Affiliation:
Centro de Biotecnologia e Química Fina (CBQF), Escola Superior de Biotecnologia, Universidade Católica Portuguesa, 4169-005 Porto, Portugal
Email: csfoliveira@ucp.pt
ORCID: https://orcid.org/0000-0002-0113-9528
Affiliation:
Centro de Biotecnologia e Química Fina (CBQF), Escola Superior de Biotecnologia, Universidade Católica Portuguesa, 4169-005 Porto, Portugal
ORCID: https://orcid.org/0000-0002-6273-6668
Explor Immunol. 2023;3:233–254 DOI: https://doi.org/10.37349/ei.2023.00100
Received: December 02, 2022 Accepted: March 17, 2023 Published: June 30, 2023
Academic Editor: Sunil K. Arora, Postgraduate Institute of Medical Education & Research, India
Besides trauma, several pathological conditions which directly affect the normal functioning of organs, require new therapeutic strategies to repair damaged or diseased tissues. Tissue regeneration is a complex and spatiotemporal process involving a plethora of cell types, including various immune cells and stem cells in a synchronized relationship. However, individual parameters, namely ageing, obesity, diabetes, and chronic conditions, have been intrinsically correlated with poor regenerative properties of adult tissues. While vast progress has been made regarding stem cell-based therapy to direct self-healing, the immune response is still the Achilles’ heel of such strategies. Whereas the role of effector immune cells has been well defined along the regenerative process, an understanding of the behavior of the main adult stem cells, namely mesenchymal stem cells (MSCs) and hematopoietic stem and progenitor cells (HSPCs), along the different phases of the regenerative process could clarify how these stem cells can be used to positively influence the immune response. In this scope, this review highlights the main interactions between these stem cells and immune cells during tissue repair, exploring the most important regenerative properties of stem cells and correlating them with the modulation of the immune response during tissue regeneration. Furthermore, the utmost strategies used to explore how the behavior and stem cell fate are affected by specific microenvironments and/or stimuli usually found during a regenerative process, are emphasized. This clarification may provide critical insight into the molecular mechanisms by which stem cells modulate the immune response in a positive feedback loop toward tissue repair.
The number of human disorders which require the repair of damaged or diseased tissues has reached global proportions [1]. Besides, individual parameters, namely ageing, obesity, diabetes, and chronic conditions have been intrinsically correlated with the lack of effective healing and poor regenerative properties of certain adult tissues [2]. To overcome such limitations, the use of stem cells has emerged as a potential alternative to improve self-healing and tissue repair. In fact, stem cell-based therapy is an important branch of regenerative medicine with the goal of enhancing the body repair machinery via stimulation, modulation, regulation, and differentiation of certain stem cells pool toward tissue regeneration [3].
Remarkably, the discovery of adult stem and progenitor cells, namely mesenchymal stem cells (MSCs) and hematopoietic stem and progenitor cells (HSPCs), residing in a multitude of human tissues and organs, such as bone marrow (BM), adipose tissue, dental pulp, and umbilical cord (UC), or circulating in the peripheral blood (PB) has supported the development of several approaches for using these cells or their products in tissue regeneration strategies [4]. Since then, the use of adult stem cells is becoming pivotal for regenerative therapies, wherein they either serve as integrated participants in the target tissue or as a platform to deliver molecular signals during the regenerative process [5]. Therefore, the main approach of stem cell-based therapy is not only to explore the self-renewal and differentiation potential as unique properties of stem cells but also their secretory potential to regenerate damaged tissues [5, 6].
Although several regenerative properties have been demonstrated by these stem cells, many challenges have still hampered their clinical applications due to the controversial immune response reported by several in vitro and in vivo studies [7, 8]. In this sense, an in-depth understanding of the interplay between MSCs, HSPCs, and immune system is of paramount importance to elucidate their reciprocal interactions during tissue healing and regeneration.
