Affiliation:
1Department of Internal Medicine, Georgetown University, Washington DC 20057, USA
ORCID: https://orcid.org/0000-0003-2162-4739
Affiliation:
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501, USA
ORCID: https://orcid.org/0000-0003-0232-065X
Affiliation:
3Department of Internal Medicine, Virginia Commonwealth University, Richmond, VA 23284, USA
ORCID: https://orcid.org/0000-0002-8441-0132
Affiliation:
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501, USA
ORCID: https://orcid.org/0000-0001-9314-5361
Affiliation:
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501, USA
Affiliation:
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501, USA
ORCID: https://orcid.org/0000-0003-3772-2616
Affiliation:
2Department of Internal Medicine, Eastern Virginia Medical School, Norfolk, VA 23501, USA
Email: dajevms@aol.com
ORCID: https://orcid.org/0000-0002-8737-0711
Explor Immunol. 2024;4:90–105 DOI: https://doi.org/10.37349/ei.2024.00130
Received: July 12, 2023 Accepted: January 04, 2024 Published: February 28, 2024
Academic Editor: Jochen Mattner, FAU Erlangen-Nürnberg and UKER, Germany
The intestinal mucosal barrier plays a critical role in maintaining the integrity of the gastrointestinal (GI) tract and protecting the body from harmful toxins and pathogens. Nutrition additionally serves as a vital component in maintaining bodily homeostasis. Macronutrients, micronutrients, and specific dietary habits exert profound effects on the immune system. The complex interactions of the immune system reflect a multifaceted, integrated epithelial and immune cell-mediated regulatory system. While several factors can influence the intestinal mucosal barrier and its pro- and anti-inflammatory processes, such as myeloid cell, regulatory T cell (Treg), or intraepithelial lymphocyte populations, there is growing evidence that macronutrients play an essential role in regulating its function. Herein this is a review of the peer-reviewed literature pertaining to dietary effects on mucosal integrity, including intraepithelial lymphocyte populations and immune function. This review is intended to explore the underlying mechanisms by which macronutrients impact and modulate the mucosal immune system.
The intestinal mucosal barrier is partially comprised of mucus organized as a bilayer composed of an inner adherent layer and a loose outer layer. Disruptions in this barrier can lead to various diseases, including inflammatory bowel disease (IBD), celiac disease, and food allergies. Multiple immune cell populations comprise the mucous-epithelial boundaries of our body, interfacing with the environment, microorganisms, and their metabolites to comprise an integrated regulatory system. This review aims to provide an analysis of the current literature on how macronutrients affect the intestinal mucosal barrier’s integrity and immune function. The paper will explore the underlying mechanisms by which these nutrients modulate the gut microbiota, regulate inflammatory responses, and impact the mucosal immune system’s development and function. The findings of this review may have significant implications for the development of dietary interventions aimed at promoting intestinal health and preventing or treating various gastrointestinal (GI) disorders.
The intestinal barrier plays a crucial role in maintaining gut homeostasis and preventing the translocation of harmful substances from the gut lumen into the bloodstream. Dietary carbohydrates have been implicated in modulating the intestinal barrier’s function and integrity. Carbohydrates are divided into four types: monosaccharides, disaccharides, oligosaccharides, and polysaccharides.
Dietary fiber comprises a diverse group of non-digestible carbohydrates that resist hydrolysis by pancreatic amylase in the small intestine and are important in maintaining the intestinal barrier [1]. It can be classified into two categories: soluble and insoluble fiber. Soluble fiber, found in foods such as oats, legumes, and fruits, dissolves in water to form a gel-like substance. Insoluble fiber, present in whole grains, vegetables, and nuts, adds bulk to the stool and aids in bowel regularity. Dietary fiber passes through the upper GI tract largely intact and reaches the colon, where it is fermented by the gut microbiota. Gut bacteria possess enzymes that can break down complex carbohydrates, such as cellulose and hemicellulose, into simpler compounds.
The main effects of these carbohydrates regarding the gut barrier include the production of short-chain fatty acids (SCFAs), the metabolism of fructose and its effect on the intestinal barrier, and the role of complex carbohydrates in maintaining barrier integrity. With this focus, the aim of this review is to highlight how different dietary carbohydrates influence the health of the intestinal barrier and subsequently, impact overall gut health.
