Affiliation:
1Otolaryngology-Head & Neck Surgery, Athens Pediatric Center, 15125 Athens, Greece
†These authors contributed equally to this work.
ORCID: https://orcid.org/0000-0003-0592-6921
Affiliation:
2Children’s Oncology Unit “Marianna V. Vardinoyannis-ELPIDA”, Aghia Sophia Children’s Hospital, 11527 Athens, Greece
†These authors contributed equally to this work.
Email: psamara@biol.uoa.gr
ORCID: https://orcid.org/0009-0001-2249-7068
Affiliation:
1Otolaryngology-Head & Neck Surgery, Athens Pediatric Center, 15125 Athens, Greece
ORCID: https://orcid.org/0009-0002-8439-618X
Explor Immunol. 2024;4:73–89 DOI: https://doi.org/10.37349/ei.2024.00129
Received: July 12, 2023 Accepted: November 23, 2023 Published: February 22, 2024
Academic Editor: Giuseppe Bardi, Istituto Italiano di Tecnologia, Italy
Autoimmune and autoinflammatory diseases affecting the inner ear can cause symptoms such as hearing loss, imbalance, vertigo, and tinnitus, presenting demanding and often underdiagnosed conditions. Diagnostic challenges arise due to their diverse manifestations, potential long-term consequences, and the absence of specific serological markers, necessitating a multidisciplinary approach combining clinical evaluation, audiological assessments, and imaging techniques. Various autoimmune disorders, including systemic lupus erythematosus, rheumatoid arthritis, and Sjogren’s syndrome, have been implicated in immune-mediated damage to auditory structures, resulting in inner ear dysfunction. Inflammatory processes in autoinflammatory diseases like Cogan’s syndrome and relapsing polychondritis can also affect the inner ear. While the exact mechanisms of inner ear involvement in these conditions are still being studied, immune-mediated inflammation, damage to auditory structures, and vascular involvement play significant roles in auditory impairments. Treatment strategies primarily focus on immunomodulation and inflammation control using corticosteroids, immunosuppressants, and targeted biologic agents to ameliorate symptoms and preserve hearing function. Hearing aids and cochlear implants may be also considered for severe hearing loss. Individualized approaches are necessary due to patient response heterogeneity. This review provides a concise overview of key autoimmune and autoinflammatory diseases impacting the inner ear, highlighting clinical manifestations, diagnostics, pathophysiology, and treatment options. Early recognition and appropriate management are crucial for optimizing patient outcomes. Further research is needed to understand underlying mechanisms and identify novel therapeutic targets. Collaboration between otolaryngologists, rheumatologists, and immunologists is crucial for improving the quality of life in these complex conditions.
Autoimmune and autoinflammatory diseases are both characterized by dysregulated immune responses, however, they differ in their underlying mechanisms and the specific components of the immune system involved. Autoimmune diseases arise when the immune system mistakenly targets and attacks the body’s own cells, tissues, or organs, considering them as foreign (non-self). This immune response is primarily mediated by autoantibodies (antibodies that target self-antigens) or autoreactive T cells. Autoimmune diseases encompass a wide range of conditions, including rheumatoid arthritis, systemic lupus erythematosus (SLE), and multiple sclerosis, among others. These diseases often encompass chronic inflammation and can affect multiple organs or systems across the entire body [1]. On the other hand, autoinflammatory diseases are characterized by episodes of uncontrolled inflammation, often without evidence of autoantibodies or autoreactive T cells. The inflammation seen in autoinflammatory diseases develops from dysregulated innate immune responses, particularly involving components such as cytokines and inflammasomes. These diseases are typically characterized by recurrent or periodic flares of inflammation, which can affect specific organs or systems. Examples of autoinflammatory diseases include familial Mediterranean fever, cryopyrin-associated periodic syndromes (CAPS), relapsing polychondritis, and Behcet’s disease. Following the emergence of autoinflammatory diseases, several autoimmune conditions have undergone reclassification as autoinflammatory diseases, or at least, they have been recognized to exhibit certain characteristics associated with autoinflammatory disease [2].
Both autoimmune and autoinflammatory diseases can affect the inner ear, causing auditory impairments and vestibular dysfunction. While the outer and middle ear can also be impacted, it occurs less frequently. The inner ear, with its intricate and delicate structure, is particularly vulnerable to damage. The specific mechanisms by which these diseases affect the inner ear are not fully understood. In autoimmune diseases, the immune system mistakenly targets the cells and tissues of the inner ear, resulting in inflammation and damage to the cochlea (responsible for hearing) and vestibular system (responsible for balance). This immune-mediated damage disrupts the transmission of sound signals to the brain, leading to sensorineural hearing loss (SNHL). SNHL can occur as a complication of non-organ-specific autoimmune diseases or as a primary focus called autoimmune inner ear disease (AIED). In autoinflammatory diseases, uncontrolled episodes of inflammation can also influence the inner ear. The inflammation may harm the blood vessels that supply the inner ear, resulting in ischemia and subsequent hearing loss [3]. Conditions like Cogan’s syndrome, characterized by inflammation of the eyes and inner ear, can cause SNHL, vestibular dysfunction, and ocular symptoms [4].
Early recognition, appropriate diagnosis, and targeted treatment strategies are fundamental in managing inner ear involvement in autoimmune and autoinflammatory diseases. Timely intervention can help preserve hearing function, alleviate symptoms, and improve the overall quality of life for affected individuals. This review aims to provide an overview of the current understanding of these diseases, focusing on their clinical presentation, diagnosis, pathogenesis, and management strategies.
These conditions often lead to inflammation within the inner ear, resulting in debilitating manifestations such as SNHL, tinnitus, ear fullness, dizziness, balance disturbances, ataxia, motion intolerance, and positional or episodic vertigo [5]. Therefore, achieving an accurate and prompt diagnosis is crucial for effective management and treatment.
