Affiliation:
Translational Immunology, Osteoimmunology & Immunoporosis Lab (TIOIL), Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), New Delhi 110029, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0009-0008-3569-5101
Affiliation:
Translational Immunology, Osteoimmunology & Immunoporosis Lab (TIOIL), Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), New Delhi 110029, India
†These authors contributed equally to this work.
ORCID: https://orcid.org/0009-0000-6966-5770
Affiliation:
Translational Immunology, Osteoimmunology & Immunoporosis Lab (TIOIL), Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), New Delhi 110029, India
ORCID: https://orcid.org/0000-0003-3643-8242
Affiliation:
Translational Immunology, Osteoimmunology & Immunoporosis Lab (TIOIL), Department of Biotechnology, All India Institute of Medical Sciences (AIIMS), New Delhi 110029, India
Email: rupesh_srivastava13@yahoo.co.in; rupeshk@aiims.edu
ORCID: http://orcid.org/0000-0002-3323-0713
Explor Immunol. 2023;3:255–275 DOI: https://doi.org/10.37349/ei.2023.00101
Received: February 14, 2023 Accepted: June 14, 2023 Published: August 04, 2023
Academic Editor: Nitin Saksena, Victoria University, Australia
The article belongs to the special issue Immunology, Immunopathology and Genomics of SARS-COV-2
The coronavirus disease-2019 (COVID-19) pandemic is a significant threat in the modern era. Clinical studies show that the most common symptom of severe COVID-19 is viral pneumonia-induced acute respiratory distress syndrome (ARDS). The underlying mechanisms by which severe respiratory disease syndrome-coronavirus-2 (SARS-CoV-2) results in ARDS and how certain host factors confer an increased risk of developing severe disease remain unknown. Therefore, identifying the distinctive features of this severe and fatal disease and the therapeutic approaches to COVID-19-induced ARDS remains an immediate need to serve as a basis for best practice models of standardized ARDS treatment. This review article aims to comprehensively discuss the immunopathology of ARDS and provides an overview of the precise role of both the innate and adaptive immune system, with emphasis on the current treatment strategies being tested in the COVID-19-induced ARDS patients. This knowledge will supposedly help in revealing further mechanistic insights into understanding COVID-19-induced ARDS.
In December 2019, a pneumonia outbreak occurred in the Chinese city of Wuhan, which quickly spread around the world and posed a serious public health emergency. It was caused by the novel coronavirus 2019 (2019-nCoV) which was later named severe respiratory disease syndrome-coronavirus-2 (SARS-CoV-2) by the International Committee on Taxonomy. The disease has been designated as coronavirus disease-2019 (COVID-19) by the World Health Organization (WHO). Before SARS-CoV-2, two other CoVs, SARS-CoV (2002–2003) and Middle East respiratory syndrome-coronavirus (MERS-CoV; 2012) were already known to cause epidemics [1]. Inhaling infectious respiratory droplets from infected individuals with symptomatic and asymptomatic states is the primary method of new coronavirus transmission. However, contaminated objects or surfaces might cause them to spread inadvertently through other channels, like post-natal and fecal-oral routes [2]. The viral DNA is translated into proteins as it replicates in the cytoplasm of host cells, including the structural protein spike domain S1, which binds to the host cells’ receptor [3]. Modifications in the genetic code due to mutations and recombination events occurring during transcription and replication lead to the generation of various mutants. A variety of the SARS-CoV-2 viruses have one or more mutations that set it apart from other variations.
The widespread outbreak of COVID-19 infection prompted many studies to concentrate on the immune system’s function in warding off viral illnesses. SARS-CoV and MERS-CoV employ a variety of immune escape mechanisms for better endurance against immune response in host cells. Pattern recognition receptors (PRRs) can identify pathogen-associated molecular patterns (PAMPs), which are evolutionarily conserved microbial structures. SARS-CoV, however, can cause the creation of vesicles without PRRs and then reproduce within those vesicles, preventing the host from being able to discern their double-stranded ribonucleic acid (dsRNA) [2, 3]. The influence of the virus on a person’s immune system determines the prevalence and progression of SARS-CoV-2. SARS-CoV and MERS-CoV have been found to inhibit or delay immune response, which typically results in exaggerated inflammatory host responses and severe lung injury [4–7]. According to the data, it is now thought that a kind of severe respiratory failure develops in around half of the 20% of patients who need hospitalization [2].
Acute respiratory distress syndrome (ARDS) which is caused by SARS-CoV and MERS-CoV, was discovered to be also instigated by the novel coronavirus, SARS-CoV-2 [8]. ARDS, also known as acute lung injury (ALI), is a severe lung injury that first appears in infected patients with flu-like symptoms. In 2012, the established specific diagnostic criteria for ARDS in 1992 were modified in the so-called Berlin definition of ARDS in adults [9]. The 2012 Berlin definition considers the following points:
Timing: acute hypoxemic respiratory failure within 1 week of a known insult or new and/or worsening respiratory symptoms.
Origin: respiratory failure not fully explained by cardiac function or volume overload (need objective criterion such as echocardiography to exclude hydrostatic edema if no risk factor is present).
Imaging: bilateral opacities on chest radiograph or computed tomography (CT) not fully explained by effusion, collapse, or nodules.
Oxygenation: acute onset of hypoxemia defined as PaO2/FiO2 < 300 mmHg on at least positive end-expiratory pressure (PEEP) 5 cm H2O.
PaO2/FiO2 of 201–300 mmHg is mild ARDS.
PaO2/FiO2 of 101–200 mmHg is moderate ARDS.
PaO2/FiO2 ≤ 100 mmHg is severe ARDS.