During the fermentation process, gut bacteria produce SCFAs, including acetate, propionate, and butyrate [2]. These SCFAs are the primary end products of fiber fermentation and have important implications for intestinal health. Notably, SCFAs play a pivotal role in maintaining the integrity and function of the intestinal barrier. Butyrate serves as a preferred energy source for colonocytes and enhances the production of mucin, a glycoprotein that is the functional component of the mucus [3]. These SCFAs also promote the expression of tight junction proteins, such as claudins and occludins, which are vital for maintaining the integrity of the intestinal barrier. Recognizably, SCFAs exert their effects on the intestinal barrier through several mechanisms [4]. They regulate the production of antimicrobial peptides, enhance the secretion of mucin, reduce oxidative stress, and modulate the immune response in the gut [4]. These actions collectively contribute to a strengthened intestinal barrier and reduced risk of intestinal inflammation/disease.
The consumption of a high-fiber diet has been associated with numerous health benefits, including improved intestinal barrier function. Increasing dietary fiber intake has the potential to enhance SCFA production, promote healthy gut microbiota, and protect against various GI disorders [2]. Further research is needed to explore the therapeutic potential of SCFAs and dietary fiber supplementation in the context of intestinal barrier dysfunction and related diseases.
Fructose, a monosaccharide extracted from fruits, vegetables, and added sugars, has become increasingly prevalent in the modern diet due to its widespread use as a sweetener in processed foods and beverages. Excessive fructose consumption has been linked to various metabolic disorders and GI disturbances.
Mechanistically, most glucose absorption occurs in the small intestine enterocytes, through the sodium-glucose co-transporter and a glucose transporter type 2 (GLUT2) mechanism [5]. The fructose pathway, however, mediated by a passive transporter through GLUT5, is also present in epithelial enterocytes. It has been demonstrated that as little as 5 g of fructose, can overwhelm this passive transporter [6]. Rather than small intestinal absorption, this luminal fructose burden instead is delivered downstream to the colon, where it can be consumed by local flora, primarily in the right colon [7]. Many studies have begun to show that cancerous tissues have a higher expression of GLUT5 transporters, including colorectal cancer (CRC) tissue [8].
Excessive fructose consumption has been shown to impair the integrity of the intestinal barrier. Studies have demonstrated increased intestinal permeability, reduced expression of tight junction proteins, and heightened inflammation in response to fructose overload [9]. Fructose metabolism results in the production of several metabolites, including advanced glycation end products (AGEs) and reactive oxygen species (ROS). These metabolites can induce oxidative stress, inflammation, and damage to the intestinal epithelial cells, compromising the integrity of the intestinal barrier. Chronic consumption of high-fructose diets has been associated with intestinal inflammation, which may contribute to the development of IBD and other GI disorders [8]. Fructose-induced disruption of the intestinal barrier may facilitate the entry of luminal antigens, triggering immune responses and promoting inflammation.
Modifying dietary habits and reducing excessive fructose intake is crucial to mitigating the detrimental effects on the intestinal barrier. A balanced diet rich in whole foods, low in added sugars, and high in fiber, can help restore intestinal barrier integrity and prevent fructose-induced GI disturbances.
Complex carbohydrates encompass a range of polysaccharides and oligosaccharides found in foods such as whole grains, legumes, and starchy vegetables [10]. They provide a slow and sustained release of glucose, along with other essential nutrients and dietary fiber. While dietary fiber represents a significant portion of complex carbohydrates, other components like resistant starch and non-digestible oligosaccharides also contribute to their health benefits. These components undergo fermentation in the colon, resulting in the production of SCFAs and other beneficial metabolites. Consumption of complex carbohydrates, particularly those rich in dietary fiber, has been associated with improved intestinal barrier function [11]. The fermentation of complex carbohydrates by gut microbiota results in the production of SCFAs, which positively influence tight junction protein expression, mucus production, and overall intestinal barrier integrity. Complex carbohydrates, including dietary fiber, act as prebiotics, selectively promoting the growth of beneficial gut bacteria. A diverse and balanced gut microbiota composition is essential for maintaining intestinal barrier health and preventing dysbiosis-associated conditions [12]. Regular consumption of complex carbohydrates, especially those high in dietary fiber, has been linked to reduced risk of GI disorders, such as IBD, CRC, and irritable bowel syndrome (IBS) [9]. The promotion of healthy gut microbiota through complex carbohydrate consumption contributes to the maintenance of intestinal barrier integrity and overall gut health. In addition to complex carbohydrates, factors such as probiotics, prebiotics, and a variety of whole foods also play a crucial role in promoting a diverse and balanced gut microbiota. This, in turn, supports a healthy intestinal barrier and optimal gut function.
