Affiliation:
1Department of Zoology, Maulana Azad National Urdu University, Hyderabad 500032, Telangana, India
Affiliation:
1Department of Zoology, Maulana Azad National Urdu University, Hyderabad 500032, Telangana, India
Email: sufaya19@gmail.com
Affiliation:
1Department of Zoology, Maulana Azad National Urdu University, Hyderabad 500032, Telangana, India
Affiliation:
2Obstetrics and Gynecology Department, Niloufer Hospital, Hyderabad 500028, Telangana, India
Affiliation:
1Department of Zoology, Maulana Azad National Urdu University, Hyderabad 500032, Telangana, India
Explor Immunol. 2025;5:1003189 DOI: https://doi.org/10.37349/ei.2025.1003189
Received: December 01, 2024 Accepted: March 20, 2025 Published: April 08, 2025
Academic Editor: Calogero Caruso, University of Palermo, Italy
Aim: To assess circulating levels of tumor necrosis factor-alpha (TNF-α) and transforming growth factor-beta (TGF-β) in mid-gestation pregnant women from South India, with (RPL) and without history of recurrent pregnancy loss (non-RPL) and its correlation with neutrophil to lymphocyte ratio (NLR).
Methods: Blood samples were collected from 400 pregnant women attending government maternity hospital, Hyderabad, and subjected to enzyme linked immunosorbent assay (ELISA) for cytokines. NLR was calculated from absolute cell counts obtained from hospital records. Mann-Whitney U and Spearman r correlation was conducted as data followed non-normal distribution.
Results: We found significantly decreased level of TGF-β and elevated TNF-α, TNF-α/TGF-β (P < 0.0001), and NLR (P = 0.0007) in patients over controls. Receiver operating curve characteristics of TNF-α/TGF-β (area under curve: 0.96) were superior to individual cytokines and NLR for patients when compared to the control group. A negative correlation was noted between NLR and TGF-β in the RPL group (P = 0.0041).
Conclusions: Our results are indicative of predominant pro-inflammatory environment during mid-gestation in patients contrary to the anti-inflammatory milieu in controls. This is first study that attempted to connect cytokines with cellular ratio in RPL. The affordability of NLR to track inflammation is promised by its inverse correlation with TGF-β. However, further longitudinal studies are warranted spanning all stages of gestation in normal pregnant and RPL women to establish our observations. The limitations of the study include other factors that drive pro-inflammatory status like emotional dysregulation in women associated with chronic pro-inflammatory status is unexplored.
Pregnancy is a state of balanced inflammation to establish immune tolerance towards a semiallogenic fetus. It is defined by immunological shift which is pro-inflammatory in the first and third trimester for implantation and parturition and anti-inflammatory during mid-gestation for fetal growth and development [1]. During initial stages along with increased pro-inflammatory cytokines, innate immune cells are also shown to facilitate trophoblastic invasion and spiral artery remodeling [2]. Later successful pregnancy outcome is favoured by local and systemic reduction in inflammatory Th1 responses [3]. Tumor necrosis factor-alpha (TNF-α) is a Th1 pro-inflammatory cytokine [4] associated with processes such as implantation, placentation, and parturition during pregnancy. TNF-α secreted by the immune cells work via Jak/Stat pathway by binding to its type 1 (R1) and 2 (R2) cell receptors [5]. Elevated and prevailing level of TNF-α has been reported in women with obstetric complications like gestational hypertension, gestational diabetes mellitus (GDM) [6], and recurrent pregnancy loss (RPL) [7] disturbing the Th1/Th2 balance at the maternal-foetal interface [8]. Another pleiotropic cytokine intimately associated with pregnancy is transforming growth factor-beta (TGF-β), which regulates hormone secretions, implantation, and placentation [9], and later helps in growth and development of the foetus by immunomodulating the mother’s immune cells [10]. Heightened levels of TGF-β have been implicated in a number of pregnancy-related disorders like preeclampsia (PE) and GDM, while a decrease can lead to recurrent abortions due to the disturbance in the immune-tolerant environment [11].