The interactions between MSCs, HSPCs, and immune cells are essential not only to maintain and restore tissue homeostasis but also to balance the inflammatory response during tissue regeneration [9]. While MSCs exert a variety of immune-regulatory functions on cells of the adaptative and innate immunity response [10, 11], hematopoietic stem cells (HSCs) give rise to all cells of the myeloid and lymphoid lineage, such as platelets, neutrophils, monocytes, macrophages, lymphocytes, dendritic cells (DCs), and natural killer (NK) cells, key players of the immune response [12]. Given the crucial duties of these stem cells in maintaining tissue integrity and driving tissue regeneration under several conditions, it is not surprising that immune cells are considered as critical components of the stem cell niches and prominent effectors of stem cells’ functions and activities [9]. In fact, tissues contain resident and circulating immune cells of the innate immune system, namely macrophages and DCs, as well as adaptative immune T cells, which together preserve tissue integrity and functions during homeostasis, infection, and trauma [13]. Therefore, immune cells exert critical influence over the processes of inflammation and repair in their clearance of cellular debris, along with their effects on the mobilization/recruitment, proliferation, activation, and differentiation of tissue specific stem and progenitor cells, including MSCs and HSPCs (Figure 1).
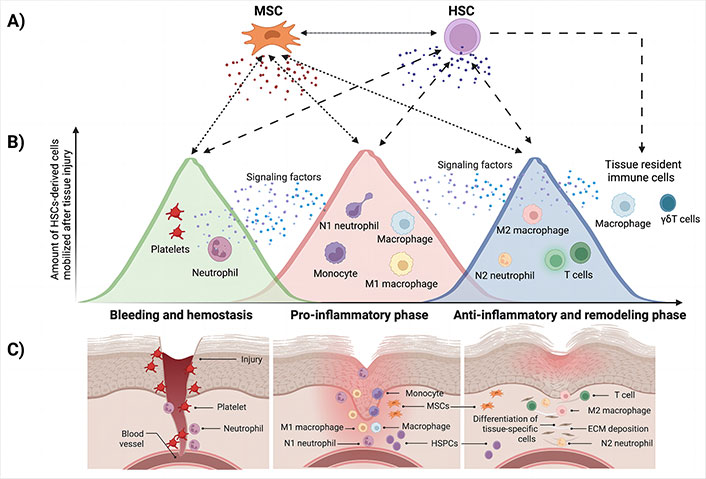
Overview of the critical interactions between adult stem cells, namely MSC and HSC, and effector immune cells during tissue repair. A) MSC has the potential to differentiate into specific-tissue cells, while also regulating the biological functions of HSC. MSC interacts with many effector immune cells in all phases of the regenerative process through cell-to-cell contact or by paracrine signaling. In a process called hematopoiesis, HSC gives rise to all cells of the myeloid and lymphoid lineage, (e.g., platelets, eosinophils, basophils, neutrophils, monocytes, macrophages, T cells, B cells, DCs, NK cells, and others), key players of the immune response required for tissue regeneration. HSC also regulates the functions of MSC; B) the graph summarizes the number of immune cells mobilized after tissue injury per phase of the regenerative process; C) representation of the different phases of the regenerative process highlighting the presence of stem cells and effector immune cells involved with tissue repair and remodeling. ECM: extracellular matrix
In this sense, the benefits of the crosstalk between stem cells and immune cells can be used to improve the efficacy of tissue repair and remodeling [14]. However, a deeper understanding of the complex stem cell behavior/function vs. immune response per phase of the regenerative process needs to be addressed.
Within this scope, the main interactions between these adult stem cells and immune cells during tissue repair, exploring the most important regenerative properties of stem cells and correlating them with the modulation and regulation of the immune response during tissue regeneration, are highlighted. Additionally, the utmost strategies used to explore how the behavior and stem cell fate are affected by specific microenvironments and/or stimuli usually found during a regenerative process, are delineated. Finally, some perspectives needing more investigation to further enhance the safety and effectiveness of stem cell-based therapies to positively modulate the immune response toward tissue repair, are also evidenced.