Clearly, there is a direct link between the types of carbohydrate intake humans consume and their intestinal barrier health. Dietary fiber, through the production of SCFAs, exerts beneficial effects on the intestinal barrier by enhancing barrier integrity and reducing inflammation [13]. On the other hand, excessive fructose consumption has been associated with compromised intestinal barrier function and increased intestinal permeability. Complex carbohydrates, particularly those high in dietary fiber, have shown promise in maintaining intestinal barrier integrity and promoting gut health. Further research and clinical studies are necessary to better define the precise mechanisms involved as well as, establish evidence-based dietary recommendations for optimal gut health. By adopting a well-balanced diet rich in complex carbohydrates, individuals can potentially support a healthy intestinal barrier and overall gut function.
With growing recognition of the microbiome and intestinal health’s role in systemic function, further research is needed to investigate key mediators in these processes. SCFA is an area of great interest as it acts as a key mediator in this role, influencing homeostasis, inflammation levels, immune system health, and gut motility. SCFA, specifically, has a vast range of potential impacts as evidenced by butyrate being shown to regulate the expression of 5–20% of human genes [14]. Recent studies have revealed a connection between SCFA and intestinal permeability and immune function, both of which may play a role in several pathologies both in and out of the GI luminal system [14].
Intestinal permeability is an area of interest as it is thought to be an indicator of overall intestinal epithelial health and is implicated in the pathophysiology of several inflammatory disorders of the GI tract [15]. However, it is not certain whether intestinal permeability is the cause of or result from inflammation. SCFA may offer insight into this process.
SCFA, specifically butyrate, has been shown to influence intestinal permeability via a multitude of factors, as summarized in Figure 1, including hypoxia-inducible factor (HIF), tight junction proteins, and interleukin-10 (IL-10) [16, 17]. HIF is a transcription factor involved in intestinal barrier protection. Several studies have shown that HIF has a key role in inflammation and barrier health, as stabilization of HIF is protective of colitis, and the loss of epithelial HIF-1α increases susceptibility [18]. Butyrate influences HIF via induction of hypoxia. In a low-oxygen colonic environment, the section of the GI tract which harbors the highest concentration of SCFA, favors anaerobic organism growth. These anaerobic bacteria in turn produce high levels of butyrate, which then shifts metabolism away from glycolysis and towards further butyrate use. This leads to physiologic hypoxia, which induces HIF stabilization and downstream effects of barrier protection [16]. This suggests that SCFA plays a prominent role in intestinal barrier health.
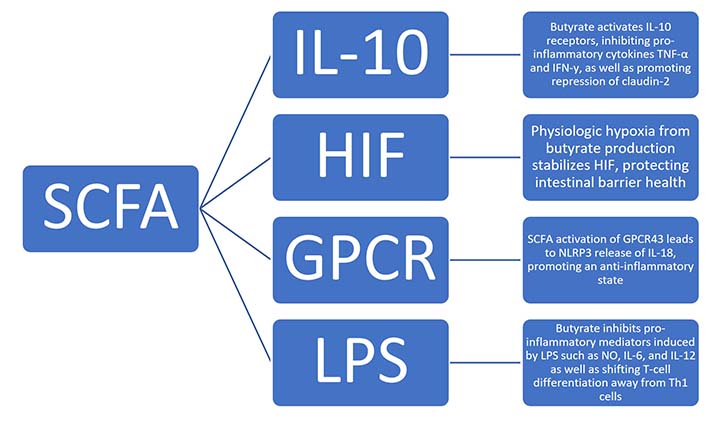
Proposed mechanisms of how SCFAs influence intestinal permeability via multiple factors, including HIF, tight junction proteins, and IL-10. GPCR: G-protein-coupled receptor; LPS: lipopolysaccharide; TNF-α: tumor necrosis factor-α; IFN-γ: interferon-γ; NLRP3: nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3; NO: nitric oxide; Th1: T-helper 1
Additionally, butyrate has been shown to reduce epithelial permeability via regulation of IL-10 receptors (IL-10Rs), occludin, zonulin, and claudins, with the latter three known as tight junction proteins. Butyrate was found to increase transepithelial electrical resistance and claudin-1 expression, as well as induce zonula occludens-1 (ZO-1) and occludin redistribution in the cellular membrane. This is mediated through the transcription factor specificity protein’s interaction within a promoter region of claudin-1 [19]. IL-10 has also been shown to interact with SCFA and tight junction proteins. One of IL-10’s major functions is to act as an anti-inflammatory cytokine, inhibiting TNF-α and IFN-γ. It does this by binding to the IL-10R on cells such as intestinal epithelial cells. Once the ligand binds, it results in the activation of the Janus kinase (JAK) signal transducer and activator of transcription (JAK-STAT) pathway which has been shown to be instrumental in the generation of anti-inflammatory processes. For example, mice without IL-10 or IL-10R have been shown to be more susceptible to colitis [17]. This is another area in which SCFA is able to influence intestinal health, as butyrate promotes epithelial barrier formation via IL-10R activation and subsequent repression of claudin-2. Claudin-2 forms paracellular channels through which water and small cations can pass through and is thought to be partially responsible for watery diarrhea in IBD as its concentration is higher in this pathology [17].