Along with the cytokines, immune cells are crucial for pregnancy success [12]. In recent years, neutrophil to lymphocyte ratio (NLR), a potent marker of systemic inflammation has gained importance as prognostic marker for acute and chronic inflammatory reproductive disorders. Neutrophils, part of innate immune system, increases cytokine production at site of inflammation, which in turn get stimulated to synthesize new neutrophils leading to further increase in inflammation [13]. Lymphocytes being a part of adaptive immune system, specially Th2 type stimulates production of anti-inflammatory cytokines like interleukins-4, 5, 9, 10, 13, etc. [14, 15]. The increase in neutrophils and decrease in lymphocytes may lead to damage of the endothelial lining of the blood vessels resulting in various complications [16].
An imbalanced inflammation likely plays a role in triggering pregnancy complications, therefore variety of cellular biomarkers like NLR, platelet to lymphocyte ratio (PLR) in pregnancy-related complications like PE [17], GDM, and RPL [18] has been studied. Recently, NLR as a potential predictive biomarker of systemic inflammation in the field of cardiovascular diseases [2], cancers [19] has gained importance but research in reproductive issues and with relation to the cytokine profile has been scarce. Therefore, in the present study, our hypothesis was to examine circulating levels of TNF-α, TGF-β, and NLR, and associate cytokines with cellular ratio which may reflect systemic inflammatory status existing in south Indian mid-gestation pregnant women. To our knowledge, it is our first study conducted with this approach in this region. To minimize the influence of confounding factors, samples were included from the same ethnicity, socio-economic status, and with matching gestational phase.
For the present case, control study 400 blood samples were collected from mid-gestation pregnant women with (RPL group) and without the history (non-RPL) of RPL from Obstetrics and Gynecology Department, Niloufer Hospital, Hyderabad, Telangana-500028, India. The study participants were recruited at the time of sample collection according to inclusion criteria for the study. For RPL group, only idiopathic recurrent abortion cases were included without autoimmune, anatomic, infectious, genetic, thyroid abnormality, and endocrine defects. Under non-RPL group, healthy pregnant women with no health concerns such as hypertension, GDM, poly cystic ovarian syndrome, or infections, visiting the obstetrics and gynaecology outpatient clinic for routine check-ups with at least one live birth, and without any history of abortion or infertility and normal ultrasonography evidence were included in the study. General vitamins and calcium supplements were prescribed and smoking and alcohol consumption was lacking in both the groups. For the present study institutional ethical clearance was obtained from Osmania Medical College, Hyderabad, Telangana-500028, India (Reg No: ECR/300/Inst/AP/2013/RR-16).
Five mL of blood sample was collected in K2 EDTA and red cap vial after taking informed consent from the patients. The absolute cell counts from hospital records were used to compute the NLR. Sera were collected after centrifuging the samples at 2,000–3,000 rpm for 10 min at 4℃. The circulating levels of TNF-α and TGF-β were measured by pre-coated anti-human TNF-α and TGF-β sandwich ELISA kits (BT LAB, Biosystems, China). The operating procedure provided by the manufacturer was strictly followed.
Shapiro-Wilk test was done to assess normal distribution. Clinical and demographic data followed normal distribution and represented as mean ± standard deviation, followed by independent student t-test. Data for TNF-α, TGF-β, and NLR did not pass the normality test and was reproduced as median (25% to 75% interquartile range). Mann-Whitney U test was performed to compare the statistical difference between the non-RPL and RPL groups. Receiver operating curve (ROC) characteristics were conducted to explore diagnostic capacity of individual parameters included in the study. Spearman r correlation coefficient was calculated between TNF-α, TGF-β, TNF-α/TGF-β with NLR in RPL and non-RPL groups. All the statistical calculations and graphical representations were done using GraphPad Prism version 9.0 (USA). Bonferroni adjustment was done and P value less than 0.005 was considered statistically significant [20]. For evaluating magnitude and significance of the findings 95% confidence interval (CI) was calculated for all the parameters and statistical tests.
In the present hospital-based case control study, we found no significant difference in demographic characteristics such as age (P = 0.023), hemoglobin levels (P = 0.544), BMI (P = 0.678), age at menarche (P = 0.019), age at first conception (P = 0.235) and, mean gestational age (P = 0.385) between patient and control group (Table 1).