It is becoming increasingly apparent that the modulation of the immune response has emerged as an attractive approach for regenerative medicine strategies. In fact, the use of stem cells has been intensively investigated with promising results [8, 10, 15]. However, an in-depth understanding of the critical interactions between adult stem cells, namely MSCs and HSPCs, and immune cells during each phase of the regenerative process is still a critical barrier to overcome (Figure 1A).
Surprisingly, the immune system can have both positive and negative regulation during tissue repair, resulting in effective tissue regeneration or fibrosis and scarring, respectively [14]. In this sense, the immune system plays a pivotal role in tissue healing and regeneration which is mediated by several interactions between tissue-specific cell populations, resident/recruited/or even implanted stem cells, and immune cells at the injury site [16]. In general, after a tissue injury or damage, the normal tissue repair process usually follows the phases:
(i) hemostasis,
(ii) pro-inflammatory phase,
(iii) anti-inflammatory phase, and
(iv) tissue repair and remodeling.
While the successful resolution of each of these phases ensures an optimal tissue healing, failures during the way can impair effective regeneration and lead to fibrosis and potential organ failure [16]. Of note, the crosstalk between stem cells and immune cells, particularly during the pro- and anti-inflammatory phases can be used to improve the efficacy of tissue repair and remodeling [14].
Succinctly, after a tissue injury, a rapid local response is induced by tissue resident cells, namely macrophages and γδT cells which trigger the mobilization of other immune cells [16]. This starts the first phase of the regenerative process, called hemostatic phase. During this phase, platelets secrete multiple growth factors and chemokines, namely platelet-derived growth factor (PDGF), platelet factor 4 (PF4), epidermal growth factor (EGF), transforming growth factor-β (TGF-β), and CXC-chemokine ligand (CXCL) 4 [17]. These platelet-derived mediators recruit inflammatory cells, such as neutrophils and monocytes (Figure 1B and C).
However, neutrophils are the first circulating immune cells recruited to the site of injury, promoting inflammation and the recruitment of monocytes and macrophages. At this step, a pro-inflammatory phase triggers a complex inflammatory response characterized by the recruitment, proliferation, and activation of a variety of hematopoietic and non-hematopoietic cells, including neutrophils, macrophages, lymphoid cells, NK cells, B cells, T cells, fibroblasts, endothelial cells, and stem cells, which together make up the cellular response that orchestrates tissue repair [17].
Firstly, the pro-inflammatory phase is characterized by neutrophil influx, monocyte, and macrophage infiltration followed by their subsequent differentiation and activation, at the damaged environment of tissue [2]. Signals from these innate cells recruit lymphocytes to the site of injury, which influence further innate immune responses. Mostly, this phase resolves naturally and it is marked primarily by neutrophils’ and macrophages’ transition from pro-inflammatory type-1 phenotype (N1, M1) to a pro-regenerative type-2 phenotype (N2, M2), respectively [18]. At this phase, MSCs play a key central role, being used to drive an effective resolution of the inflammatory phase mediated by the macrophage transition to a pro-regenerative population [19]. Although the detailed mechanisms of MSC-macrophage polarization during tissue repair are still under intensive investigation, its critical role in the repair and regeneration process has highlighted the importance of MSC in the design of new stem cell-based regenerative therapies [8, 20, 21]. Despite the classical interplay between stem cells and immune cells, the involvement of the complement system, particularly during the inflammatory phase, has also been addressed. In this sense, there is a strong evidence that during inflammation, the activation of the complement system contributes to tissue regeneration at several levels ranging from chemo-attraction of stem and progenitor cells, increase of the survival of various immune cells in the presence of split products of complement, and production of trophic factors by cells activated by the anaphylatoxins C3a and C5a [22]. For instance, C3a and C5a seem to play a crucial role in MSC-dependent tissue repair by recruiting MSC to injured tissue, stimulating its differentiation potential, and increasing its survival and secretion of trophic, angiogenic, and anti-inflammatory factors [22, 23]. Despite the complement system activation and its interplay between adult stem cells during tissue repair being described, the molecular mechanisms behind these processes, need further investigation.