Focusing on their role in immune function, it is understood that SCFA plays a significant role in both pro- and anti-inflammatory states via interactions with cytokines and ILs. One study has revealed that SCFA binds to GPCR43 and leads to a multitude of downstream effects [20]. In addition to influencing the migration of neutrophils, GPCR43 has also been shown to be deficient in mice with inflammatory conditions such as colitis, as well as an increased level of inflammatory mediators in cells without the receptor [20, 21]. This is significant as the stimulation of GPCR43 by SCFA yields an area of inflammatory regulation and intervention [21]. One possible mechanism of an SCFA and GPCR43-mediated anti-inflammatory state is through the protein NLRP3. NLRP3 is an inflammasome-activating protein that induces the release of IL-18, which in turn promotes an anti-inflammatory state and contributes to gut homeostasis [22]. Given their impact on immune cells and inflammation, studies have investigated SCFA interaction with LPS, a key mediator in pro-inflammatory states [22]. Butyrate has been shown to inhibit pro-inflammatory mediators induced by LPS, such as nitric oxide, IL-6, and IL-12. Butyrate is also able to influence T-cell differentiation, inhibiting the differentiation of T-cells into pro-inflammatory Th1 producing IFN-γ and instead shunting differentiation towards regulatory T cells (Tregs) [20].
As the understanding of the microbiome and its significant effect on gut and systemic health grows, potential factors affecting the microbiome are of great interest. Dietary intake of fiber results in the production of SCFA, which has been evidenced to have the ability to affect intestinal permeability and immune response through mediations such as HIF, tight junction proteins, and ILs. These effects may lead to therapeutic interventions for many inflammatory GI pathologies.
There has been an increasing understanding of the role of long-chain fatty acids (LCFAs) in immune modulation, particularly with regard to their role as antimicrobial agents. These LCFAs are straight chain fatty acids with twelve or more carbon atoms [23]. They constitute a large portion of the gut and act as nutrients for various gut microbial organisms. Additionally, they play a role in cellular signaling to modulate physiological and pathological processes throughout the body, particularly the gut microbiome [24]. Gut bacteria have also been shown to produce various LCFAs that, in turn, have been shown to attenuate gut inflammatory processes [25]. Given this wide array of functions, LCFAs have become a highly studied subject matter for their potential therapeutic uses.
Long-chain polyunsaturated fatty acids (PUFAs) are major constituents of the cellular membrane so they modulate T-cell activity by modifying T-cell receptor interactions with antigen-presenting cells [26]. In addition to being essential for cellular membrane synthesis and structure, LCFAs regulate virulence responses of various enteric pathogens, including Salmonella enterica, Listeria monocytogenes, Vibrio cholera, and enterohemorrhagic Escherichia coli (E. coli) [24]. One study found that LCFAs mitigate Staphylococcus aureus virulence by destabilizing cellular membrane integrity [27]. Given these findings, LCFAs show potential as possibly supplementation or alternatives for antimicrobial agents.