Among the other symptoms caused by these diseases, regarding the ear, hearing loss is the most common. It can be diverse and may arise due to various underlying mechanisms. These conditions can lead to auditory impairments that range from mild to severe, affecting one or both ears. The onset of hearing problems can be sudden, occurring rapidly within a short period, or it may develop gradually over time, making it harder to notice initially. Characteristically, AIED causes bilateral, asymmetric, and fluctuating hearing loss that worsens rapidly over weeks or months. Balance issues may or may not be present. One intriguing aspect of hearing issues in autoimmune and autoinflammatory diseases is the potential for selective frequency-specific hearing loss. This means that individuals may experience hearing loss only in specific frequencies or pitches, while other frequencies remain unaffected [6].
Inner ear diseases associated with autoimmune and autoinflammatory conditions present considerable diagnostic challenges due to their intricate symptomatology and overlapping clinical features. Often, the diagnosis involves ruling out other disorders with similar symptoms, like Meniere’s disease [7]. Diagnosing these conditions necessitates a systematic and comprehensive approach, integrating clinical evaluation, specialized audiological assessments, laboratory investigations, imaging modalities, and multidisciplinary collaboration (Figure 1). At present, there are no universally accepted diagnostic criteria or definitive pathognomonic tests available for accurate diagnosis. Therefore, the diagnosis is often supported by observing a positive response to immunosuppressive treatment, which serves as a valuable indicator in confirming the presence of the disease. The diagnostic process commences with a thorough patient evaluation, encompassing a detailed medical history and physical examination [8]. Recognizing potential underlying autoimmune or autoinflammatory factors is essential, guiding further investigations and aiding in reaching a precise diagnosis. Understanding the patient’s clinical presentation and identifying potential triggers or co-existing autoimmune conditions are vital for tailoring appropriate diagnostic strategies.
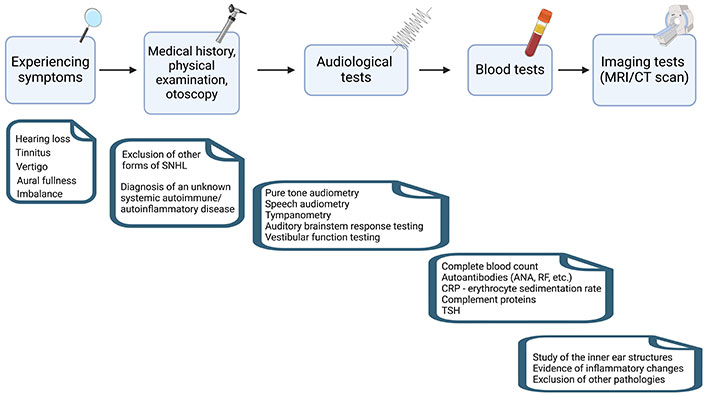
Diagram outlining the steps for diagnosing inner ear diseases in autoimmune and autoinflammatory conditions. This diagram provides a general overview of the diagnostic steps and may vary depending on individual cases and healthcare professionals’ recommendations. MRI: magnetic resonance imaging; CT: computed tomography; ANA: antinuclear antibody; RF: rheumatoid factor; CRP: C-reactive protein; TSH: thyroid-stimulating hormone. Created with BioRender.com
Specialized audiological assessments play a pivotal role in evaluating hearing impairment, a common manifestation of inner ear diseases in autoimmune and autoinflammatory contexts. Fundamental tests such as pure-tone audiometry and speech audiometry assess the nature and extent of hearing loss, while tympanometry rules out middle ear pathology. Audiometric tests, including auditory brainstem response testing, provide valuable insights into the integrity of auditory pathways, differentiating peripheral from central auditory disorders. Given the frequent occurrence of dizziness and balance disturbances in inner ear diseases, comprehensive vestibular function testing becomes indispensable. Caloric testing, videonystagmography, and rotational chair testing are commonly employed to assess vestibular function and identify specific patterns of vestibular dysfunction. These tests aid in localizing the site of pathology within the inner ear and help exclude central vestibular disorders [9].
Laboratory investigations play an instrumental role in detecting markers of immune system dysregulation. Measurement of autoantibodies, such as ANAs, anti-microsomal antibodies, anti-gliadin antibodies (for celiac disease), smooth muscle antibodies (SMA), RF, or specific inner ear antibodies, provides critical evidence for an autoimmune etiology. Additionally, the analysis of inflammatory markers, like CRP and erythrocyte sedimentation rate, complement C1Q, or TSH helps identify autoinflammatory processes [10]. Imaging studies, particularly MRI, also prove valuable in the diagnostic process. High-resolution MRI with gadolinium enhancement can reveal inflammatory changes, including enhancement of the labyrinth or vestibular nerve, indicative of active inflammation within the inner ear [11]. This information assists in confirming the diagnosis and guiding appropriate treatment strategies.
Given the complex nature of these conditions, a multidisciplinary approach involving collaboration between otolaryngologists, rheumatologists, immunologists, and other relevant specialists is indispensable. This collaborative effort ensures a comprehensive evaluation of the patient, optimal interpretation of diagnostic findings, and informed treatment decisions. Advancements in diagnostic technologies and increased awareness of the intricate interplay between immune system dysregulation and inner ear pathology promise improved diagnostic accuracy and enhanced patient care in the future. As research progresses, further understanding of these diseases will aid in refining diagnostic approaches and treatment strategies for inner ear diseases related to autoimmune and autoinflammatory conditions.
While the inner ear was traditionally considered “immune-privileged” due to the presence of the blood labyrinthine barrier, research has revealed the existence of immune cells within the inner ear, challenging this notion. Autoimmune and autoinflammatory diseases can affect the inner ear through various mechanisms, although the precise details for all conditions are not fully determined. Presented below are some of the primary mechanisms involved [12]:
(a) Autoantibody-mediated damage. In autoimmune diseases, the immune system produces antibodies that mistakenly target the body’s own tissues. Within the inner ear, these autoantibodies can attack structures like the cochlea or vestibular system, leading to inflammation and damage. For example, in AIED, autoantibodies can cause hearing loss, dizziness, vertigo, tinnitus, and balance problems.