Based on its specificity, the severity of COVID-19-induced ARDS is now classified into three stages: mild, moderate, and severe. It is observed that COVID-19-induced ARDS patients require mechanical ventilation for longer than patients with non-COVID-19-induced ARDS. The primary approach to managing ARDS involves implementing lung-protective ventilation strategies, such as low tidal volumes and PEEP which aim to minimize ventilator-induced lung injury while maintaining adequate oxygenation. Apart from that, the most widely used adjuvant therapy in normal ARDS includes recruitment maneuvers (RMs), high-dose corticosteroids, and continuous neuromuscular blocking medications. RMs involve the application of airway pressure to increase transpulmonary pressure transiently. Once non-aerated lung units are reopened, gaseous exchange improves resulting in better respiratory system mechanics. RMs can be both fast RM (sustained inflation) and slow RM (stepwise inflation). While this technique can transiently improve oxygenation in some patients with ARDS, their routine use as a mainstay treatment is not recommended as they can be associated with potential risks and complications, including hemodynamic instability, barotrauma (excessive pressure-related lung injury), and oxygen desaturation during the procedure [10]. Since corticosteroids have anti-inflammatory properties, they are a potential treatment for ARDS. The WHO highly advised systemic corticosteroid therapy for patients with severe and critical COVID-19 and discouraged it for those with non-severe COVID-19. Although several medications are being tested in clinical trials to treat COVID-19, there are presently no proven effective treatments available.
The immune system plays a major role in the pathogenesis of COVID-19-induced ARDS and can be targeted for successful therapy. This review first describes the role of the immune system in COVID-19-induced ARDS and then intends to assess the current status of promising emerging therapies for patients with ARDS, especially via the immune system henceforth also highlighting the importance of immunotherapeutic here.
Lung vascular injury is the major cause of ARDS. Numerous pieces of evidence have indicated that high endothelial cell activity and intravascular hydrostatic pressure cause an excessive build-up of pulmonary edema leading to significant leakage of plasma, including big proteins like albumin [11, 12]. Protein-rich pulmonary edema, leads to severe hypoxia and poor carbon dioxide excretion, restricting gas exchange and thereby causing hypoxemia. Neutrophils are the major players behind this. They release toxic mediators such as proteases, reactive oxygen species, procoagulant molecules, and proinflammatory cytokines in the lung microvasculature thereby leading to loss of endothelial barrier integrity [13].
Diffuse alveolar destruction is a typical histological pattern associated with ARDS that can be grouped into two episodes. The first or exudative phase, which corresponds to the first 10 days of viral infection, is primarily characterized by the formation of a hyaline membrane as a result of the fibrin polymerization of plasma liquid that leaked into the interstitial/alveolar space, alveolar-capillary barrier injury caused by red blood cell extravasation, and intense inflammatory cell infiltration into the intra-alveolar space. To eliminate the pathogen, activated innate immune cells such as alveolar epithelial cells (AECs), alveolar macrophages (AMs), and dendritic cells (DCs) secrete cytokines that alert the resident lymphocyte population. In response to the released cytokines [interferon (IFN), tumor necrosis factor (TNF), interleukin (IL)-12, IL-23, and IL-1β], these tissue-resident lymphoid cells which include cytotoxic γδT cells, T helper (Th) 1 cells, Th17 cells, natural killer (NK) cells, NKT cells, and innate lymphoid cells (ILCs) are engaged in the early primary effector response leads towards the secondary phase of infection [14–16]. The second phase, which is also known as the proliferative phase, is described by an accelerated proliferation of fibroblasts and myofibroblasts. This results in acute fibrinous organizing pneumonia followed by extracellular matrix deposition, which results in parenchymal remodeling and pulmonary fibrosis [7].
ARDS is one of the most serious side effects of SARS-CoV-2 infection. A significant COVID-19 complication that is foreseeable and requires prompt identification and thorough therapy is COVID-19-induced ARDS [4, 17]. Therefore, it is essential to comprehend the basic molecular mechanisms and pathophysiology of ARDS in COVID-19 to create efficient management plans and therapeutic designs. In the course of the infection, SARS-CoV-2 triggers both innate and adaptive immune responses, this response may also lead to extremely severe inflammatory reactions causing damage to tissues which can be on the systemic and local levels, such as lung diseases [18]. Thus, abnormal inflammatory response results from the host’s immunological response to SARS-CoV-2 heightens the illness. Patients with COVID-19 have been found to have elevated levels of proinflammatory cytokines, which are mediators of ARDS. Patients with COVID-19-induced ARDS exhibit elevated cytokine production as a result of cytokine dysregulation, which is also known as the “cytokine storm” stage of hyperinflammation. Analysis of the bronchoalveolar lavage fluid (BALF) also revealed enhanced chemokine expression. The cytokines and chemokines generated draw more effector cells, which accelerates the progression of inflammatory response and lung injury in COVID-19 patients [11–13, 19, 20]. The innate immune system is the initial defense against the virus, which plays a crucial role in viral immunity [6]. Primarily, neutrophils, AMs, and pulmonary epithelial cells get activated, which stimulates B and T lymphocytes involved in the adaptive immune system (Figure 1).