Clinical and demographic characteristics of the study groups
| Category | Controls(n = 200)Mean ± SD | 95% CI | Patients(n = 200)Mean ± SD | 95% CI | P value |
|---|---|---|---|---|---|
| Age (years) | 23.346 ± 2.398 | 17.326–27.212 | 24.320 ± 1.819 | 17.321–31.235 | 0.023 |
| Hb (g/dL) | 10.899 ± 0.254 | 8.235–12.291 | 11.894 ± 1.293 | 8.991–13.313 | 0.544 |
| BMI (kg/m2) | 26.129 ± 3.391 | 23.917–27.120 | 27.250 ± 3.320 | 24.175–32.189 | 0.678 |
| Age at menarche (years) | 12.010 ± 1.212 | 10.102–14.231 | 12.960 ± 1.120 | 10.452–15.022 | 0.019 |
| Age at first conception (years) | 20.754 ± 2.633 | 18.179–23.213 | 21.658 ± 3.15 | 18.899–24.211 | 0.235 |
| Mean gestational age (months) | 5.890 ± 0.711 | 3.900–7.660 | 6.261 ± 2.010 | 4.100–8.040 | 0.385 |
t-test was carried out for clinical and demographic data. Hb: hemoglobin; BMI: body mass index; CI: confidence interval
For the considered parameters we obtained a significant increase in TNF-α, TNF-α/TGF-β, and NLR and decreased TGF-β in the patient group when compared to controls (P < 0.0001) (Table 2, Figure 1).
Comparison of cytokines levels, TNF-α/TGF-β, and NLR between RPL and non-RPL group
| S. No. | Parameters (pg/mL) | Median (25th–75th percentile) | P value | |||
|---|---|---|---|---|---|---|
| Non-RPL (n = 200) | 95% CI | RPL (n = 200) | 95 % CI | |||
| 1. | TNF-α | 140.3 (115.8–169.0) | 133.9–149.1 | 287.0 (238.5–336.4) | 279.6–297.0 | *** |
| 2. | TGF-β | 917.3 (698.1–1,048.0) | 874.2–945.4 | 356.9 (169.1–511.4) | 311.9–396.5 | *** |
| 3. | TNF-α/TGF-β | 0.1596 (0.124–0.205) | 0.150–0.1713 | 0.824 (0.538–1.594) | 0.697–0.9703 | *** |
| 4. | NLR | 2.945 (2.233–3.565) | 2.80–3.13 | 3.26 (2.57–3.928) | 3.04–3.41 | 0.0007 |
Mann-Whitney U test was performed for the above-mentioned parameters in the table. TNF-α: tumor necrosis factor-alpha; TGF-β: transforming growth factor-beta; NLR: neutrophil to lymphocyte ratio; CI: confidence interval; ***: P < 0.0001
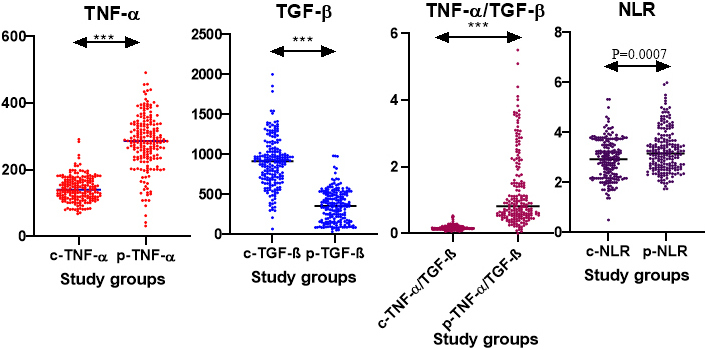
Represents circulating levels of TNF-α, TGF-β, TNF-α/TGF-β ratio, and systemic neutrophil to lymphocyte ratio in RPL and non-RPL groups. ***: P < 0.0001; c-: control; p-: patient; TNF-α: tumor necrosis factor-alpha; TGF-β: transforming growth factor-beta; RPL: recurrent pregnancy loss
ROC analysis was carried out for all the considered parameters to assess their diagnostic potential with area under curve (AUC), specificity (Sp %), and sensitivity (Sn %) (Table 3, Figure 2) in the order TNF-α/TGF-β > TNF-α > TGF-β > NLR with AUC having the value 0.966, 0.931, 0.930, and 0.6 respectively, indicating TNF-α/TGF-β has the potential to work as predictive marker of inflammation in women with a history of RPL (Table 3, Figure 2).