(b) Immune complex deposition. Immune complexes are formed when antibodies bind to antigens, forming clusters that can deposit in tissues, including the inner ear. This deposition triggers an inflammatory response and disrupts the normal functioning of inner ear structures. Immune complex deposition is observed in certain autoimmune conditions, such as SLE, contributing to inner ear involvement.
(c) T cell-mediated inflammation. Dysregulation of T cells is common in autoimmune and autoinflammatory diseases. In some cases, activated T cells infiltrate the inner ear and initiate an inflammatory response, leading to tissue damage. This mechanism is believed to contribute to the development of conditions like Cogan’s syndrome.
(d) Cytokine imbalance. Cytokines, which regulate immune responses, can become imbalanced in certain autoimmune and autoinflammatory diseases. An imbalance of pro-inflammatory and anti-inflammatory cytokines can result in chronic inflammation affecting the inner ear. For instance, Behcet’s disease involves abnormal cytokine production, contributing to inner ear involvement.
The immune response within the inner ear involves interactions between antibodies and antigens, leading to activation of the complement system, vasculitis, micro-thrombosis, and electrochemical reactions. Innate immune cells like neutrophils, macrophages, and dendritic cells recognize antigens, releasing immune mediators such as interleukin-1β (IL-1β), IL-2, and tumor necrosis factor-alpha (TNF-α), which promote an adaptive immune response. This, in turn, leads to the migration of activated circulating lymphocytes into the inner ear through chemotaxis, causing irreversible tissue damage (Figure 2) [13]. Studies have indicated an association between sudden SNHL and the presence of antibodies against specific inner ear antigens, including heat shock protein 70 (HSP-70), cochlin, β-tectorin, connexin 26, β-actin, choline transporter-like protein 2, and types II and IX collagen [14, 15].
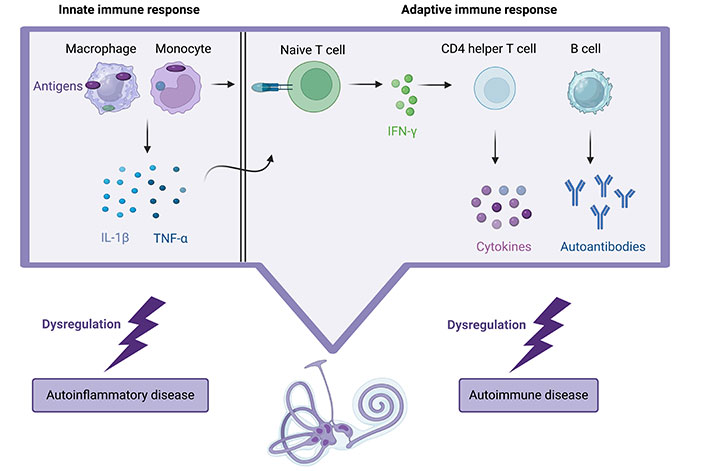
Description of the immune system attack on the cochlea in autoimmune and autoinflammatory diseases: inflammation is triggered, leading to irreversible damage as activated lymphocytes migrate, while autoantibodies also contribute to hearing loss and vestibular symptoms. Created with BioRender.com
Specifically, western blot analysis in these patients has revealed the presence of antibodies against cochlin and β-tectorin, which are distributed across various regions in the inner ear, such as the spiral limbus, spiral ligament, basal hair cells, supporting cells, cochlear, and vestibular labyrinths [16, 17]. Additionally, other proteins like Kresge Hearing Research Institute-3 (KHRI-3), expressed in the wall of the saccule, utricle, endolymphatic sac, supporting cells, as well as type II collagen, expressed in the subepithelial layer of the endolymphatic duct and the spiral ligament, could also be potential targets in immune-mediated hearing loss [18]. The inner ear contains hair cells that are responsible for converting sound vibrations into electrical signals, which are then transmitted to the brain for interpretation. When these hair cells are damaged or destroyed, it can result in hearing loss. The autoimmune attack on these hair cells disrupts the delicate process of sound transmission, causing varying degrees of hearing impairment. Furthermore, some autoimmune and autoinflammatory diseases can also affect the blood vessels supplying the inner ear, leading to reduced blood flow and oxygenation of the cochlea. This reduced blood supply can contribute to hearing problems and may be a factor in the development of hearing loss in certain cases [19].
Patients with immune-mediated SNHL have shown aberrant T cell expression patterns, characterized by reduced cytotoxic T lymphocytes, CD4+ T cells, and naive T cells during the onset of hearing loss. Corticosteroid treatment led to the normalization of T cell populations in those who responded to the treatment. However, non-responders exhibited a decline in naive T cells and an increase in memory T cells, attributed to sustained immune activation due to the inability to clear self-antigens within the vestibulocochlear system. This process subsequently triggered robust effector functions in both B and T cells, as noted by Berrocal and Ramírez-Camacho [20]. Studies have found associations between the presence of autoantibodies targeting vestibulocochlear proteins and SNHL [21]. These studies have also demonstrated that hearing recovery and the clearance of these antibodies can occur with corticosteroids [22], suggesting a potential role in the disease’s pathogenesis.
Quaranta et al. [23] revealed that initially elevated levels of circulating autoantibodies and immune complexes in patients with AIED responded positively to treatment with corticosteroids or cyclophosphamide. After receiving immunosuppressive treatment, these patients experienced hearing recovery, as evidenced by an improvement in the mean pure-tone audiometry, the presence of evoked otoacoustic emissions, and the restoration of normal levels of circulating immune complexes. The auditory dysfunction observed in AIED was attributed to immune complex deposition in the stria vascularis, resulting in a type III hypersensitivity reaction. This deposition caused damage to the capillary endothelium, leading to increased vascular permeability, ultimately resulting in endolymphatic hydrops and disruption of the outer hair cells. This proposed mechanism finds support in histopathologic observations from temporal bone studies in both humans and animals [12]. Another study demonstrated that AIED often involves dysfunctional immune responses in peripheral blood mononuclear cells to cochlear antigens. This research involved a comparison using autologous perilymph-stimulated peripheral blood mononuclear cells from AIED patients and controls. Furthermore, the induction of a protective molecule, membrane-bound IL-1 receptor type II (IL-1R2), seemed to correlate with clinical hearing improvement in responsive AIED patients, suggesting its potential as a biomarker for the condition [24].