Although there is limited knowledge of the exact mechanism, LCFAs have also been linked to decreased bouts and shorter duration of IBD flares [28]. One study found that supplementing colitis-induced rats with n-3 PUFAs decreased intestinal inflammation, ulceration, and necrosis by reducing TNF-α and nuclear factor kappa B (NF-κB) levels [29]. Salaga et al. [30] found that supplementing dextran sodium sulphate-induced colitis models with oleic acid, an omega-9 LCFA, delayed diarrhea, and rectal bleeding onset but did not histologically change disease progression. Additionally, the study found that docosahexaenoic acid supplementation in these models showed decreased inflammatory cell and cytokine infiltration [30]. Dietary fish oils contain high concentrations of n-3 LCFAs and have also been shown to decrease colitis severity [31]. Given the promising data, these studies have demonstrated the possibility of therapeutic LCFA use during IBD flares.
Long-chain PUFAs act as precursors to eicosanoid production, which gives rise to resolvins and protectants, both of which contribute to the resolution of inflammatory processes [32]. Additionally, long-chain PUFAs compete with arachidonic acid to act as substrates for cyclooxygenase and lipoxygenase, leading to the reduction of prostaglandin E2 (PGE2) levels [26]. PGE2 is involved in many pro and anti-inflammatory pathways involving T-, B-, dendritic cells, and macrophages [33]. Therefore, modifying PGE2 levels has implications for various cells constituting the body’s innate and adaptive immune system.
Other studies have found that long-chain PUFAs have a concentration-dependent effect on immune response [34]. Low concentration intake of long-chain n-3 fatty acids, such as fish oils, has been shown to enhance immune function, while high concentrations can reduce various immune functions, such as antigen-presentation, adhesion molecule expression, and Th1 and Th2 response [34]. High concentrations of PUFA intake have even been shown to induce lymphocyte apoptosis [34]. From this study, it is evident that further randomized control studies on dosing must be conducted in order to fully understand the therapeutic usage of LCFAs for the various ailments that they can mitigate.
With regards to intestinal permeability, certain LCFAs, such as oleic acid, have been shown to dilate the tight junctions, modify the cytoskeleton of intestinal epithelial cells, and permit higher drug absorption [35]. On the other hand, Salaga et al. [30] found that increased free fatty acid receptor-4 (FFAR-4), a GPCR that detects LCFAs, correlates with decreased intestinal permeability caused by inflammation and restoration of tight junctions. Another study by Mokkala et al. [36] explored various interventions to mitigate the increased intestinal permeability in pregnant women by supplementing pregnant women with long-chain PUFAs and measuring intestinal permeability by zonulin concentration. However, they found no change in pregnancy-induced intestinal changes after supplementation [36]. This study showed that hormonal and weight shifts during pregnancy might outweigh the influence of LCFAs on intestinal epithelium. This varying data highlights that not all LCFAs exert the same effect on intestinal permeability so further studies need to be performed to understand how various characteristics of LCFAs cause differing responses in the gut.
Medium-chain fatty acids (MCFAs) are defined as unsaturated fatty acids with 6–12 carbon atoms and are naturally found in milk fat and coconut oil. MCFAs are primarily utilized in the body for immediate energy or stored as lipid supply for later usage. MCFAs are more rapidly absorbed in the intestine than LCFAs because their smaller size allows them to be transported directly through the membrane without a shuttle [37]. MCFAs require less energy to be absorbed, MCFAs decrease intestinal metabolic stress, alleviating symptoms of various intestinal pathologies, including IBS [38]. Although the exact mechanisms are unknown, MCFAs have been shown to modify the gut microbiota, particularly in the jejunum and colon [37]. These alterations, in turn, can have wide ranging effects on gut permeability and immune function. For example, Kono et al. [39] found that MCFAs improved gut integrity and improved immunoglobulin A (IgA) secretion, boosting mucosal immunity and maintaining homeostasis in congruence with gut microbiota. Dierick et al. [40] found that supplementing piglets with Cuphea, a natural dietary MCFA source, increased intestinal villus height and decreased crypt depth. These changes maintain gut integrity and allow for greater mucosal protection against pathogens. Another study by Kono et al. [41] found that medium-chain triglycerides not only decreased induced colitis but also improved metalloproteinase and inflammatory marker activity in the colon. On the other hand, Haghikia et al. [42] found that dietary MCFAs, such as lauric acid, enhance Th1 and Th17 cell differentiation, increasing experimental autoimmune encephalomyelitis severity. These varying results demonstrate the complex relationship of MCFAs within our body, reducing autoimmune responses in the gut while increasing inflammatory responses in the central nervous system.