The subsequent repair phases, anti-inflammatory and remodeling phases, are characterized by angiogenesis, synthesis of collagen, and deposition of ECM [16]. These events support the maturation of the newly formed vessels and the remodeling of tissue [16]. Immune cells exert critical influence over the processes of remodeling and regeneration in their clearance of cellular debris, along with their effects on the mobilization/recruitment, proliferation, activation, and differentiation of tissue-specific stem and progenitor cells, including MSCs and HSCs. On the other hand, while MSCs exert a variety of immune-regulatory functions on cells of the immune system, including cells involved with both adaptative and innate immunity [10, 11], HSCs give rise to all cells of the myeloid lineage, such as erythrocytes, platelets, eosinophils, basophils, neutrophils, monocytes, macrophages, and mast cells, as well as all the lymphoid lineage cells, including T cells, B cells, and NK cells [12].
Overall, MSCs regulate the proliferation and function of effector T cells with concomitant induction of their apoptosis [7, 15], while enhancing the generation of regulatory T cells [24, 25]. These stem cells also inhibit the activation and proliferation of B cells [26], while suppressing the differentiation, maturation, and antigen presentation of DCs [8]. Furthermore, MSCs inhibit the proliferation and cytotoxicity of NK cells [27], regulate the switch from pro-inflammatory M1 to anti-inflammatory M2 macrophages [28], while also having many effects on neutrophils such as the suppression of nitric oxide (NO) secretion, inhibition of apoptosis, reduction of their infiltration, and stimulate the conversion of pro-inflammatory neutrophils to anti-inflammatory phenotype [10, 19]. Besides the capacity of MSCs to differentiate into specific-tissue cells, these stem cells also exert key-regulatory effects in the activity, self-renewal, and differentiation of HSCs [29].
At the same time, HSCs not only stimulated the proliferation of MSCs but also stimulate their tri-lineage differentiation potential, suggesting the existence of stemness signals that are also transduced from HSCs to MSCs [30]. Furthermore, HSCs originate several multipotent progenitors (MPPs) and effector immune cells directly involved in all phases of the regenerative process.
Although it has been possible to highlight the relative amount of each cell type, i.e., resident, and recruited/mobilized cells per phase in a chronological sequence of the regenerative process (Figure 1B), a detailed understanding of their individual contributions to healing and their interactions with other cells, particularly stem and immune cells, is urgently required. Taking into consideration stem cell-based therapies for tissue regeneration, this clarification is still critical to provide insight into the molecular mechanisms that modulate the immune response in a positive feedback loop.
MSCs are undifferentiated cells mostly present in the BM, adipose tissue, dental pulp, UC, and placenta tissue [31, 32]. According to the International Society for Cellular Therapy, isolated and purified MSCs have the following characteristics:
(i) Adhere to plastic under standard culture conditions;
(ii) Present cluster of differentiation (CD) 90, CD105, and CD73 cell surface markers, and lack hematopoietic and endothelial cell markers, namely CD34, CD45, CD14, and CD31 [32–34]; and
(iii) Have the capacity to differentiate into adipocytes, osteocytes, and chondrocytes under specific in vitro stimulation [33, 35]. Beyond its well-known tri-lineage capacity, additional in vitro conditions promote smooth and striated muscle, cardiac, liver, and nerve cells [31, 34].
For a long time, MSCs have been widely used in stem cell-based therapies due to their great potential in repairing damaged tissues and organs compromised by several reasons, ranging from acute causes and trauma to chronic or degenerative conditions [5, 28, 31, 35–37]. Generally, this kind of stem cell may contribute to the regeneration of injured tissues through at least three main mechanisms:
(i) Capacity to differentiate into specific-tissue cells.