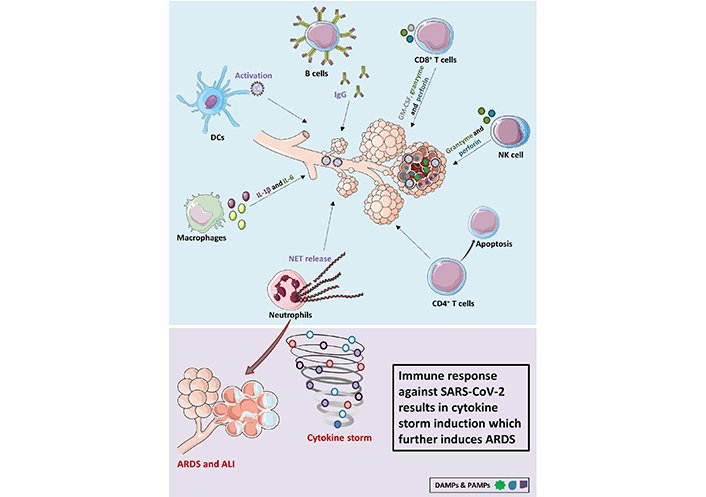
COVID-19-induced ARDS. SARS-CoV-2 entry into the lungs leads to the activation of several adaptive and innate immune cells. Neutrophils and macrophages are recruited from the circulation to the site of infection. Macrophages produce inflammatory cytokines IL-6 and IL-1β and neutrophils are induced to produce NET resulting in tissue damage. CD4+ T cells are observed to undergo apoptosis. Immune cells such as DCs, NK cells, B cells, and CD8+ T cells on the other hand help in virus clearance. SARS-CoV-2 leads to uncontrolled activation of the immune system resulting in cytokine storm which further induces ARDS and ALI. DAMPs: damage-associated molecular patterns; NET: neutrophil extracellular trap; IgG: immunoglobulin G; GM-CSF: granulocyte macrophage colony stimulating factor
The chronology of COVID-19-induced ARDS can vary among individuals and depends on several factors, including the severity of the infection, underlying health conditions, and access to medical care. However, a general pattern can be provided based on the majority of the cases:
Onset of COVID-19 symptoms: the initial symptoms of COVID-19, such as fever, cough, sore throat, and fatigue, usually appear within 2 to 14 days after exposure to the virus. Some individuals may also experience shortness of breath or difficulty breathing.
Progression to severe disease: for some individuals, COVID-19 can progress to a more severe form within 7 to 10 days after symptom onset. Severe disease may be characterized by worsening respiratory symptoms, persistent fever, and the need for hospitalization.
Hospitalization and diagnosis of ARDS: if respiratory symptoms worsen and oxygen levels decrease significantly, the individual may be admitted to the hospital for further evaluation and treatment. ARDS associated with COVID-19 is typically diagnosed based on clinical criteria, including severe respiratory distress, imaging findings (such as bilateral lung infiltrates), and decreased oxygen levels.
Intensive care unit (ICU) admission: in severe cases of COVID-19-induced ARDS, patients may require admission to the ICU for closer monitoring and advanced respiratory support. This can include the use of mechanical ventilation to assist with breathing.
Disease progression or recovery: the course of COVID-19-induced ARDS can be highly variable. Some patients may continue to deteriorate despite medical interventions, experiencing respiratory failure and multiple organ dysfunction. Unfortunately, in some cases, this can lead to death. On the other hand, with appropriate medical care and supportive treatment, many individuals with ARDS can gradually improve over time.
Patient discharge or prolonged hospitalization: the duration of hospitalization for COVID-19-induced ARDS can vary widely. Some patients may recover and be discharged from the hospital after several weeks, while others may require an extended period of intensive care and respiratory support [21].
Multiple epidemiological research strongly implies a correlation between age and health issues severity [22]. Additionally, the death rates of patients with co-morbidities such as diabetes, cardiovascular illnesses, hypertension, and cancer are much greater. Not only these but recent research from numerous nations suggests that obesity may be a standalone factor in predicting the risk and prognosis of COVID-19 individuals [23, 24]. Simply because individuals with larger waistlines occasionally exhibit intensified inflammatory markers. The so-called inflammatory outbreak, for instance, can lead to a hyperactive immune system, and these people may have higher levels of inflammation due to increased IL-1, IL-6, or C-reactive protein (CRP) levels [23, 24].
This shows that severe COVID-19 etiology may be caused by a particularly dysregulated immune response. Below we have discussed in detail the role of both innate and adaptive immune systems in the pathogenesis of COVID-19-induced ARDS.
The innate immune response produces an antiviral state in the primary infection site, which prevents viral reproduction within infected cells. This action sets off adaptive responses. However, in the case of SARS-CoV-2, an abnormal inflammatory innate response is induced which heightens the illness [25, 26]. The SARS-CoV-2 manifestations, notably fever, and dyspnea, are influenced by the activation of the inflammatory response, particularly the IFN response. Restricted and delayed IFN-I response triggers the production of proinflammatory cytokines and causes widespread cellular infiltrates in the respiratory tract along with persistent viral load in blood. Lack of IFN synthesis can affect T-cell responses as IFNs help T cells survive and perform their effector duties [27–29]. In a study of the spatio-temporal evolution of SARS-CoV infection which was done in rhesus macaques, it was demonstrated that as predicted, early viral replication and spread are significantly influenced by innate immunity [30]. It has also been found that connecting networks between innate immune cells aids in systemic viral transmission [30]. The role of various innate immune cells in the pathophysiology of SARS-CoV-2 is discussed below (Figure 2).