Gregor et al. [43] found that caprylate, also known as octanoic acid, formed from microbiota can prevent colitis activation in the distal colon, though the mechanism is unknown. Additionally, IBD patients showed decreased levels of MCFAs, including caproate, heptanoate, and caprylate, suggesting MCFA might be essential to propagate anti-inflammatory pathways in the gut [44]. Similar to other fatty acids, MCFAs are also being studied for their antimicrobial properties. Martínez-Vallespín et al. [45] did an introductory study of various MCFAs, including caprylic, capric, and lauric acid, and found that these fatty acids exhibit an inhibitory effect on E. coli and Salmonella enteritidis (S. enteritidis) growth. Additionally, this study found that MCFAs downregulate occludins and increased claudin-4 expression in intestinal epithelium, both of which act together to create tight junctions and aid in cell signaling [45]. Small intestinal bacterial overgrowth (SIBO) has been linked to decreased cholic acid absorption and MCFAs have been found to boost cholic acid metabolism, decreasing the likelihood of SIBO development and further emphasizing MCFAs antimicrobial potential capabilities [38]. Preliminary studies on dietary MCFAs have shown promising results for their use in both anti-inflammatory and anti-bacterial pathways, however, further studies must be completed to fully understand their potential.
Amino acids (AAs) are not only essential for tissue growth and repair but also play multifaceted roles in regulating various physiological processes. Recent studies have uncovered the remarkable influence of specific AAs on intestinal permeability and immune function [46–48]. Among the myriad of AAs, glutamine, tryptophan (Trp), and branched-chain AAs (BCAAs) have emerged as particularly intriguing candidates due to their unique properties and bioactive effects as summarized in Table 1 [49–51].
The influence of specific AAs on intestinal permeability and immune function
| AAs | Mechanism of immune optimization |
|---|---|
| Glutamine | Glutamine maintains the structural integrity of intestinal tissue: enterocyte proliferation, optimization of growth factors such as EGF, IGF-I, and TGF-α, and induces expression of tight junction proteins |
| Trp | Serves as a precursor for serotonin, melatonin, and various metabolites in the KYN pathway: modulation of tight junction proteins, T-cell differentiation, the production of IL-17 and IL-22, the maintenance of ILC3 in the gut, and the antioxidant response |
| BCAAs: leucine, isoleucine, and valine | Shown to influence intestinal development, tight junctions, nutrient transportation, cell migration, and barrier function: increased expression of β-defensin |
EGF: epidermal growth factor; IGF-I: insulin-like growth factor-I; TGF-α: transforming growth factor-α; ILC3: type 3 innate lymphoid cells; KYN: kynurenine
Glutamine, the most abundant AA in the human body, holds a significant position in the realm of nutrition and health. It serves as a critical fuel source for various cells, including enterocytes and immune cells, and plays a vital role in protein synthesis, cellular energy production, and nitrogen balance [52–54]. However, its impact on intestinal permeability and immune function has garnered particular attention.
Glutamine exhibits regulatory influence on various cellular pathways, including the oxidative stress, innate immune response, and inflammatory response, which collectively contribute to the regulation of intestinal permeability [55]. Glutamine is known to maintain the structural integrity of intestinal tissue through various mechanisms [49, 56, 57]. It promotes enterocyte proliferation, activates mitogen-activated protein kinases (MAPKs), including extracellular signal-regulated kinases (ERK1/2) and c-Jun N-terminal kinases (JNK1/2) [55]. Glutamine influences the actions of growth factors such as EGF, IGF-I, and TGF-α, and induces the expression of tight junction proteins including claudin-1, claudin-4, occludin, and ZO [55, 56].
Glutamine deprivation reduces the expression of tight junction proteins and increases epithelial cell permeability, while glutamine supplementation rescues the impaired barrier functions and improves tight junction integrity [55]. This protective effect of glutamine has significant implications for reducing the risk of immune activation and inflammation in various conditions such as parenteral nutrition, sepsis, infection, and radiation exposure [58–60].
