, improved objective and/or subjective sleep outcomes among individuals with GERD and NHB (Table S1). Notably, in one study of the H2RA famotidine, the treatment improved subjective acid reduction or reflux and subjective sleep outcomes [38]; another study of the PPI esomeprazole given once a day prior to breakfast also demonstrated improvement in GERD-related sleep disturbances that resulted in improved work productivity compared with placebo [33]. A study of the combination of an H2RA with a PPI likewise demonstrated an improvement in subjective acid reduction/reflux and subjective sleep outcomes [37].
A new approach to acid suppression is by potassium-competitive acid blockers, which decrease acid section by parietal cells through the reversible binding of H+/K+-adenosine triphosphatase [42]. In vivo animal data and in-human pharmacology studies suggest that this approach may result in more rapid acid suppression with a longer duration than PPIs. In a study of the agent tegoprazan [42], 46 patients with erosive esophagitis, NHB, and sleep disturbances were randomly assigned to receive 50 mg of tegoprazan or 40 mg of esomeprazole for 2 weeks. Tegoprazan resulted in a similar time to first NHB-free interval and percentage of NHB-free days as esomeprazole, suggesting that tegoprazan may be another option to treat NHB.
Additionally, some studies suggested that non-pharmacologic interventions of acid or reflux management including laparoscopic fundoplication, reduction of simple sugars in the diet, bed head elevation, and use of positional therapy devices improved both objective and subjective acid reduction outcomes or subjective measures of sleep [11, 40, 41].
In addition to reviewing published studies, internal studies were examined that may be unpublished to better understand the effect of H2RAs on NHB. There were 3 unpublished studies and 1 published study identified that evaluated famotidine for NHB prevention (Table S2–S4). Two of the studies were double-blind and compared 10 mg and 20 mg of famotidine to placebo in patients with a history of food-induced heartburn for at least 2 months. In study 1 (Table S2), 794 adults with at least 3 episodes of moderate to severe heartburn per week were randomly assigned to receive 10 mg or 20 mg of famotidine or placebo prior to a provocative evening meal. Both doses of famotidine statistically significantly reduced nighttime awakenings due to heartburn. There were 43.1% of patients in the placebo group who reported no awakenings compared with 57% in the 10 mg famotidine group (P = 0.001) and 60% in the 20 mg group (P < 0.001). In study 2 (Table S3), 1,229 patients with at least 3 episodes a week of frequently severe (≥ 30%) heartburn were randomly assigned to receive 10 mg or 20 mg of famotidine or placebo, given before a provocative evening meal. Consistent with the findings in study 1, famotidine statistically significantly reduced nighttime awakenings due to heartburn. In the placebo group, 53% of patients reported no awakenings compared with 69% (P < 0.001) and 70% (P < 0.001) in the 10 and 20 mg famotidine groups, respectively. Similar results were reported in a third double-blind study (study 3) of 304 patients with frequent heartburn (≥ 3 times per week for ≥ 2 months), in which 10 mg of famotidine was given 1 h prior to a provocative meal at home significantly improved heartburn during the evening hours (P < 0.001), throughout the night (P < 0.0001), and improved the ability to fall asleep (P < 0.0001) (Table S4).
Additionally, a published study [38] of famotidine demonstrated similar and favorable improvements in sleep. In a double-blind study of 309 patients with frequent heartburn (≥ 3 times per week for ≥ 2 months), 10 mg of famotidine given 1 h before the evening meal at home decreased heartburn frequency during the evening (P < 0.0001) and sleep (P < 0.0001), improved the ability to fall asleep (P = 0.0156), and reduced the number of awakenings (P = 0.0001) compared with placebo [38].
Sleep directed medications have also been used to reduce awakenings associated with NHB. For example, zolpidem significantly reduced sleep arousals by 49% compared with placebo (P < 0.01), but significantly prolonged the duration of nocturnal acid reflux events (P < 0.01) [43]. Although the prolonged exposure of the esophagus to acid reflux among patients treated with zolpidem in that study [44] could theoretically raise concern for an increased risk of GER-related complications, the sample size was small with 16 patients with GER and 8 controls. Another small study found that treatment of patients with heartburn and/or regurgitation at least 3 times a week and insomnia for at least 3 months with ramelteon significantly improved daytime and nighttime heartburn, 24-hour acid regurgitation, and insomnia (all P < 0.05). There were no significant adverse events reported.
Supplemental melatonin has been associated with improving sleep, and conditions related to inflammation [10]. An increase in the understanding of melatonin secretion by the GI tract and its potential role in GI health has led to an interest in determining the potential role of melatonin as a treatment for GER and GERD and associated sleep disturbance. The GI tract is the second most important source of endogenous melatonin, with the pineal gland the first [45]. The most well-known function of melatonin is its role in maintaining the circadian rhythm and promotion of sleep. However, in the GI tract, melatonin is released primarily after ingestion of food and functions to modulate GI motility and gastric emptying [45, 46]. In the upper GI tract, melatonin is thought to be involved in not only circadian functions, but scavenging free radicals, protecting mucosa, and promoting healing of the mucosa [45]. One study evaluated plasma levels of melatonin among patients with non-erosive GER, GERD, functional dyspepsia, recurrent duodenal ulcer, and healthy subjects [47]. Melatonin concentrations were highest among patients with non-erosive GER (P < 0.01) and functional dyspepsia (P < 0.05) and lowest among patients with GERD (P < 0.05) and duodenal ulcers (P < 0.01), which the authors concluded suggests that melatonin has a protective effect. Other studies of animal models suggest that supplementation with melatonin may have a protective effect against the progression from erosive GERD to Barrett’s esophagus and adenocarcinoma [48]. Although prospective studies are needed to further elucidate the role of melatonin in GER and the potential role of melatonin in treating occasional NHB, the evidence to date is promising.