Although the exact mechanisms of autoimmune and autoinflammatory diseases affecting the inner ear are not fully elucidated, these conditions can impact the inner ear through multiple mechanisms such as autoantibody-mediated damage, immune complex deposition, T cell-mediated inflammation, cytokine imbalance, and the presence of antibodies against inner ear antigens. The lack of an ideal animal model, variable peripheral blood study data, and the challenges associated with assessing inner ear anatomy contribute to the ongoing uncertainty regarding the exact immune mechanisms. Identifying real-time molecular markers for inner ear autoimmune damage in humans is a formidable challenge. A “liquid biopsy” of the inner ear, such as perilymph sampling, could potentially replace the need for invasive tissue biopsy, although it is applicable in cases requiring surgical procedures like cochlear implantation. Alternatively, future research could explore non-invasive serological biomarkers for AIED. Analyzing blood serum samples from AIED patients may unveil specific antibodies, cytokines, and immune cell markers associated with disease severity, progression, and treatment response, ultimately establishing reliable serological indicators. Further research will deepen—understanding of these mechanisms and aid in the development of targeted treatments.
Genetic factors can also play a role in the development of autoimmune and autoinflammatory diseases affecting the inner ear. Certain genetic variations have been associated with an increased susceptibility to these conditions. The human leukocyte antigen (HLA) system has long been recognized as a crucial indicator for autoimmune and autoinflammatory diseases, serving as a valuable disease marker [25]. In recent years, there has been a growing body of research investigating the intriguing association between HLA molecules and inner ear diseases within the context of these abovementioned conditions. HLA molecules, an essential part of the immune system, serve as key players in presenting antigens to T cells, thereby influencing immune responses. This pivotal role makes HLA molecules potentially influential in the development and progression of inner ear disorders. Their presence or absence can provide vital insights into the susceptibility and development of such disorders, thereby contributing to advancements in diagnostics and potential treatment strategies.
Within the HLA types, certain ones, such as HLA B27, B35, B51, C4, C7, and DRB1*04, have come to the forefront as particularly significant in the context of AIED [26]. Emerging evidence suggests that certain HLA alleles may confer susceptibility or protection to inner ear diseases. For instance, specific HLA alleles have been linked to an increased risk of developing AIED. Conversely, other HLA variants may exhibit a protective effect, shielding individuals from inner ear ailments associated with autoimmune and autoinflammatory processes.
The implications of unraveling the relationship between HLA molecules and inner ear disease are profound. By gaining a deeper understanding of how these genetic factors interact with the immune system in the context of inner ear disorders, researchers and healthcare professionals can potentially unlock new avenues for early diagnosis, personalized treatment strategies, and even the development of targeted therapies. However, the complexity of the immune system and the diverse array of HLA molecules call for further investigations to fully comprehend the intricate mechanisms underlying the association between HLA molecules and inner ear diseases. As ongoing research continues to shed light on this captivating interplay, the potential for improved patient outcomes and tailored interventions holds great promise for the future of inner ear disease management.
Unfortunately, the confirmation of an autoimmune mechanism for inner ear disease remains challenging due to the limited availability of living patient tissue specimens for histopathologic analysis. Postmortem temporal bone studies have revealed evidence of previous inflammation through osteoneogenesis, but a direct link to disease onset remains unproven [27]. Insights into potential injury mechanisms primarily come from animal models, such as genetically-modified research mice, Lewis rats, and guinea pigs, as they allow for more extensive investigation.
In the inner ear, numerous antigens are expressed, making them potential targets for immune surveillance. Recognition of these antigens by innate immune cells triggers the release of IL-1β, initiating an ongoing adaptive immune response. Immunocompetent tissues in the endolymphatic sac have been identified, capable of mounting immune responses [28]. Animal models have demonstrated that when sensitized animals are immune-challenged by delivering antigens to the inner ear, substantial inflammation and associated hearing loss occur. Additionally, the roles of cell-mediated and antibody-mediated immunity have been highlighted. Lymphocytes and immunoglobulins (Igs) enter the ear in response to antigenic challenge, with vestibulocochlear autoantibodies, predominantly IgG, found in the perilymph (albeit at lower titers than in serum). The involvement of IgG immune complexes has been implicated, and AIED development has been observed in recipient animals through T cell transfer from test subjects.
In a compelling study by Ma et al. [29], a sterile immune response was induced in guinea pigs through the use of keyhole limpet hemocyanin to assess the structural and functional repercussions in the cochlea. As a consequence of this immune response, inflammatory cells migrated from the bloodstream into the cochlea. Examination of cochlear sections, conducted 1–2 weeks after the onset of inflammation, unveiled the degeneration of sensory and supporting cells within the cochlear turns where inflammatory cells were prevalent, whereas structures in less affected cochlear regions displayed better preservation. At the five-week mark, the majority of infiltrated cells underwent apoptosis, and the inflammatory matrix in the scala tympani commenced calcification. The extent of hearing impairment varied depending on the level of inflammation.
While animal models provide valuable insights, further research is necessary to bridge the gap between animal studies and the complex autoimmune mechanisms at play in human AIED patients. Challenges in studying AIED include limited access to the human cochlea for clinical investigation, potential discrepancies between observations of the peripheral blood immune system and immune reactions within the inner ear, and the initial scarcity of appropriate immunologic reagents for characterizing complex immune responses accurately. Therefore, it is crucial to fulfill Witebsky’s postulates by reproducing the disease in animal models for them to be representative of human autoimmune disease [3].