Several studies have reported benefits associated with glutamine supplementation in various diseases. For example, it has been shown to improve immunologic aspects in trauma patients, alleviate mucositis in post-chemotherapy patients, and ameliorate symptoms of IBS [61–63]. In patients with acute critical illness and Crohn’s disease, reduced plasma glutamine concentrations have been observed [64, 65]. Leading to the hypothesis that glutamine supplementation could improve clinical outcomes. Nevertheless, conflicting evidence and mixed results from clinical studies have contributed to the ongoing debate [66–69].
Furthermore, glutamine plays a vital role in supporting immune function within the gut-associated lymphoid tissue (GALT). Experimental data have shown that glutamine influences cytokine production by various immune cells, including macrophages, lymphocytes, and intestinal epithelial cells [66, 70, 71]. Glutamine deprivation exacerbates the production of pro-inflammatory cytokines in intestinal epithelial cells, while glutamine supplementation limits the inflammatory response in vitro [52]. These effects are mediated through various signaling pathways, including the inhibition of NF-κB signaling and modulation of peroxisome proliferator-activated receptor gamma (PPARγ), which contribute to the inhibition of intestinal inflammation and maintenance of gut barrier integrity [72]. Additionally, glutamine enhances the production of secretory IgA (sIgA), an essential antibody involved in mucosal immunity, thereby enhancing immune surveillance and defense against pathogens at mucosal surfaces [73–75].
Overall, these findings emphasize the critical role of glutamine in preserving the structural integrity of tight junctions and regulating intestinal permeability. By promoting the expression of tight junction proteins and attenuating their disruption, glutamine contributes to the maintenance of proper gut barrier function and the prevention of immune activation and inflammation. However, further research and well-designed clinical trials are necessary to ascertain the true efficacy and safety of glutamine supplementation in these conditions.
Trp, an essential AA, holds significant importance as a precursor for the synthesis of several bioactive compounds. The KYN pathway is the major Trp metabolism pathway in the body, and the metabolites act as important immune system modulators. Among these compounds are serotonin, melatonin, and various metabolites in the pathway [76, 77]. All of these have been linked to crucial functions such as immune regulation, neuroendocrine signaling, and the maintenance of intestinal homeostasis.
Recent investigations have shed light on the substantial involvement of Trp and its three primary metabolic pathways, namely the indole pathway, the KYN pathway, and the 5-hydroxytryptamine (5-HT) pathway, in the modulation of intestinal inflammation [50, 78, 79].
The indole pathway, predominantly mediated by the gut microbiota, plays a pivotal role in maintaining intestinal homeostasis [80]. This pathway exerts its effects by activating the aryl hydrocarbon receptor (AhR), a key regulator of cellular responses. Upon activation, the Trp-AhR pathway triggers the expression of downstream cytokines, including IL-22 and IL-17, which contribute to the fine-tuning of intestinal homeostasis [81]. The production of indoles by the gut microbiota is disrupted in individuals with IBD, contributing to the pathogenesis of the disease [79]. Nikolaus et al. [82], in their analysis of serum samples from over 500 IBD patients, identified a negative correlation between serum levels of Trp and disease activity, as well as levels of C-reactive protein.
The KYN pathway serves as the major route for Trp metabolism (95%), converting Trp into various neuroactive compounds [83]. These metabolites generated through the KYN pathway have shown the ability to modulate the expression of tight junction proteins, exerting potent biological effects by interacting with specific receptors [84]. Notably, certain metabolites of the KYN pathway impact immune cell functions through the activation of the AhR. AhR activation is involved in essential processes such as T-cell differentiation, the production of IL-17 and IL-22, the maintenance of ILC3 in the gut, and the antioxidant response [85, 86].
Disruptions in the KYN pathway and alterations in Trp metabolism have been linked to increased intestinal permeability and immune dysregulation. These disturbances contribute to the development and progression of various GI disorders, including IBD, CRC, cardiovascular diseases, nervous system disorders, infectious diseases, and hepatic fibrosis [78, 80, 87–92]. The influence of these metabolites on the pathogenesis of these conditions underscores their significant role in GI health and highlights the importance of maintaining proper KYN pathway function and Trp metabolism.