ROC analysis of RPL and non-RPL group
| S. No. | Parameters | AUC | Sp (%) | Sn (%) | 95% CI | Cut-off | P value |
|---|---|---|---|---|---|---|---|
| 1. | TNF-α | 0.931 | 96 | 88 | 0.90–0.95 | 199.6 | *** |
| 2. | TGF-β | 0.930 | 82.76 | 93.4 | 0.91–0.95 | 635.9 | *** |
| 3. | TNF-α/TGF-β | 0.966 | 96 | 92.5 | 0.94–0.98 | 0.318 | *** |
| 4. | NLR | 0.6 | 27.14 | 92.61 | 0.54–0.65 | 2.278 | 0.0007 |
ROC characteristics to assess the diagnostic potential of parameters. ROC: receiver operating curve; RPL: recurrent pregnancy loss; AUC: area under curve; Sp: specificity; Sn: sensitivity; CI: confidence interval; ***: P < 0.0001
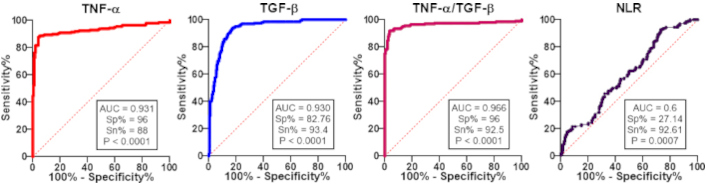
Depicts ROC for tumor necrosis factor-alpha (TNF-α), transforming growth factor-beta (TGF-β), TNF-α/TGF-β ratio, and neutrophil to lymphocyte ratio (NLR) in RPL and non-RPL group. RPL: recurrent pregnancy loss
A significant negative correlation (r = –0.203, P = 0.0041) was noted between NLR and TGF-β in RPL group whereas no correlation was observed in non-RPL group (Table 4).
Spearman correlation between TNF-α, TGF-β, TNF-α/TGF-β ratio, and NLR in non-RPL and RPL groups respectively
| Groups | Spearman correlation | |||
|---|---|---|---|---|
| Non-RPL | r | 95% CI | P value | |
| TNF-α | NLR | –0.041 | –0.184–0.103 | 0.565 |
| TGF-β | 0.104 | –0.039–0.243 | 0.143 | |
| TNF-α/TGF-β | –0.13 | –0.273–0.010 | 0.061 | |
| RPL | r | 95% CI | P value | |
| TNF-α | NLR | 0.071 | –0.213–0.072 | 0.320 |
| TGF-β | –0.203 | –0.337––0.061 | 0.0041 | |
| TNF-α/TGF-β | 0.110 | –0.034–0.2503 | 0.122 | |
Spearman r correlation coefficient was carried out between cytokine and its ratio and NLR between non-RPL and RPL group. TNF-α: tumor necrosis factor-alpha; TGF-β: transforming growth factor-beta; NLR: neutrophil to lymphocyte ratio; RPL: recurrent pregnancy loss
Reproduction is a complex physiological process necessary for species survival and sustenance. Modulation of maternal immune responses via regulatory and anti-inflammatory mechanisms predominantly through cytokine signaling prevents the rejection of semi-allogeneic fetus [21]. Deregulation of these mechanisms can lead to adverse pregnancy outcomes like spontaneous or recurrent abortion, PE, preterm labor, and intrauterine growth restriction [22].