Several studies have shown that MSCs derived from different sources can be induced to differentiate into a variety of cells, including osteo-lineage cells, chondrocytes, adipocytes, hepatocyte-like cells, cardiomyocytes, muscle cells, and neuronal cells [31, 33, 35].
(ii) Secretion of key factors that promote tissue healing and regeneration.
Both in vitro and in vivo studies, MSCs can sense the microenvironment and secrete biomolecules according to the needs of the damaged tissue [35, 38–40]. These molecules include: 1) growth factors: hepatocyte growth factor (HGF), fibroblast growth factor (FGF), vascular endothelial growth factor (VEGF); 2) chemokines: CXC-chemokine receptor 3 (CXCR3), CC-chemokine receptor 5 (CCR5) ligands, CXCL9, CXCL10, CXCL11, CXCL12; 3) anti-inflammatory cytokines: interleukin (IL)-4, IL-6, IL-10; 4) immunosuppressive factors: indoleamine 2,3-dioxygenase (IDO), prostaglandin E2 (PGE-2), cyclooxygenase 2 (COX-2), TGF-β; and 5) extracellular vesicles (EVs) and exosomes: small membrane vesicles (ranging from 50 to 1,000 nm) derived from multi-vesicular bodies or from the plasma membrane. These vesicles are enriched with proteins, lipids, and nucleic acids, commonly used for intercellular communication including immune modulation [2, 11, 34, 41, 42].
(iii) Modulation of the immune response.
MSCs have been considered one of the main actors in the regulation of both innate and adaptive immune response [37]. Depending on the context, they can have an immunosuppressive or immune-stimulating capacity mediated by the secretion of soluble factors [43]. However, the generation of a pro-regenerative microenvironment through the paracrine secretion of trophic factors is now the most explored therapeutic mechanism associated with the immunomodulation property of MSCs [38, 40].
Succinctly, MSCs interfere with the maturation and the antigen-presenting function cells (APCs), inhibiting T cell proliferation and decreasing CD8+ T cells cytotoxicity [35]. Additionally, MSCs reduce the presence of pro-inflammatory neutrophils (N1 type) and stimulate their conversion to an anti-inflammatory phenotype (N2 type) [10, 44]. Similarly, MSCs promote the modulation of macrophage polarization from a pro-inflammatory phenotype (M1) to a pro-regenerative phenotype (M2) [20, 21]. The elevated secretion of IL-10 by MSCs is also responsible for the block of neutrophils recruitment and influx of these cells to the damaged tissue [28], playing a critical role in the resolution of the inflammatory phase.
In summary, MSCs can influence the activation, maturation, proliferation, polarization, and function of immune cells, namely neutrophils, monocytes, macrophages, DCs, NK cells, T-lymphocytes, and B-cells [2, 10, 19, 34, 43].
Many studies have demonstrated that the regenerative ability of MSCs is not intrinsically determined but is also stimulated and regulated by several components from the local microenvironment, namely cells, signals (i.e., growth factors, cytokines, chemokines, EVs), and physical conditions [6, 39, 40, 45]. In fact, to mimic the complexity of the in vivo microenvironment during the different steps of the tissue regeneration process, several in vitro approaches have been designed to explore how MSCs respond to specific environments and different stimuli. The main strategies with physiological relevance are then subdivided as follows:
The interactions between MSCs and immune cells are essential not only to maintain and restore tissue homeostasis but also to balance the inflammatory response during tissue regeneration. Within this scope, the crosstalk between MSCs and immune cells, namely neutrophils and macrophages, has been intensively investigated to understand how these interactions can be used to positively modulate and regulate the tissue regeneration process [10, 37, 46].