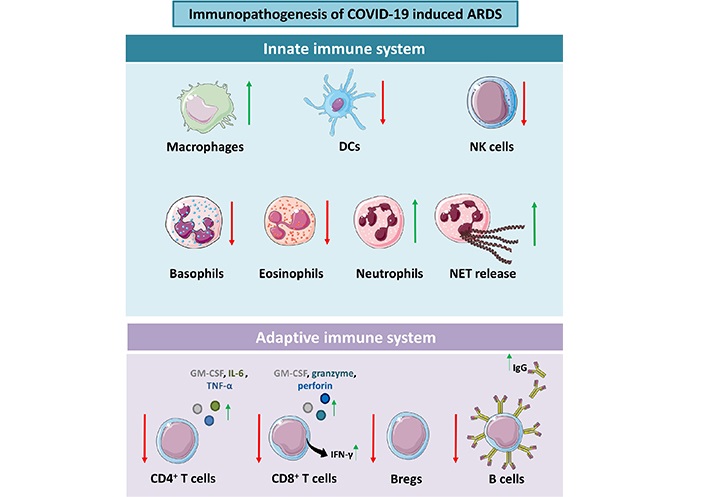
Immunopathogenesis of COVID-19-induced ARDS. SARS-CoV-2 infection results in the dysregulation of both innate and adaptive immune cells. Bregs: regulatory B cells
NK cells are the effector lymphocytes that regulate various microbial infections by preventing their progression and resulting in tissue damage. Several recent studies have also shown that NK cells interact with other immune cells, highlighting that NK cells may reduce or intensify immunological reactions [31]. Macrophages and DCs become activated during viral infection and release IL-12, IFN-γ, and IL-18, which then activate NK cells. NK cells can induce cell apoptosis, degranulation, and antibody-dependent cell-facilitated cytotoxicity (ADCC) [31]. In a recent study, it was concluded that a decrease in NK cell number and activity in patients with severe symptoms decreases the clearance of infected and activated cells [32]. This leads to an unregulated rise in tissue-damaging inflammatory markers [32]. These inflammatory responses are responsible for a significant risk of mortalities linked to ARDS [33]. In a study, it was observed that NK cells may help immune-immune cell interactions to prevent excessive inflammation and tissue damage [34]. But they are not observed to involve in the increased inflammatory responses seen in ARDS [34]. Therefore, in COVID-19, NK cells play a crucial role in the transition from a beneficial to a detrimental immune response.
There is growing evidence that suggests macrophages, both recruited blood macrophages, and resident AMs are important players in the etiology of ARDS. Macrophages are found to play a subtle role in the development of inflammatory responses. There are two types of AMs involved in ARDS, i.e., M1 and M2. M1 produces IL-1β, IL-6, monocyte chemoattractant protein 1 (MCP-1), and TNF-α which participate in tissue injury by neutrophilic inflammation and induce pro-inflammatory conditions. M2, on the contrary, produces anti-inflammatory cytokines such as arginine 1 (Arg-1), IL-10, and tumor growth factor β (TGF-β) by inducing tissue repair and remodeling [35, 36]. After clearance of infection from the body, the rehabilitation phase occurs where M1 macrophages change into M2 macrophages which plays a pivotal role in repairing the damaged lung tissue [37]. The late stage of ALI/ARDS, pulmonary fibrosis, is brought on by excessive collagen deposition and fibroblast proliferation. The ratio of M1 to M2 macrophages at this stage determines the progress and severity of pulmonary fibrosis. Therefore, it is possible to anticipate that controlling macrophage polarisation will accelerate the development of fibrosis in ALI/ARDS [38].
It is feasible for SARS-CoV-2 to infect macrophages and monocytes through angiotensin-converting enzyme 2 (ACE2)-independent and ACE2-dependent routes. If this occurs, the infected cells will lose their ability to fight off viruses and elicit adaptive immune responses [38]. Macrophages also play a crucial role in evoking the adaptive immune response to tackle the infection. However, their dysregulated activities can cause organ harm in patients, primarily by escalating acute inflammation, encouraging cytokine storms, and lengthening fibrotic consequences. As a result, they significantly aggravate the condition and cause ARDS, which frequently results in the death of people with COVID-19 [39–41]. Infiltration of interstitial mononuclear inflammation into the lung tissues was also validated by clinical-pathological examination of COVID-19 patients’ biopsy samples [42, 43]. Nevertheless, the molecular mechanisms behind the aberrant inflammatory responses in histology and serology brought on by SARS-CoV-2 infection are yet unknown.
DCs are well-known professional antigen-presenting cells (APCs) they can either serve as a primary player in the innate immune response defense against pathogen infection or they can also behave as masterminds in ensuing adaptive immune response. These are found to be connecting links between innate and adaptive immune systems. It is found that SARS-CoV-2 pneumocytes release viruses, PAMPs, and DAMPs. DCs get stimulated by PAMPs and DAMPs and this leads to naive DCs maturation [44, 45]. In a recent study, it is already been noted that there is a real increase in mature DCs in BALF of patients, this indicates that DCs are involved in lung immune response during SARS-CoV-2 infection [46].
ILCs are the newly discovered innate immune subsets. According to the proposed nomenclature, these cells are of 3 types, ILC1, ILC2, and ILC3, these are known to be mirror images of Th cells Th1, Th2, and Th17 respectively [47]. They are the ones who upon initial encounter with an incoming pathogen regulate the immune response and hence have a crucial role in immune surveillance. These lack specific antigenic receptors and therefore are found to be activated under the influence of cytokines or there might be some unknown receptors triggering their activation. Alarmins such as IL-25, IL-33, and thymic stromal lymphopoietin (TSLP) are released by damaged epithelium, these alarmins work on ILC2 to promote the proliferation and function of effector cells. ILC2 response is inhibited by IFN-β from restored epithelium and IFN-α from macrophages. Additionally, ILC2 converts to T-bet expressing ILC1 under the presence of cytokines IL-12, IL-18, and IL-1β [48]. ILC2 recruits’ eosinophils to the inflammation site resulting in tissue recovery. It has been showed that that C-C chemokine receptor (CCR) 10+ ILC2 expanded under recovery condition, although the mechanism driving lung repair are not entirely understood, concluding that ILCs have become important participants following acute injuries [49–51]. ILC2 has been described as a double-edged sword in the context of respiratory infections, aiding lung pathogenicity and homeostasis in both chronic and acute contexts. Similarly, IL-22 is observed to be increased post-infection indicating a positive role of ILC3 in tissue repair [49–51]. A thorough understanding of ILCs in respiratory tract infection is still required to understand their intricate cellular and molecular interactions under host-pathogen encounters.