The current South Indian case-control study found that mid-gestation pregnant women with a history of RPL had higher levels of TNF-α than the non-RPL group. In healthy pregnancy, a study found a significant rise in levels of TNF-α from 1st to 3rd trimester [23, 24]. Spence et al. [21] stated, it is likely that the TNF-α concentration rise steadily with advancing gestation as primary source of TNF-α is placenta [25] catering to the increased metabolic needs of the growing fetus. Studies have shown increased TNF-α levels in first trimester of pregnancy is associated with RPL [4, 26, 27]. Further, impact of up-regulated levels of TNF-α with increased abortion rate was seen in pregnant mice model by Samudra et al. [28]. Jang et al. [29] observed that Korean women with TNF-α gene (−863C>A) polymorphism are at higher risk for RPL. A meta-analysis stated the association of -308G/A polymorphism in TNF-α with susceptibility to RPL [30]. TNF-α plays an important role both in inflammatory responses and immunomodulation occurring at an appropriate stage of gestation and also regulates several processes required for the embryogenesis [31], placenta differentiation, and parturition [32]. Elevated TNF-α may influence the maternal-fetal cross-talk infuriating a shift in the secretory profile of placenta-derived immune-modulating factors, affecting the maternal immune response [6]. Moreover, excessive levels of TNF-α result in activation of cytotoxic activity of decidual natural killer (NK) cells [33] which impairs decidualization thereby changing the microenvironment of maternal-fetal interface, resulting in spontaneous abortion [26] thus comprehensive studies with shorter intervals between evaluations of TNF-α concentration from the beginning to the end of trimesters associated with both favourable and adverse outcomes have to be conducted to note TNF-α as an appropriate marker for diagnosis and prognosis of RPL.
Apart from a pro-inflammatory cytokine, we sought to see the circulating levels of anti-inflammatory cytokine TGF-β with the aim of assessing the relative concentration of pro to anti-inflammatory cytokine milieu in mid-gestation pregnant women with a history of RPL. We observed decreased levels of TGF-β in the RPL group compared to non-RPL group. TGF-β promotes immune tolerance by inhibiting the proliferation of Th1 and Th17 T cell subsets and regulates inflammatory response by promoting the proliferation of T-regulatory cells rendering an immunosuppressive environment thereby protecting fetus from maternal immune rejection [34]. Studies have shown decreased levels of TGF-β in pregnant women results in increased proinflammatory Th17 type of cells and decreased T-regulatory cells causing fetal loss [35]. Previous studies from our research group by Jameel et al. [36] and Dirisipam et al. [37] also have shown decreased levels of TGF-β during the second and first trimester respectively in RPL cases. Similarly, Mishra et al. [38] observed decreased serum levels of TGF-β in RPL patients. Further, Dirisipam et al. [39] reported an association of TGF-β -509 C/T promoter polymorphism in RPL women with adverse pregnancy outcome. In contrary, to our findings, few other studies in Japanese and Iranian women reported elevated levels of TGF-β in recurrent miscarriage group compared to gestational age match controls [40, 41]. In placental samples of women with sporadic miscarriages, Ball et al. [42] noted no variation in the expression of TGF-β.
The non-unanimity in the above studies could be due to the factors that affect cytokine signaling, differences in gestational period, ethnicity, source, sample size, and evaluation techniques; it is difficult to make firm conclusions about the use of TGF-β as an independent marker for RPL.
In order to strengthen the potential relevance of our findings, the relative concentration of pro to anti-inflammatory cytokines were calculated from individual values of TNF-α and TGF-β. It was found that mid-gestation pregnant women with a history of RPL had a higher TNF-α/TGF-β ratio when compared to non-RPL group. A recent review by Piccinni et al. [43] inferred that unexplained recurrent spontaneous miscarriage is associated with a greater bias towards pro-inflammatory cytokine profiles. Kwak-Kim et al. and Raghupathy [44, 45] reported significantly higher Th1/Th2 ratios in first trimester pregnant women with recurrent spontaneous abortions when compared to controls. By using placental antigens to co-cultivate the peripheral blood mononuclear cells of healthy pregnant women and RPL cases, Makhseed et al. and Raghupathy et al. [46, 47] assessed multiple cytokines. They found that recurrent aborters with a successful pregnancy had lower proinflammatory cytokine bias than women who experienced another abortion. Recently, Madduru et al. [48] reported a higher TNF-α/IL-4 ratio in the peripheral circulation of second trimester pregnant women with a history of RPL as compared to normal healthy pregnancies. The aforementioned findings corroborate our observations that mid-gestation pregnant women with a history of RPL had a higher pro-inflammatory milieu. TNF-α/TGF-β ratio displaying inordinate ROC characteristics like AUC (0.966), specificity (96%), and sensitivity (92.5%, P < 0.0001), proved it to be a better diagnostic marker with clinical significance likewise according to a study, the pro/anti-inflammatory cytokine ratio had superior diagnostic characteristics for monitoring pregnancy and predicting its outcomes [49, 50]. Therefore, host of cytokines and cells downstream of TNF-α and TGF-β may be influenced by their relative levels. Thus studies focussing on relative levels of TNF-α and TGF-β across various stages of gestation may help in developing better management strategies for RPL.