Nevertheless, in 2004, the murine cochlin-peptide vaccination model was introduced, successfully validating AIED as an autoimmune disease [30]. In brief, the authors demonstrated that cochlin, as well as β-tectorin, can trigger an experimental autoimmune hearing loss in mice. Within five weeks of immunization with these proteins, the mice experienced substantial hearing loss at all tested frequencies. These peptides activated particular CD4+ T cells displaying a pro-inflammatory Th1-like phenotype. To further confirm the involvement of T cells, peptide-activated CD4+ T cells were transplanted into different mice, leading to increased hearing loss six weeks later, accompanied by the infiltration of white blood cells into the inner ear. Additionally, the identification of high levels of anti-cochlin antibodies in a subset of patients further support all these findings [16].
In the elegant review authored by Mancini et al. [31], a comprehensive list of autoimmune and autoinflammatory diseases that can affect the ear and lead mainly to hearing loss is presented. Furthermore, the list has been expanded and systematically classified, organizing it into Table 1 to enhance understanding and facilitate a detailed analysis of the distinct impacts on auditory health.
Comprehensive list of characteristic autoimmune/autoinflammatory diseases targeting various organs, including the inner ear
| Disease (histological/molecular basis) | Primary affected organ | Ear involvement | Molecules/antibodies/histological features |
|---|---|---|---|
| Systemic sclerosis (R) | Abnormal connective tissue thickening and protein accumulation in organs/tissues | Hearing impairment prevalence: 20–77% | Endothelin-1/ATA, ACA, anti-RNAP antibodies/excessive deposition of extracellular matrix, fibrosis |
| Multiple sclerosis (N) | Primarily affects the myelin in the CNS | Vertigo/dizziness occurs in around 33% of patients while hearing loss is rare but can happen due to brainstem involvement | IgG, complement, and Fc gamma receptors in the active lesions |
| Rheumatoid arthritis (R) | Prominent symptoms related to joints and surrounding tissues, impacting various organs including the heart, lungs, skin, and eyes | SNHL is the prevalent form of hearing impairment, with a prevalence ranging from 25–72% | RF, ACPA, anti-CarP antibodies/synovial inflammation, pannus formation, neutrophil infiltration |
| Hashimoto’s thyroiditis (E) | Thyroid gland | Anti-thyroid antibodies significantly impact hearing loss, particularly at lower frequencies, impairing cochlear activity | Anti-TPO and anti-TG antibodies/lymphocytic infiltration, thyroid follicular damage |
| Sjogren’s syndrome (I) | Primarily affects the salivary and lacrimal glands, resulting in dry eyes and mouth | Notable connection between SNHL and primary Sjogren’s syndrome, particularly in the higher frequency ranges | IL-6, TNF-α/anti-Ro (SSA) and anti-La (SSB) antibodies/parenchymal and ductal changes, lymphocyte infiltration, proliferation of the lining cells, formation of epimyoepithelial cell islands, acinar atrophy |
| SLE (I) | Multiple tissues/organs including joints, skin, kidneys, blood cells, brain, heart, and lungs | Development of SNHL | C3, C4/ANA, anti-DNA, anti-Smith and anti-phospholipid antibodies/immune complex deposition |
| Mixed cryoglobulinemia (V) | Cryoglobulins in the bloodstream damage or inflame affected blood vessels and surrounding tissues | 22% of patients experience bilateral SNHL | Circulating cryoprecipitable immune complexes in the serum, C4/association with infectious agents like hepatitis C/leukocytoclastic vasculitis |
| Antiphospholipid antibody syndrome (V) | Recurrent clotting episodes in arteries/veins due to antiphospholipid antibodies | Limited incidence and prevalence of sudden SNHL, with only a few case reports available, including both unilateral and bilateral occurrences | Lupus anticoagulant, ACA, anti-beta-2 glycoprotein I antibodies/thrombotic microangiopathy |
| Behcet’s syndrome (V) | Vasculitic disorder characterized by recurrent oral aphthous ulcers, genital ulcers and uveitis, various systemic manifestations (neurologic, articular, and gastrointestinal) | Sudden deafness and vertigo episodes, with SNHL prevalence ranging from 30–63% | No specific/pathognomonic biomarker, histopathology feature, or laboratory test, pathergy test positivity is suggestive, diagnosis is primarily clinical |
| Giant cell arteritis (V) | Large blood vessel inflammation with multinucleated giant cell formation from fused macrophages particularly affecting the temporal arteries in the head | SNHL is an uncommon complication, with hearing impairment present in 25% of patients. Vertigo and other indications of eighth cranial nerve involvement, such as dizziness and nystagmus | Erythrocyte sedimentation rate, CRP, thrombocytosis/transmural inflammatory infiltrate comprised of lymphocytes, macrophages, and giant cells |
| Wegener’s granulomatosis (or granulomatosis with polyangiitis) (V) | Formation of granulomas and inflammation of blood vessels, primarily affecting the upper and lower respiratory tracts in early stages | Middle ear disease commonly leads to conductive hearing loss, with around 10% of patients experiencing SNHL, primarily affecting low frequencies due to basilar membrane stiffness | Proteinase 3/ANCA/formation of granulomas featuring giant cells, encompassed by plasma cells, lymphocytes, and dendritic cells |
| Cogan’s syndrome (V) | Involvement of the inner ear and ocular structures, corneal/stromal interstitial keratitis, inflammation of the blood vessels | Hearing loss, tinnitus, and spontaneous sudden vertigo | No particular marker/ANA, SMA, lupus anticoagulant, cryoglobulins, ANCA, RF/infiltration by lymphocytes and plasma cells |
| Polyarteritis nodosa (V) | Systemic vasculitis primarily affects small and medium-sized arteries, leading to localized necrosis and inflammatory changes in the arterial wall | Hearing loss can be conductive, mixed, or sensorineural. SNHL usually appears bilaterally and symmetrically, with sudden onset or rapid progression | No definitive laboratory marker/localized necrotizing arteritis along with a mixed inflammatory infiltrate |