NHB is common among patients with occasional heartburn. However, there is limited clinical data in the published literature specific to occasional NHB, making it difficult to understand the patient experience versus that of a patient with GERD. The symptom of NHB is analogous between patients with GERD and those with occasional heartburn, and so the approach to occasional episodes of heartburn has been extrapolated. It is clear, however, that more studies are needed to characterize the nature of occasional NHB that is not associated with GERD and its effective management.
Importantly, NHB has been shown to be causal with sleep disturbances, including arousals that may or may not be remembered [35, 36]. Many individuals with NHB complain of difficulty falling asleep, difficulty staying asleep, and next-day sleepiness or not feeling rested, and studies show that these arousals substantially impact next-day function, including the ability to drive safely and work productivity [5, 19, 33–35, 49]. Sleep disturbances and inadequate sleep have also been associated with long-term negative health effects, particularly on the cardiovascular system [9]. Although frequent NHB may be associated with more complicated GERD, the evidence accumulated to date has shown little risk of progression to Barrett’s esophagus or adenocarcinoma among patients with normal or mild esophageal changes [11, 50–52]. The risk of developing serious esophageal sequelae is likely even less for patients with occasional or trigger-provoked NHB.
In otherwise healthy adults, short-term consequences of sleep disruption include increased stress responsivity, somatic pain, reduced quality of life, emotional distress and mood disorders, and deficits of cognition, memory, and performance [53]. Next-day functioning can also be affected. Analysis of the data from the 2009–2010 Behavioral Risk Factor Surveillance System found that 4.2% of adults reported to have fallen asleep while driving at least once during the last 30 days, and individuals who reported insufficient sleep or short sleep duration were twice as likely to fall asleep while driving [49]. Furthermore, evidence suggests that even one night of sleep disruption may impact next-day functioning and could require up to 2 days to return to baseline, and 10 days of sleep disruption requires more than 7 days of sleep recovery [54, 55].
Negative longer-term health effects have also been associated with poor sleep [8]. A cross-sectional study of the National Health and Nutrition Examination Survey 2005–2008 data found that cardiovascular disease, including congestive heart failure, coronary heart disease, myocardial infarction, and stroke were significantly associated with sleep problems, daytime sleepiness, insufficient sleep, and/or prolonged sleep-onset latency after controlling for confounders in a multivariate analysis [9]. Other factors that are potentially involved include obesity and the intestinal microbiome [10].
Accumulating evidence suggests that NHB can be effectively managed with acid suppression therapy, including PPIs and H2RAs [15–19, 39]. These studies demonstrated subjective improvement in sleep parameters and next-day functioning. Although one study suggested that the sleep-promoting medication zolpidem may improve sleep disturbance associated with symptoms of NHB, it also increased the duration of acid reflux events [43]. Therefore, zolpidem and other sleep-promoting medications that may exacerbate heartburn should be avoided unless otherwise indicated.
In conclusion, NHB symptoms can cause sleep dysfunction that can have a profound adverse downstream effect on quality of life, next-day functioning, and health-related outcomes. The current approach to managing occasional NHB is similar to that for NHB associated with GERD, highlighting the need for studies specific to the episodic heartburn population. Health care providers should investigate NHB as one of the potential causes of sleep complaints, and patients with heartburn should be questioned about sleep quality, recalled arousals, next-day vitality, early fatigue, and next-day functioning. Potential treatments that have been shown to improve heartburn symptoms, should be discussed with patients, specifically with the more expansive adverse health-related effects of NHB and related sleep disruption. Additionally, lifestyle and behavioral modifications in conjunction with acid suppression medicines should be included in provider-patient discussions to help manage the impacts of occasional NHB.
GER: gastroesophageal reflux
GERD: gastroesophageal reflux disease
GI: gastrointestinal
H2RA: histamine type-2 receptor antagonist
NHB: nocturnal heartburn
PPI: proton pump inhibitor
The supplementary Tables for this article are available at: https://www.explorationpub.com/uploads/Article/file/1001191_sup_1.pdf.
DAJ, LS, and NFW: Conceptualization, Methodology, Writing—review & editing, Visualization. AMPD: Conceptualization, Methodology, Validation, Formal analysis, Resources, Writing—original draft, Writing—review & editing, Supervision, Project administration, Visualization, Funding acquisition. EA: Conceptualization, Methodology, Resources, Writing—review & editing, Supervision, Visualization. HD: Methodology, Validation, Formal analysis, Writing—review & editing. ASBP: Validation, Formal analysis, Writing—review & editing, Visualization. All authors read and approved the submitted version.
DAJ is a consultant to Johnson & Johnson Consumer Inc. and ISOThrive. NFW is a consultant to Johnson & Johnson Consumer Inc., Idorsia, Harmony Biosciences, Jazz, and Takeda. HD is an employee of ClinChoice Canada which received funding from Johnson & Johnson Consumer Inc. for the conduct of systematic literature searches and preparation of summaries of identified published literature. ASBP is an employee of Medica Communications, LLC, which received funding from Johnson & Johnson Consumer Inc. for the conduct of systematic literature searches and preparation of summaries of identified published literature. LS is a retired employee of Johnson & Johnson Consumer Inc. and was a consultant to Johnson & Johnson Consumer Inc. through Source One Technical Solutions, LLC. AMPD and EA are employees of Johnson & Johnson Consumer Inc.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