Along with the above-mentioned innate immune subsets, granulocytes are also found to be involved in COVID-19-induced ARDS. According to a few studies, neutrophils number is increased in COVID-19 infection, this can be the signature of increased immune defense but the incidence of alveolus damage is also increased due to respiratory burst, degranulation, and NET formation [52–54]. These immune responses via neutrophils lead to inflammation in the lungs and cause damage to the endothelium that ultimately leads to impaired oxygenation [52–54]. Not only neutrophils but eosinophils and basophils are also found associated with lung damage during the COVID-19 infection. These granulocytes are a major marker of inflammatory home. In the case of eosinophils, their involvement in lung injury is still not clear, their count is found to be decreased during the initial phase of ARDS but increases in the late phase. They may emerge as effector cells that cause tissue damage or may be simply involved in an immune response to lung injury and repair [9, 55, 56].
Specialized immune response against foreign invaders is elicited by adaptive immune response via specialized immune cells and antibodies, it also keeps a memory of how they appear in the future and mounts a distinct immune response. This is firmly regulated by cross-talk between innate immune systems. Most acute viral infections result in the activation, expansion, differentiation, and start of secretion of effector molecules by B and T cells that can interact via antigen receptors with viral proteins. B cells, CD4+ T cells, and CD8+ T cells have a fundamental role in immunological defense against SARS-CoV-2 and were all shown to be lower in COVID-19 patients [9, 10, 57, 58].
The body’s B cells primarily carry out humoral immunity. Upon antigen activation, they can develop into plasma cells, making certain antibodies and playing a little part in immune defense. The generation of antibodies that block the invasion of SARS-CoV-2 into cells is carried out by B cells, however, it is yet unclear how long recovering COVID-19 patients would retain their immunological memory [59, 60]. B cells can be directly activated by antigens via B cell receptor (BCR) or Th2 cells. Plasma B cells secrete IgM, IgA, and IgG which mediates the neutralization of antigens by recognizing the spike protein. Also, in recovered patients these increase with time as observed in the data obtained. B cells can connect to viral proteins through their antigen receptors in response to most viral infections, helping to control viral infections by secreting effector molecules (IL-2, IL-4, IL-6, IFN-γ, and TNF-α) [61]. Enhanced IgG and IgA-secreting B cells and abnormalities in the circulating B cell subsets, and decreased Bregs immuno-suppressiveness might lead to respiratory tract inflammation and several lung pathologies [62]. The relative significance of the major players engaged in the pathophysiology of ARDS is still difficult to determine. More research is required to link and clarify the diverse cloud of B cell responses in COVID-19-induced ARDS.
The CD4+ and CD8+ subsets of T cells are essential for both autoimmune and inflammatory responses. There have been some theories about how SARS-CoV-2 causes lung damage. SARS-CoV-2 might primarily affect T cells, which would worsen the patient’s condition. It has been shown that SARS-CoV-2 induces immunological activation of T cells to produce antiviral responses. CD8+ T lymphocytes were a frequent marker that was observed as an independent predictor of COVID-19 severity and therapy response [63]. According to several studies, it was concluded that the CD8+ T cell response and CD4+ T cell response are different in the case of mild and severe disease respectively [64].
The majority of recovered patients’ CD4+ and CD8+ T cells produce a substantial number of antiviral immune responses against spike proteins, which clearly shows the importance of T lymphocytes in viral clearance and recovery [65]. In COVID-19 patients, Grifoni et al. [66] assessed CD4+ and CD8+ T cells’ responses to SARS-CoV-2. All cases showed SARS-CoV-2-specific CD4+ T cell and antibody responses, and the majority also showed SARS-CoV-2-specific CD8+ T cell responses. A significant finding was the presence of pre-existing SARS-CoV-2-cross-reactive T-cell responses in healthy donors, suggesting the possibility of pre-existing immunity in the general population [66, 67]. Hence, they were able to positively correlate the T-cell responses with the antigen titers. A pathogenic synergy of T cell-associated bystander effects leads to aberrant or overactive immune responses in SARS-CoV-2 infected patients which further leads to significant tissue damage [68, 69]. With regard to understanding the potential involvement of the immune system in the disease, these data together give light on the potential differences in T-cell responses as a function of disease severity.
Excessive immune response against SARS-CoV-2 is the primary reason responsible for COVID-19-induced ARDS. Therefore, inhibiting the inflammatory response caused by a dysregulated immune response may be an effective therapy for COVID-19-induced ARDS prevention. We discussed below some of the immunotherapeutic strategies that are recently being employed for COVID-19 treatment options along with some general antiviral treatments (Figure 3).