| Relapsing polychondritis (R) | Cartilage of the upper airway (ear, nose, trachea) | Conductive or SNHL | Matrilin-1, cartilage oligomeric matrix protein/antibodies against collagens II, IX, and XI/fragmented cartilaginous tissue surrounded by fibrous connective tissue with mononuclear inflammatory infiltrates |
| Takayasu’s arteritis (V) | Vasculitis that affects large and medium vessels | Hearing loss is a rare occurrence | Matrix metalloproteinases/transmural fibrous thickening of the arterial walls, degeneration of elastic fibers, reactive fibrosis |
| Vogt-Koyanagi-Harada’s disease (I) | Immune-mediated destruction of melanocytes by T cells, inflammation in pigmented organs | Auditory and vestibular manifestations in two-thirds of patients, including bilateral, rapidly progressive SNHL, tinnitus, and vertigo | Tyrosinase peptide/non-necrotizing granulomatous inflammation |
| Pyoderma gangrenosum (I) | Neutrophilic dermatosis characterized by chronic ulcers | Few cases of SNHL, several cases of sensorineural deafness in association with ulcerative colitis | Inflammasomes, Janus kinase 2, IL-23/proliferation of clonal T cells, ulceration of the epidermis and dermis associated with intense neutrophilic infiltrate, neutrophilic pustules, and abscess formation |
| Inflammatory bowel disease (I) | Chronic inflammation of the gastrointestinal tract | SNHL is commonly seen in patients with IBD, especially ulcerative colitis | Significant infiltration of the lamina propria by neutrophils, macrophages, dendritic, and NK cells |
| Celiac disease (I) | Chronic immune-mediated enteropathy of the small intestine, triggered by ingestion of gluten in genetically predisposed individuals | SNHL, vertigo with nystagmus | Tissue transglutaminase IgA and IgG, endomysial IgA, deamidated gliadin peptide IgA and IgG/epithelium infiltration with lymphocytes, crypt hyperplasia, villous atrophy |
| Ankylosing spondylitis (R) | Chronic inflammation that leads to progressive new bone formation in the spine, particularly in the sacroiliac joints | SNHL primarily results from cochlear damage, vestibular pathologies from the dysfunction of various neural pathways | Presence of HLA B27 |
| CAPS [familial cold autoinflammatory syndrome, Muckle Wells syndrome and neonatal onset multi-system inflammatory disease (N, R, I)] | Cutaneous, neurological, ophthalmologic, and rheumatologic manifestations | Progressive SNHL | NLRP3 gene mutations, IL-1β-mediated systemic inflammation |
| Susac syndrome (retinocochleocerebral vasculopathy) or SICRET (V) | Endotheliopathy causes dysfunction in the vestibule, cochlea, retinal damage, and multifocal encephalopathy | SNHL occurs in 43% of cases, primarily affecting the lower and mid-frequency ranges. Bilateral involvement is observed in 50% of cases. Additional common symptoms include tinnitus, vertigo, and potential gait impairments | AECA/ischemic infarcts, lack of viable endothelial cells in the blood vessels, thickening of the arterial walls due to amorphous material |
| Autoimmune chronic hypertrophic pachymeningitis (N) | Significant thickening of the cranial dura mater | Cranial nerve palsies, particularly involving the acoustic nerve, progressive SNHL, episodes of dizziness, recurrent bouts of vertigo | Inflammatory infiltrate of B and T lymphocytes, fibroblast activation, collagen deposition, tissue hypertrophy |
| Autoimmune encephalitis (N) | Brain inflammation by immune response against self-antigens expressed in the CNS | Rarely bilateral hearing loss of sudden onset | IgG antibodies against the NR1 subunit of the anti-N-methyl-d-aspartate (NMDA) receptor |
| Autoimmune hepatitis (I) | Chronic inflammatory liver disease | Rapidly progressive, bilateral, asymmetrical, and asynchronous SNHL (case report [32]) | Liver enzymes, IgG/lymphocytic inflammatory infiltration of the liver |
R: rheumatic; N: neurologic; E: endocrine; I: immunologic; V: vascular; ATA: anti-topoisomerase antibodies; ACA: anticentromere antibodies; RNAP: RNA polymerase; CNS: central nervous system; ACPA: anti-citrullinated protein antibodies; CarP: carbamylated protein; TPO: thyroid peroxidase; TG: thyroglobulin; La: lupus antibodies; SSA: Sjogren’s syndrome-related antigen A; C3: complement component 3; ANCA: anti-neutrophilic cytoplasmic antibodies; IBD: inflammatory bowel disease; NK: natural killer; NLRP3: nucleotide-binding and oligomerization domain-like receptor protein 3; SICRET: small infarctions of cochlear, retinal and encephalic tissue; AECA: anti-endothelial cell antibodies (The abbreviations “R”, “N”, “E”, “I”, “V” for “rheumatic”, “neurologic”, “endocrine”, “immunologic” and “vascular”, respectively, are only applicable in the table)
Due to the low incidence of these conditions, there are only a few studies with small cohorts and varying results on specific therapies.
Corticosteroids serve as the primary treatment option for sudden SNHL, when individuals experience acute hearing decline. Studies indicate that there is a significant likelihood of spontaneous hearing recovery within the initial 14 days following the onset of hearing loss [33]. However, healthcare professionals emphasize the importance of timely treatment to maximize the chances of hearing restoration, as delayed intervention may lead to an inadequate response.
Similarly, in the case of AIED, early initiation of treatment is decisive in order to prevent irreversible hearing loss. If AIED is suspected, treatment should be considered after ruling out other potential causes. Scientific evidence strongly supports the effectiveness of steroids in improving hearing outcomes in AIED. Typically, oral prednisolone is prescribed at an initial dose of 60 mg (1 mg/kg per day) and continued for a minimum of 4 weeks. If hearing improvement is observed, the treatment should be continued and gradually tapered over a period of 6 months. In cases where no response is observed after 4 weeks, tapering occurs over 12 days [34]. To ensure optimal control of the condition and minimize the risk of relapse, it is crucial to maintain appropriate dosing and treatment duration. Corticosteroids exert their therapeutic effects primarily through their anti-inflammatory properties, which play a vital role in their mechanism of action. By facilitating the reparative process of the pathological breakdown at the blood-labyrinthine barrier and assisting in restoring strial function, corticosteroids contribute to hearing restoration.