Serotonin plays a crucial role in regulating intestinal permeability, immune system function, and the development of intestinal diseases. While only a small fraction of dietary Trp contributes to 5-HT production through the 5-HT pathway (1–2%), the majority of 5-HT is synthesized in the gut by enterochromaffin (EC) cells [83]. Research has shown that serotonin directly and indirectly affects immune cells, influencing their activation, proliferation, cytokine production, and immune function regulation [83]. Additionally, it plays a role in various intestinal functions including the regulation of epithelial barrier function, fluid and electrolyte transport, mucin secretion, and motility [93]. Dysregulation of serotonin signaling can disrupt intestinal homeostasis, leading to inflammation, dysregulated immune responses, altered gut microbiota, and compromised epithelial barrier function. In patients with IBS and IBD alterations in enteric 5-HT signaling have been observed [83, 93, 94].
In conclusion, the relationship between Trp metabolism, intestinal permeability, and the immune system is a complex area that requires further investigation. The role of Trp metabolites, such as serotonin, indole, and KYN, in modulating immune responses and intestinal barrier function is evident, but there is still much to uncover about the specific molecular mechanisms involved.
Leucine, isoleucine, and valine constitute the BCAAs, which play pivotal roles in multiple physiological processes, including energy homeostasis, nutrition metabolism, gut health, immune function, and disease regulation [51, 95]. These regulatory effects are mediated through specific signaling networks, particularly the phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT)/mammalian target of rapamycin (mTOR) signaling pathway [96, 97]. Understanding the complex interplay between BCAAs, intestinal permeability, and immune system function is crucial for unraveling their therapeutic potential in various health conditions.
BCAAs have demonstrated significant effects on intestinal health and immune system function. They have been shown to promote intestinal development, enhance the integrity of tight junctions, aid in the transportation of nutrients, stimulate cell migration, improve barrier functions in the intestine, and enhance immunity by increasing the expression of β-defensin, up-regulating pro-inflammatory cytokines and down-regulating anti-inflammatory cytokines [98, 99]. Several studies have established the association between BCAAs and chronic inflammatory conditions such as diabetes, insulin resistance, obesity, and cardiovascular disease [97]. However, specific to ulcerative colitis, a study conducted by Papada et al. [100] revealed that valine, one of the BCAAs, is associated with inflammation localized to the bowel. The mechanisms underlying the link between BCAAs and disease severity in ulcerative colitis and other intestinal diseases remain to be fully understood. The effects of specific AAs on intestinal permeability and immune function are multifaceted and intricately interconnected.
The intestinal mucosal barrier consists of a mucus bilayer and involves immune cells interfacing with microorganisms, crucial for regulation. Macronutrients influence the barrier’s integrity and immune function, affecting gut microbiota, inflammatory responses, and the development of the mucosal immune system. Dietary fiber, through the production of SCFAs, exerts beneficial effects on the intestinal barrier by enhancing barrier integrity and reducing inflammation. Glutamine, Trp, and BCAAs exert distinct influences on the maintenance of gut barrier integrity and immune responses within the GI tract. The review’s findings might inform dietary interventions for promoting intestinal health and managing GI disorders. Understanding these mechanisms is crucial for developing targeted interventions to promote gut health and combat associated diseases.
5-HT: 5-hydroxytryptamine
AAs: amino acids
AhR: aryl hydrocarbon receptor
BCAAs: branched-chain AAs
CRC: colorectal cancer
GI: gastrointestinal
GLUT2: glucose transporter type 2
GPCR: G-protein-coupled receptor
HIF: hypoxia-inducible factor
IBD: inflammatory bowel disease
IBS: irritable bowel syndrome
IFN-γ: interferon-γ
IL-10: interleukin-10
IL-10Rs: IL-10 receptors
KYN: kynurenine
LCFAs: long-chain fatty acids
LPS: lipopolysaccharide
MCFAs: medium-chain fatty acids
NLRP3: nucleotide-binding oligomerization domain (NOD)-like receptor family pyrin domain containing 3
PGE2: prostaglandin E2
PUFAs: polyunsaturated fatty acids
SCFAs: short-chain fatty acids
Th1: T-helper 1
TNF-α: tumor necrosis factor-α
Trp: tryptophan
LBC: Conceptualization, Data curation, Formal analysis, Writing—original draft, Writing—review & editing. MV: Writing—review & editing. KH: Data curation, Writing—original draft, Writing—review & editing. MS, AV, and SMD: Data curation, Formal analysis, Writing—original draft, Writing—review & editing. DAJ: Conceptualization, Supervision, Writing—original draft, Writing—review & editing.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