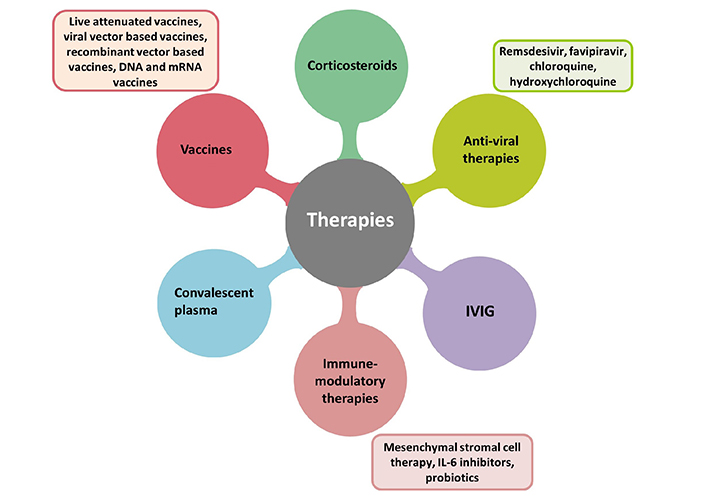
Therapies adopted for treatment and management of COVID-19-induced ARDS. mRNA: messenger ribonucleic acid; IVIG: intravenous immunoglobulin
Various already existing antiviral therapies have been utilized for the management of COVID-19. Some of these therapies exhibited fruitful results in decreasing the pathogenesis of COVID-19 and thus can be implemented for the management of COVID-19-induced ARDS.
Remdesivir is the first Food and Drug Administration (FDA)-approved drug for COVID-19 hospitalized patients. It is an adenosine analog that acts as an inhibitor of viral RNA polymerase and thereby, disrupts viral replication. Both in vivo and in vitro, remdesivir has been reported to suppress MERS-CoV and SARS-CoV [70, 71]. In a recent study, remdesivir was demonstrated to be highly efficient against SARS-CoV-2 infection in vitro [72]. However, in a randomized clinical trial (RCT), Wang et al. [73] showed that remdesivir did not exhibit any significant clinical benefits in hospitalized people. Contrastingly, the final results of another RCT concluded that remdesivir is superior to placebo and speeds up recovery in patients with lower respiratory tract infections who were hospitalized with COVID-19 [74].
Favipiravir is a viral RNA polymerase inhibitor, which has exhibited a crucial impact against influenza A and B [75]. Recent studies show that favipiravir significantly improved viral clearance and pulmonary CT scan outcomes in COVID-19 patients [76]. Takahashi et al. [77] suggested that favipiravir may help improve respiratory function even in serious or critical circumstances by preventing the progression of pneumonia and the generation of cytokines.
Chloroquine (Clq) and its hydroxylated version, hydroxy Clq (HClq) are two antimalarial medications that interfere with ACE2 binding, which prevents viral entrance. They also alter the pH of endosomes and lysosomes, which can prevent viral fusion with host cells [78]. Additionally, these drugs prevent the release of cytokines that promote inflammation. Clq has been particularly demonstrated to reduce lung damage induced by influenza A H5N1 virus in preclinical animal models [79] and SARS-CoV-2 infection in vitro [72]. The addition of Clq/HClq to routine therapy for patients hospitalized with severe COVID-19, on the other hand, dramatically exacerbated their clinical state in an RCT. According to the findings of this study, people who have a more serious case of COVID-19 pneumonia shouldn’t use Clq/HClq [80]. Another controlled trial reported that Clq/HClq does not add anything to the therapy of COVID-19 patients [81]. Since the HClq trial failed to show any benefit, the National Institute of Health and WHO dropped the trial for hospitalized COVID-19 patients [81].
Since the immune system is the major player in the pathogenesis of SARS-CoV-2, immunomodulatory therapies become game changers in the management of COVID-19-induced ARDS.
In cases of SARS-CoV-2-induced cases of cytokine storm, corticosteroids with anti-inflammatory, antifibrotic, and vasoconstrictive actions have been explored for the potential improvement of lung damage caused by inflammation in patients with ARDS [82]. The duration of corticosteroid therapy is a very important parameter and should be considered. The efficacy of corticosteroid therapy in critically ill COVID-19 patients was assessed in several clinical studies. A clinical trial that assessed the effectiveness of intravenous dexamethasone administration in COVID-19 patients found that it increased days alive and free of mechanical ventilation over 28 days by a statistically significant amount compared to standard treatment alone [83]. Villar et al. [84] found in a multicentre, randomized controlled study that early dexamethasone medication might reduce overall deaths and ventilator time in patients with established moderate-to-severe ARDS. According to some preliminary trial results, methylprednisolone and dexamethasone can be used for the severe form of COVID-19 [85]. In a different study, it was discovered that methylprednisolone performed better than dexamethasone in treating COVID-19 hypoxic patients [86]. Currently, several phase 2/3 clinical trials are looking into the efficacy and safety of methylprednisolone in patients with COVID-19 ARDS. These studies should hopefully shed further light on the role of steroids in these patients.
Nearly all COVID-19 patients who have recovered have convalescent plasma from their blood, which contains neutralizing antibodies against viral proteins [87]. In light of this, investigating the safety and effectiveness of convalescent plasma treatment in COVID-19 patients may be advantageous [88]. Hoffmann et al. [89] showed that SARS-CoV serum from convalescent patients conferred defense against SARS-CoV-2 infection, and this approach may be successful if employed preventatively. In a study by Shen et al. [90], convalescent plasma was used to treat 5 critically ill patients with laboratory-confirmed COVID-19 and ARDS. This resulted in a rapid reduction in viral load and improved patient outcomes. Furthermore, according to Allahyari et al. [91], early convalescent plasma delivery can aid in easing the symptoms of severely ill COVID-19 patients who are experiencing mild to moderate ARDS and are in danger of deteriorating into a critical condition.