Neutrophils are usually the first inflammatory cells recruited at a site of injury, enhancing host defenses while removing contaminants [2, 16]. The existence of different subsets of neutrophils was first characterized according to their polarization states in the tumor microenvironment, namely N1 (pro-inflammatory subtype) and N2 (anti-inflammatory subtype) [47]. Whereas the N1 subtype has potent anti-tumor activity due to its capacity to release pro-inflammatory factors, namely IL-12, tumor necrosis factor-α (TNF-α), chemokine ligand 3 (CCL3, also known as macrophage inflammatory protein 1-alpha), and CXCL9, which facilitates recruitment and activation of CD8+ T cells, the N2 subtype has strong immunosuppressive and tumor-promoting activity characterized by secretion of oncostatin M, reactive oxygen species (ROS), reactive nitrogen species (RNS), matrix metalloproteinases (MMPs), and neutrophils elastase [48]. Despite the main properties of the N1/N2 subtypes being demonstrated in tumor microenvironments, their exact role, behavior, activation, recruitment, and temporal polarization during tissue repair are under investigation. Considering the importance of such cells, it has been demonstrated that MSCs are able to modulate the phenotype and activity of neutrophils in all phases of tissue repair and regeneration [44]. A co-culture of MSC-neutrophil showed that MSCs enhance the phagocytic ability of neutrophils, which contributes to the efficient removal of necrotic tissue and cellular debris [10]. Moreover, MSCs, via up-regulation of the extracellular superoxide dismutase (SOD3), prevent neutrophil death and enhance neutrophil-dependent elimination of microbial pathogens and cellular remnants. Furthermore, during the resolution phase of tissue repair, MSCs reduce the presence of N1 type neutrophils and stimulate their conversion to an N2 phenotype [10, 44]. In fact, the co-culture of MSC-neutrophil showed that MSCs secrete TNF-α stimulated gene/protein 6 (TSG-6), a key molecule correlated with the decrease of acute inflammation [46]. Notably, the reduction of ROS and the increased expression/secretion of IL-10 by neutrophils, favoured their polarization to an N2-like phenotype [46].
Compared to other immune cells, the crosstalk between MSCs and macrophages has become an attractive alternative for immunomodulation strategies [20, 40, 49, 50]. Similar to the N1/N2-like neutrophil paradigm, recent interest has grown around the concept of an M1/M2-like macrophage spectrum of polarization states during tissue regeneration. While M1 macrophages secrete monocyte chemoattractant protein-1 (MCP-1), MMP-12, NO, inflammatory cytokines (e.g., TNF-α, IL-1β, IL-12), and various other inflammatory chemokines, which stimulate the recruitment of circulating immune/stem cells to the site of injury [51], M2 macrophages, through the production of numerous growth factors, including PDGF, TGF-β1, insulin growth factor (IGF)-1, and VEGF-α, promote stem cells recruitment, neo-angiogenesis, deposition of collagen and ECM proteins [52]. Additionally, in case of severe injury, M2 phenotype also stimulates the activation and differentiation of tissue resident stem and progenitor cells [18, 51]. In this scope, different macrophage phenotypes play distinct roles at different stages of tissue repair and regeneration. Remarkably, MSCs support the phagocytic functions of M1 macrophages in the initial phases of tissue repair, while, in the last phases, promoting the expansion of M2 macrophages [2, 53].