In addition to the cytokines level, we also intended to assess simple potent cellular markers of inflammation by NLR, and we observed that the RPL group had a greater NLR than the non-RPL group. Neutrophil’s recruit, activate, and programme other immune cells, secreting proinflammatory cytokines and chemokine capable of enhancing the recruitment and effector functions of other immune cells, such as dendritic cells, B cells, NK cells, and T cells [51]. Higher levels of NLR can increase cytotoxic activity of NK cells, dendritic cells etc., leading to maternal immune rejection of semiallogenic foetus. Majority of studies showed similar results in case of early pregnancy loss and during first trimester when compared to control group [22, 52–54] whereas Taskomur and Aydın [55] in 2022 reported no significant difference between the control and abortion group. Klement et al. [56] conducted population-based study reporting no significant difference between high risk and normal population. Similarly, Christoforaki et al. [57] showed lack of significant variation between NLR in women with miscarriages compared to women with live births but higher value (> 5.8) of NLR was seen in the miscarriage group. NLR due to its core significance to reflecting inflammatory load and accessibility to be determined from blood picture [18] is advantageous, however, to establish its clinical relevance further studies have to be carried out to use it as an inexpensive marker to monitor inflammation across various stages of gestation in women with ongoing pregnancy having a history of RPL.
The negative correlation between TGF-β and NLR indicates TGF-β to have inhibitory effect on the proliferation and differentiation of lymphocytes and other leucocyte activation and induces anti-inflammatory phenotype in neutrophils [58]. This is evident from the fact that placental and decidual neutrophils promote tolerance at the maternal-fetal interface [59]. Thus, lower levels of TGF-β may lead to activation of proinflammatory phenotype in neutrophils and loss of immune tolerance due to activation of adaptive immunity underlying pathophysiology of RPL. However, local and systemic immunological profiles (cellular and cytokine) defined across all stages of gestation for identification of markers is necessary that would facilitate prevention of adverse pregnancy outcomes. As RPL is multifactorial disorder, the psycho-pathophysiological aspects accompanying pregnant women with history of RPL such as emotional dysregulation, fear, anxiety, etc. are unexplored. Considering these in future studies would also give more insights about the pathophysiological aspects of RPL. Matrix table describing research on the considered parameters included in the study is depicted in Table 5.
Publications on TNF-α, TGF-β, and NLR in recurrent pregnancy loss
| Author and year | Title | Ethnicity | Gestational age | Levels |
|---|---|---|---|---|
| Tumor necrosis factor-alpha (TNF-α) | ||||
| Beckmann et al. 1997 [24] | Circulating bioactive tumor necrosis factor-alpha, tumor necrosis factor-alpha receptors, fibronectin, and tumor necrosis factor-alpha inducible cell adhesion molecule VCAM-1 in uncomplicated pregnancy | Netherlands | All three gestational ages | ↑ |
| Calleja-Agius et al. 2012 [5] | The role of tumor necrosis factor-receptors in pregnancy with normal and adverse outcome | Review | All three gestational ages | ↑ |
| Moreli et al. 2012 [25] | Interleukin 10 and tumor necrosis factor-alpha in pregnancy: aspects of interest in clinical obstetric | Brazil | During pregnancy | ↑ |
| Azizieh et al. 2015 [27] | Tumor necrosis factor-α and pregnancy complications: a prospective study | Kuwait | All three gestational ages | ↑ |
| Siwetz et al. 2016 [6] | TNF-α alters the inflammatory secretion profile of human first trimester placenta | Austria | First trimester | ↑ |
| Jang et al. 2016 [29] | Polymorphisms in tumor necrosis factor-alpha (−863C>A, −857C>T and +488G>A) are associated with idiopathic recurrent pregnancy loss in Korean women | Korea | ND | ↑ |