Intratympanic steroid injection has been investigated as an alternative treatment option for autoimmune hearing loss. This method involves delivering steroids directly through the round window membrane, enabling a concentrated dose of medication to reach the inner ear without causing systemic toxicity. Among the various steroid options, intratympanic methylprednisolone has demonstrated the highest concentration in the perilymph, making it particularly effective for patients who did not respond well to intratympanic dexamethasone. The typical administration of intratympanic methylprednisolone involves a dosage of 0.3–0.5 mL of a 40 mg/mL solution at weekly intervals over an 8-week period [35]. Both oral and intratympanic administration of corticosteroids has shown comparable effectiveness in treating sudden SNHL and AIED. Combining both routes of administration may lead to improved outcomes. During the initial stages of both conditions, response rates to corticosteroid treatment range from 60% to 70%. However, it is noteworthy that the degree of hearing recovery in AIED is relatively limited despite corticosteroid therapy.
The mechanism by which corticosteroids reduce inflammation in the inner ear is believed to involve multiple factors, although further research is needed to fully understand the intricacies of their action.
It is observed that approximately 30% of patients do not show any response to corticosteroid treatment, and some individuals may initially respond well but eventually develop corticosteroid resistance. In such cases, alternative treatment options are considered, and the most effective biologic agents are those that target specific pathways involved in inflammation and immune response. Among these agents, the most effective biologic agents are those that block TNF-α (adalimumab, infliximab, etc.), IL-1 (anakinra), or B lymphocytes (rituximab) [36].
TNF-α is indeed a cytokine known for its pro-inflammatory properties and its involvement in various cellular processes that regulate inflammation and cellular balance. However, the precise connection between TNF signaling and hearing loss remains inadequately comprehended and knowledge in this field is limited. Current research primarily focuses on investigating the effectiveness of TNF antagonist therapies in inner ear disorders where autoimmunity may play a significant role, such as AIED [37]. While corticosteroids have traditionally been the primary treatment for Cogan’s syndrome, emerging evidence suggests the potential benefits of TNF antagonist therapy in managing the condition. This highlights the possible role of TNF signaling in the treatment of Cogan’s syndrome.
IL-1 is a molecule that exerts a substantial influence on innate immunity and inflammation by activating genes associated with both innate and adaptive immune responses. It initiates the inflammatory process by binding to IL-1R1 on cell surfaces, leading to the activation of nuclear factor kappa B (NF-κB) signaling and promoting inflammation. Targeting IL-1 therapeutically has shown promise in alleviating symptoms in patients with autoinflammatory diseases such as Muckle Wells syndrome. In advanced cases of AIED among individuals undergoing cochlear implantation, immune cells in their peripheral blood exhibit a distinct expression of the IL-1 decoy receptor (IL-1R2) when exposed to their own perilymph. Further investigation has revealed increased expression of IL-1β in AIED patients who do not respond to steroid treatment compared to those who show a positive response [38]. Notably, dexamethasone fails to inhibit the release of IL-1β, while the IL-1 receptor antagonist anakinra successfully achieves this outcome. This suggests that exploring anti-inflammatory agents, such as IL-1R antagonists, holds promise for the treatment of sudden SNHL but requires further study.
Rituximab, a CD20 antagonist that inhibits the production of autoantibodies, has shown some improvement in hearing for individuals with AIED. However, further research is needed to determine the long-term sustainability of this response. In the treatment of AIED, rituximab has shown promise in reducing the dosage of steroids and alleviating symptoms. Nevertheless, currently, there is insufficient evidence to support the use of these biologic agents as a replacement for steroid therapy in AIED [39]. In cases where high doses of steroids are not recommended or when hearing loss relapses during the maintenance or steroid reduction phase, adjunctive therapy with immunosuppressive agents may be considered. Constant study is necessary to fully understand the efficacy and potential benefits of rituximab and other biologic agents in the management of AIED.
The effectiveness of methotrexate, an immunomodulator used to manage autoimmune diseases, in treating AIED is limited. While methotrexate inhibits TNF and IL-1, enhances IL-10, suppresses T cell activation cytokines, and modulates the NF-κB pathway, its specific mechanism of action in AIED and its impact on pro-inflammatory cytokines are not fully elucidated. Combination therapy involving methotrexate and other agents is generally more effective than using methotrexate alone [40]. In cases where patients are unresponsive to prednisone or unable to discontinue steroid use, cyclophosphamide may be considered as a treatment option. However, it carries a significant risk of toxicity [41]. In some cases, patients may choose to pursue a cochlear implant (CI) if their hearing loss progresses to severe or profound levels instead of opting for cyclophosphamide. The effectiveness and safety of other immunosuppressant drugs for AIED have not been thoroughly evaluated, and they often come with notable risks of toxicity. Methotrexate and cyclophosphamide have been explored as potential alternatives or additional treatments to steroids. These drugs are administered alongside steroids to minimize the adverse effects associated with high steroid doses and are used as alternative therapies for individuals who do not respond to steroids. In addition, azathioprine has been found to have a significant positive impact on hearing improvement in the majority of patients with AIED when it is administered alongside prednisone therapy [42].
Mycophenolate mofetil (MMF) has shown the ability to suppress a wide range of cytokines in monocytes, including IL-1α, IL-1β, and TNF. It also reduces the production of nitric oxide through inducible nitric oxide synthase. However, the extent of its potential benefits in immune-mediated hearing loss, including AIED, remains uncertain. It is important to note that MMF is considered a teratogen, and there have been reports linking its use to microtia and external auditory canal atresia [43].
Antioxidants, such as N-acetylcysteine (NAC), have shown potential benefits in cases of hearing loss and acoustic trauma. NAC, specifically, has demonstrated a protective role in autoimmune disorders and human idiopathic sudden hearing loss. Combining NAC with oral corticosteroids in cases of sudden SNHL has been studied and found to enhance the effectiveness of corticosteroid treatment outcomes. Moreover, NAC effectively reduces TNF-α release in patients with AIED and controls, indicating its potential as an adjunct therapy for AIED [44]. Further clinical exploration is needed to validate these findings.