Mesenchymal stromal cells (MSCs) have demonstrated immunoregulatory abilities that can reduce inflammatory responses. According to a study by Chan et al. [92], MSC treatment has favorable impacts on acute lung damage caused by H5N1 and may be helpful for patients who have severe pulmonary infections brought on by influenza viruses like H5N1 and H7N9. The results of a trial conducted by Lanzoni et al. [93] on the safety and effectiveness of allogeneic umbilical cord-derived MSC (UC-MSC) infusions in patients with ARDS associated with COVID-19 proved the safety of UC-MSC infusions for COVID-19 patients with ARDS and that when compared to controls, UC-MSC therapy decreased mortality and recovery time. Another double-blind, placebo-RCT found that administering UC-MSC accelerated the recovery of lung lesions and enhanced the function of the integrated reserve [94]. In preclinical ARDS model investigations, MSCs were proven to be efficient and safe, and the outcomes of the COVID-19 clinical trials revealed their potential effectiveness. However, more extensive studies are required to confirm MSC’s efficacy, particularly in ARDS patients who have been diagnosed with COVID-19.
It has been discovered in the pathogenesis of COVID-19 pneumonia, that there is a cytokine release syndrome with significant proinflammatory cytokine secretion, including IL-6, IL-1, and TNF-α [95]. It has been demonstrated that the immunomodulatory medication thalidomide, which acts to increase apoptosis, has positive benefits in experimental bacterial- and viral-induced ARDS. It is a potent inhibitor of IL-6 and enhances T-cell responses [96]. A study of 21 patients with SARS-CoV-2-induced ARDS showed that tocilizumab (TCZ), an IL-6 receptor antagonist improved lung opacity and lung oxygenation and decreased the number of white blood cells [97]. A single center-based study with 100 patients in Italy observed improvements in their clinical outcomes in more than three-quarters of patients after evaluating the efficacy of intravenous administration of TCZ in the treatment of severe COVID-19 pneumonia and ARDS patients [98, 99]. Pinzon et al. [100] evaluated the data about the efficacy of IL-6 inhibitors in the treatment of COVID-19 by conducting a systematic review and meta-analysis. IL-6 medications have demonstrated efficacy in decreasing mortality in COVID-19 patients, especially in those with severe illness.
The exact mechanisms of action of IVIG in immune-mediated diseases are still not fully understood, but IVIG may interact with immune cell receptors, pathogenic proteins, or other proteins that are linked to inflammation, thus acting on the host’s hyperimmune reactions [101]. A double-blind RCT demonstrated that the administration of IVIG to patients with severe COVID-19 infection who did not respond to initial therapy massively improved their clinical outcome [102]. In another study, early IVIG treatment reduced mortality and shorter ventilator time in patients with COVID-19-related ARDS [103]. Monoclonal antibodies targeting the spike protein are also preferred for immunotherapy to prevent the attachment and entry of SARS-CoV-2. The combination of numerous monoclonal antibodies that each recognise a different epitope on the viral surface, called a monoclonal antibody mixture, may help neutralise the virus. Several studies have shown that treatment with monoclonal antibodies can reduce the viral load [104].
Multiple vaccines have recently been developed and nearly every country is currently immunizing its population to tackle the COVID-19 pandemic. Even though several COVID-19 vaccines have been developed, more potent vaccines are still required to meet the demand on a global scale. Intranasal COVID-19 vaccines are also now being produced, and they have demonstrated a promising capacity to elicit a considerable amount of antibody-mediated immune response, robust cell-mediated immunity, and the additional capacity to elicit protective mucosal immunity. COVID-19 vaccines can be classified into vaccines based on attenuated SARS-CoV-2 virus, viral vector vaccines, nucleic acid-based vaccines, and vaccines based on subunit particles. At the forefront of vaccine development right now there are many COVID-19 vaccine candidates based on the original Wuhan-Hu-1 strain. Vaccines including NVX-CoV2373 (∼96%), BNT162b2 (∼95%), mRNA-1273 (∼94%), Sputnik V (∼92%), AZD1222 (∼81%), BBIBP-CorV (∼79%), Covaxin (∼78%), Ad26.CoV.S (∼66%), and CoronaVac (∼51%) have shown efficacy over 50% against symptomatic COVID-19 patients. Promising COVID-19 vaccines with various modes of action have shown good safety and clinical efficacy profiles in clinical trials (Table 1).
Vaccines for treatment of COVID-19
| Developer | Name of vaccine | Type of vaccine | Target antigen |
|---|---|---|---|
| Sinopharm | BBIBP-CorV | Inactivated virus | Whole virus |
| Sinovac Biotech | CoronaVac | Inactivated virus | Whole virus |
| Bharat Biotech | Covaxin | Inactivated virus | Whole virus |
| University of Oxford and AstraZeneca | AZD1222 | Chimpanzee adenoviral vector | S protein |
| Janssen and Johnson & Johnson | Ad26.CoV2.S | Human adenoviral vector | S protein |
| Gamaleya | Sputnik V | Human adenoviral vectors (two serotypes) | S protein |
| Moderna and NIAD | mRNA-1273 | mRNA | S protein |
| Pfizer and BioNTech | BNT162b2 | mRNA | S protein |
| Novavax and CEPI | NVX-CoV-2373 | Protein subunit | S protein |
| Bharat Biotech | BBV154 (1st intranasal vaccine) | Recombinant adenoviral vector | S protein |
Growing evidence links the pathophysiology of severe COVID-19 respiratory failure to abnormal coagulation, most especially, pulmonary microvascular thrombosis [105]. Other studies are being conducted to see if nebulized heparin can lower mortality in severe COVID-19 patients.