| Subha et al. 2016 [23] | Decreased baroreflex sensitivity is linked to sympathovagal imbalance, low-grade inflammation, and oxidative stress in pregnancy-induced hypertension | India | All three gestational ages | ↑ |
| Alijotas-Reig et al. 2017 [7] | Tumor Necrosis Factor-Alpha and Pregnancy: Focus on Biologics. An Updated and Comprehensive Review | Review | ND | ↑ |
| Samudra et al. 2018 [28] | CD39 and CD73 activity are protective in a mouse model of antiphospholipid antibody-induced miscarriages | Mouse model | ND | ↑ |
| Begum et al. 2021 [8] | Impact of TNF-α profile in recurrent pregnancy loss pathogenesis | Assam (India) | Term delivery | ↑ |
| Spence et al. 2021 [21] | Maternal Serum Cytokine Concentrations in Healthy Pregnancy and Preeclampsia | Review | All three gestational age | ↑ |
| Zhong et al. 2022 [4] | Case Report: TNF-Alpha Inhibitors to Rescue Pregnancy in Women With Potential Pregnancy Loss: A Report of Ten Cases | China | First trimester | ↑ |
| Dai et al. 2022 [26] | TNF-α/anti-TNF-α drugs and its effect on pregnancy outcomes | China | ND | ↑ |
| Present study | Predominant pro-inflammatory environment in mid-gestation pregnant women with history of recurrent pregnancy loss: a South Indian study | South India | Mid-gestation | ↑ |
| Transforming growth factor-beta (TGF-β) | ||||
| Ogasawara et al. 2000 [40] | Elevation of transforming growth factor-beta1 is associated with recurrent miscarriage | ND | ND | ↑ |
| Ball et al. 2007 [42] | Expression of TGF beta in the placental bed is not altered in sporadic miscarriage | UK | First and second trimester | No significant difference |
| Abdulkhaliq et al. 2018 [41] | The Role of IL-6 and TGF-β1 in Iraqi Women with Recurrent Abortion | Iraq | First trimester | ↑ |
| Yang et al. 2021 [10] | Role of Transforming Growth Factor-β1 in Regulating Fetal-Maternal Immune Tolerance in Normal and Pathological Pregnancy | China | ND | ↓ |
| Dirisipam et al. 2023 [37] | Can circulating levels of transforming growth factor-β1 in early pregnancy serve as a predictive marker of unfavourable outcome? | India | First trimester | ↓ |
| Jameel et al. 2024 [36] | Circulating levels of cytokines (IL-6, IL-10 and TGF-β) and CD4+CD25+FOXP3+Treg cell population in recurrent pregnancy loss | India | Second trimester | ↓ |
| Mishra et al. 2025 [38] | The Impact of Inflammatory Cytokines on Recurrent Pregnancy Loss: A Preliminary Investigation | India | ND | ↓ |
| Present study | Predominant pro-inflammatory environment in mid-gestation pregnant women with history of recurrent pregnancy loss: a South Indian study | South India | Mid-gestation | ↓ |
| TNF-α/TGF-β ratio | ||||
| Raghupathy et al. 1999 [47] | Maternal Th1- and Th2-type reactivity to placental antigens in normal human pregnancy and unexplained recurrent spontaneous abortions | Kuwait | First trimester | ↑ |
| Makhseed et al. 2001 [46] | Th1 and Th2 cytokine profiles in recurrent aborters with successful pregnancy and with subsequent abortions | Kuwait | First trimester | ↑ |
| Kwak-Kim et al. 2003 [44] | Increased T helper 1 cytokine responses by circulating T cells are present in women with recurrent pregnancy losses and in infertile women with multiple implantation failures after IVF | Chicago | First trimester | ↑ |
| Peng et al. 2021 [49] | Significance of the ratio interferon-γ/interleukin-4 in early diagnosis and immune mechanism of unexplained recurrent spontaneous abortion | China | ND | ↑ |
| Piccinni et al. 2021 [43] | Cytokines, Hormones and Cellular Regulatory Mechanisms Favoring Successful Reproduction | Review | ND | ↑ |
| Madduru et al. 2021 [48] | Association of reduced maternal sHLA-G5 isoform levels and elevated TNF-α/IL-4 cytokine ratio with Recurrent Pregnancy Loss: A study on South Indian women | India | First and second trimester | ↑ |
| Raghupathy 2022 [45] | Cytokines and pregnancy complications: modulation for prevention and treatment | Review | First trimester | ↑ |
| Present study | Predominant pro-inflammatory environment in mid-gestation pregnant women with history of recurrent pregnancy loss: a South Indian study | South India | Mid-gestation | ↑ |