Recent findings suggest that c-Jun N-terminal kinase (JNK) ligands, which act downstream of the JNK, have emerged as a promising avenue for improving sudden SNHL [45]. JNK is recognized as the principal mechanism triggering cell death in response to cochlear inflammation, and it holds the potential in addressing immune-mediated hearing loss as well.
In the future, there is speculation about the potential for interventions that directly modulate the immune system within the inner ear to restore tissue organization and enhance hearing in patients with SNHL. One potential target for modulating the immune response in the inner ear, particularly in the pathophysiology of SNHL, is the tissue macrophages present in the inner ear.
Plasmapheresis significantly contributes to the management of AIED by eliminating circulating antibodies, antigens, immune complexes, and other immune mediators from the blood. A study by Leutje and Berliner [46] showed positive changes in audiometric results for eight patients who underwent plasmapheresis. This treatment is particularly beneficial for severe cases of autoimmune diseases involving vasculitis or organ damage, even when adequate immunosuppressive therapy has been administered. In cases where steroid treatment does not produce satisfactory outcomes, plasmapheresis can serve as an adjunctive therapy for primary AIED patients with high antibody titers or secondary AIED with co-existing systemic autoimmune disease.
Currently, there is a scarcity of studies examining the utilization of hearing aids specifically for AIED. The primary challenge lies in the fluctuating nature of hearing loss experienced by AIED patients. Initially, individuals often decline the use of hearing aids due to poor speech recognition scores in the affected ear. Moreover, frequent adjustments to the device are necessary due to the fluctuating hearing loss and adverse reactions to intense sounds, resulting from cochlear damage and distortion of intensity perception. Consequently, many professionals hesitate to consider traditional hearing aids as a viable option for AIED patients due to the constant need for adaptation and adjustments. While it is reasonable to assume that the response to conventional hearing aid rehabilitation in AIED might be similar, specific studies addressing this topic are currently lacking.
Cochlear implantation is a well-established treatment choice for individuals who have severe to profound SNHL who do not benefit from conventional hearing aids [47]. While most candidates for CI typically have hearing loss caused by genetic factors, acquired conditions, or aging, there are cases where autoimmune and autoinflammatory conditions can cause significant hearing loss that may require consideration for cochlear implantation. As a result, decisions regarding candidacy and management should be tailored to each individual, taking into account their specific needs and associated risks. However, determining the optimal timing for cochlear implantation depends on individual circumstances.
While CIs are generally considered beneficial for individuals, limited data is available specifically regarding their effectiveness for AIED due to the rarity of such cases. The studies conducted on AIED have predominantly been retrospective with small sample sizes, primarily consisting of single case reports or uncontrolled case series. In the current literature, there are no additional risks that are universally recognized for AIED beyond those already accepted for cochlear implantation. It is important to note that some AIED patients may experience cochlear ossification, which can impact the surgical placement of CI electrodes. This could result in partial insertion, increased difficulty, or a more traumatic procedure, potentially leading to a higher likelihood of residual hearing loss [48]. Additionally, hearing loss in AIED can be characterized by fluctuations, making both diagnosis and hearing rehabilitation more challenging. In cases of bilateral profound SNHL associated with Cogan’s syndrome, early implantation is recommended to prevent endosteal reaction and new bone formation within the cochlear ducts.
Malik et al. [49] conducted a study to investigate the progression of hearing loss in patients with immune-mediated inner ear disease and identify factors associated with CI performance, the study involved patients experiencing rapid progressive hearing loss and varying clinical presentations. The rate of functional decline differed depending on whether the patients had isolated inner ear disease, inner ear disease associated with Cogan’s syndrome and relapsing polychondritis, or inner ear disease associated with other systemic autoimmune diseases. Despite profound hearing loss, patients achieved significant and sustained recovery of speech perception using a cochlear prosthesis over a follow-up period of up to 2 years. Better speech perception outcomes were associated with the presence of ear-specific versus organ-specific disease, younger age at CI, and complete electrode insertion.
According to Lee et al. [50]’s systematic review, cochlear implantation proves to be an effective treatment option for hearing loss associated with primary or secondary AIED. Most patients experienced substantial hearing improvement post-CI, with one-third showing continuous improvement, one-third reaching a plateau, and one-third maintaining stability. Perioperative complications in AIED patients were comparable to general cochlear implantation, although device failure and poorer audiological outcomes were observed in secondary AIED. Early CI may provide long-lasting hearing improvement and potentially reduce reliance on long-term immunosuppressant therapy, but caution is advised for cases involving cochlear ossification or fibrosis.
Careful evaluation, multidisciplinary collaboration, and individualized decision-making are crucial to ensure the safety and efficacy of the procedure, taking into account the patient’s overall health and immune status.
Autoimmune and autoinflammatory diseases impacting the inner ear pose significant diagnostic challenges and can lead to debilitating symptoms. The pursuit of accurate diagnosis and effective treatment requires a multidisciplinary approach. While current strategies focus on immunomodulation and inflammation control, further research is imperative to unravel the underlying mechanisms and discover breakthrough therapies. Collaborative efforts among specialists hold the key to transforming the lives of individuals grappling with these intricate conditions.
AIED: autoimmune inner ear disease
ANA: antinuclear antibody
CI: cochlear implant
HLA: human leukocyte antigen
Igs: immunoglobulins
IL-1β: interleukin-1β
IL-1R2: interleukin-1 receptor type II
JNK: c-Jun N-terminal kinase
MRI: magnetic resonance imaging
NAC: N-acetylcysteine
RF: rheumatoid factor
SLE: systemic lupus erythematosus
SNHL: sensorineural hearing loss
TNF-α: tumor necrosis factor-alpha
MA and PS equally contributed to: Investigation, Writing—original draft. IA: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Victor Ivanovich Seledtsov ... Alexei A. von Delwig