Probiotic strains have gained more and more recognition as a potent ally in the fight against and prevention of respiratory tract infections in recent times. It has been demonstrated that treatment with probiotic bacteria affects the production of inflammatory cytokines in the lungs, which has been associated with COVID-19 lethality [106]. According to a controlled trial, probiotics may lower the incidence of ventilator-associated pneumonia (VAP) and reduce the duration of antibiotic use for VAP [107]. Numerous preclinical research on probiotics has thus far concentrated on influenza and pneumonia, showing advantages from oral or nasal administration of probiotics, which increased survival, decreased weight loss, decreased viral loads in the lung, and minimized bronchial epithelial damage [108–110]. The best evidence supporting the effectiveness of probiotics was discovered for five disorders, including acute respiratory tract infections, according to a meta-analysis of 52 published studies that looked into the ability of probiotics to prevent or treat a variety of pathological conditions [111]. Another recent study showed that a daily oral diet with Lacticaseibacillus rhamnosus for a short time modulates the inflammatory response in a murine model of acute lung inflammation [112]. Raheem et al. [113] have reported the preventative effect of Lactobacillus casei on ALI induced by lipopolysaccharide. The beneficial role of probiotics has been linked to many processes, including their capacity to support intestinal integrity and maintain intestinal permeability, competition with pathogens for nutrients and attachment sites, regulation of immune cell activity against invading pathogens, and the prevention of excessive immune responses and inflammation. There are a quite number of clinical trials which have been conducted or are currently being conducted to decipher the therapeutic role of probiotic supplementation in COVID-19. Probiotics are also being exploited to ameliorate the inflammatory axis in COVID-19-induced ARDS. Although the outcomes of these investigations are encouraging, more thorough research must be done to reach firm conclusions.
Depending on the vaccination rate over a certain period, social acceptability or resistance, and insufficient social distance, the virus can persist in a population for too long before it mutates, even in the presence of effective vaccines. Whilst the administration of the SARS-CoV-2 vaccine generally cannot completely prevent infection, it does offer immunization to lessen severe illness and ARDS [114, 115]. Therefore, it is pertinent and important to find cures that, alone or as adjuvant therapies, alleviate this severe lung disease.
Omega-3 polyunsaturated fatty acids (PUFAs), in particular, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), have recently been discovered to support the immune system to fight inflammation, promote the generation of pre-resolving mediators, and regulate platelet aggregation and thrombosis [116]. It has been discovered that fatty acids (FAs) cause B cells to respond and develop into plasma B-cells that produce IgA or IgG. To combat pathogens, PUFAs also encourage the production of effector T-cells like Th1 and Th17 during infection. Because of this, n-3 PUFAs have attracted a lot of attention as possible treatments for both the prevention and treatment of inflammatory diseases [24]. Currently, some researchers suggest using n-3 PUFAs as immunomodulatory and antiviral medications to treat COVID-19, its consequences, and its progression, including ARDS [117, 118].
COVID-19 is a severe respiratory illness brought on by the SARS-CoV-2 virus. In severe cases, ARDS can lead to lung damage and hasten patient deaths. This review examines the potential involvement of the immune system in SARS-CoV-2-causing ARDS to lessen COVID-19 mortality. Severely ill patients suffering from ALI or ARDS are at an increased risk of complications. Clinically severe hypoxia, pulmonary edema, decreased lung compliance, reduced residual capacity, and pulmonary infiltrations are all its defining signs. Other causes of ARDS include the SARS-CoV-2-driven dysregulation of immunological responses, including cytokine storm, macrophage activation syndrome, and lymphopenia. All these observed discrepancies in clinical symptoms depend on the patient’s age, virus load, and extent of hyperactivity of the immune system. Future research and attention are still needed in various areas related to SARS-CoV-2 and ARDS.
Multiple potential drug therapies are showing promise in the treatment of ARDS, including anticoagulant medications, immune response modifiers, specific inflammatory pathway blockers, modulators of epithelial and channel function, endothelial and vascular dysfunction treatments, and therapies that help in the resolution of ARDS. It is hoped that substantial global research into potential therapies for severe COVID-19 patients will aid in the rapid identification of effective treatments for ARDS as well.
ACE2: angiotensin-converting enzyme 2
ALI: acute lung injury
AMs: alveolar macrophages
ARDS: acute respiratory distress syndrome
Clq: chloroquine
COVID-19: coronavirus disease-2019
DAMPs: damage-associated molecular patterns
DCs: dendritic cells
HClq: hydroxychloroquine
IFN: interferon
IgG: immunoglobulin G
IL: interleukin
ILCs: innate lymphoid cells
IVIG: intravenous immunoglobulin
MERS-CoV: Middle East respiratory syndrome-coronavirus
mRNA: messenger ribonucleic acid
MSCs: mesenchymal stromal cells
NET: neutrophil extracellular trap
NK: natural killer
PAMPs: pathogen-associated molecular patterns
PUFAs: polyunsaturated fatty acids
RCT: randomized clinical trial
RMs: recruitment maneuvers
SARS-CoV-2: severe respiratory disease syndrome-coronavirus-2
Th: T helper
TNF: tumor necrosis factor
UC-MSC: umbilical cord-derived mesenchymal stromal cell
WHO: World Health Organization
SD, TS, AB, and RKS acknowledge the Department of Biotechnology, AIIMS, New Delhi, India for providing infrastructural facilities. AB thanks ICMR for the research fellowship. Figures are created with the help of Servier Medical Art, provided by Servier, licensed under a Creative Commons Attribution 3.0 unported license (https://smart.servier.com).
SD and TS equally contributed to: Writing—original draft. AB: Writing—review & editing. RKS: Conceptualization, Writing—review & editing, Supervision. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was financially supported by projects: DBT [
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 8706
Download: 71
Times Cited: 0
Nitin Saksena ... Thyago H. Cardoso
Naima G. Cortes-Perez
Junming Chen ... Jianshe Yang
Hana Ratnawati ... Steven Felim