| Neutrophil to lymphocyte ratio (NLR) | ||||
| Klement et al. 2018 [56] | Neutrophils to lymphocytes ratio and platelets to lymphocytes ratio in pregnancy: A population study | Israel | All three gestational stages | No significant difference |
| Christoforaki et al. 2020 [57] | First trimester neutrophil to lymphocyte ratio (NLR) and pregnancy outcome | Greece | First trimester | No significant difference |
| Oğlak and Aydın 2020 [22] | Are neutrophil to lymphocyte ratio and platelet to lymphocyte ratio clinically useful for the prediction of early pregnancy loss? | Turkey | ND | ↑ |
| Jiang et al. 2021 [53] | Neutrophil and Neutrophil-to-Lymphocyte Ratio as Clinically Predictive Risk Markers for Recurrent Pregnancy Loss | China | First trimester | ↑ |
| Onat et al. 2020 [52] | Can hematologic inflammation markers be the indicator of early pregnancy loss? | Turkey | First trimester | ↑ |
| Taskomur and Aydın 2022 [55] | Evaluation of Inflammatory markers in threatened abortions and spontaneous abortions | Turkey | 6–20 weeks of gestation | No significant difference |
| Hantoushzadeh et al. 2024 [54] | Diagnostic value of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio to predict recurrent pregnancy loss and abortion; a systematic review and meta-analysis | Review | First trimester | ↑ |
| Present study | Predominant pro-inflammatory environment in mid-gestation pregnant women with history of recurrent pregnancy loss: a South Indian study | South India | Mid-gestation | ↑ |
Matrix table for TNF-α, TGF-β, TNF-α/TGF-β, and NLR. ND: not defined; ↑: high; ↓: low
In conclusion, there is wealth of literature where the role of NLR and individual cytokines has been studied mostly during early pregnancy but there has been dearth of studies in later phases of gestation and their association in women with ongoing pregnancy having history of RPL. This is the first approach to explore the link between cytokine and simple cellular ratio. The salient findings of our study such as heightened TNF-α/TGF-β ratio and inverse relationship between TGF-β and NLR indicates studying relative levels of pro to anti-inflammatory cytokines due to their pleiotropic nature and cross talk between cytokines and immune cells at maternal fetal interface will assist in better understanding of RPL pathophysiology and establishing their impending clinical significance in developing management strategies.
AUC: area under curve
CI: confidence interval
GDM: gestational diabetes mellitus
NK: natural killer
NLR: neutrophil to lymphocyte ratio
PE: preeclampsia
PLR: platelet to lymphocyte ratio
ROC: receiver operating curve
RPL: recurrent pregnancy loss
TGF-β: transforming growth factor-beta
TNF-α: tumor necrosis factor-alpha
We are grateful to the blood donors who have helped us with this study. During the preparation of this work, authors used the Quillbot for improving the readability and fluency of the language. After using the Quillbot, authors reviewed and edited the content as needed and take full responsibility for the content of the publication.
RB: Investigation, Methodology, Project administration, Resources, Software, Writing—original draft, Data curation, Formal analysis. SJ: Data curation, Formal analysis. FR: Writing—review & editing. RB: Methodology, Resources. PJ: Conceptualization, Visualization, Writing—original draft, Writing—review & editing. Prior to submission, the work was reviewed and approved by all authors.
The authors declare that they have no conflicts of interest.
This study was approved by the Institutional Ethical Committee from Osmania Medical College, Hyderabad, Telangana-500028, India (Ethical approval reference no: Reg No: ECR/300/Inst/AP/2013/RR-16).
Informed consent to participate in the study was obtained from all participants.
Not applicable
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
This research was funded by the Indian Council for Medical Research (ICMR ID no. [2019-1022]), New Delhi, India. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