For example, successful bone fracture healing is based on carefully coordinated crosstalk between macrophages and bone-forming cells, more specifically MSCs [52]. In particular, the key role that macrophages play in the recruitment, regulation, and differentiation of MSCs as well as the influence of MSCs in the macrophage function, activity, and polarization during bone tissue regeneration have been brought to focus. To clarify this, Zhang et al. [50] co-cultured MSCs with M0 (naive), M1, and M2 macrophages and measured alkaline phosphatase (ALP) activity at 7, 14, and 28 days. The results revealed that M1 and M2 macrophages were able to promote osteogenic differentiation at 7 and 28 days, respectively. Interestingly, the co-culture of MSCs with M2 macrophages, but not with M1 or M0 macrophages, resulted in a significant increase of MSC mineralization caused by secretion of soluble factors. Moreover, M2 macrophages also promoted proliferation and osteogenic differentiation of MSCs, while the subtypes M0 and M1 solely stimulated the osteogenic differentiation of MSCs in the early and middle stages of co-culturing. In terms of molecular mechanisms, the secretion of specific exosomes by the different subtypes of macrophages has been correlated with the osteogenic differentiation of MSCs [40]. However, in comparison to M2 macrophages, the exosomes secreted by M0 and M1 macrophages seem to be more effective in the osteoblastic differentiation of MSCs [49]. These results suggest that M0 and M1 phenotypes are essentials in early stages of bone injury playing a critical role in the osteogenic differentiation of MSCs, while M2 subtype can stimulate proliferation of MSCs, osteogenic differentiation, and mineralization in late stages of the bone tissue regeneration process.
Furthermore, Cho et al. [21] demonstrated that a transwell co-culture with MSCs and macrophages significantly prevented M1 macrophage polarization and induced M2 polarization. Additionally, another in vitro approach demonstrated that macrophages cocultured with MSCs consistently showed high-level expression of CD206, a marker of activated macrophages, while also expressing high levels of IL-10 and low levels of IL-12, compared to controls [54]. Functionally, these macrophages cocultured with MSCs showed a higher level of phagocytic activity and polarization to the M2 phenotype. Using three-dimensional (3D) models, a gelatin methacryloyl (GelMA) hydrogel encapsulating BM-MSCs and macrophages showed optimal effects on the proliferation, migration, osteogenic and chondrogenic differentiation, while also showing significant M2 macrophage polarization [55]. Inspired by these in vitro results, the in vivo implantation of hydrogels promoted osteochondral repair through an intrinsic crosstalk between MSCs and macrophages at the bone injury. Likewise, another approach showed that the transplantation of decellularized human amniotic membrane seeded with MSC-educated macrophages into animal models improved healing processes in incisional wounds by decreasing the inflammatory phase, while increasing angiogenesis, and reducing scar tissue development [56].
For instance, these findings support the powerful crosstalk between MSCs and myeloid cells, namely neutrophils and macrophages, during different phases of the regenerative process. The capacity of MSCs to switch the polarization of these cells conjugated with the effect of myeloid cells exert in MSCs can be used to locally inhibit conventional pro-inflammatory pathways while enhancing alternative anti-inflammatory responses at the injury site. Consequently, several in vitro/in vivo combinations are still necessary to explore different cellular parameters with the phase of the regenerative phase. This know-how could provide the development of several alternatives for immunomodulatory strategies using specific pool populations of MSCs, neutrophils, or macrophages, according to the individual necessity, target tissue, and regenerative phase.
It is known that during the inflammatory phase, the amount of pro-inflammatory cytokines is significantly elevated. Taking into consideration that the biological activities of MSCs are not only influenced by the direct contact with immune cells but also by the presence of biomolecules, several in vitro studies have investigated the survival of MSCs, as well as their migration, proliferation, differentiation, and secretome profile in the presence of pro-inflammatory factors [36, 38, 57, 58].
In this sense, Wedzinska et al. [57] explored the biological functions of MSCs derived from Wharton’s jelly (WJ-MSCs) exposed to pro-inflammatory cytokines, namely interferon-γ (IFN-γ), TNF-α, and IL-1β at different concentrations and under different aerobic conditions (21% O2 vs. 5% O2), key factors of the inflammatory phase. While both aerobic conditions did not influence the dynamic proliferation of MSCs, the presence of pro-inflammatory signals stimulated the secretion of anti-inflammatory cytokines, namely IL-4 and IL-10 by MSCs.
Similarly, human BM-MSCs cultured with a cocktail of pro-inflammatory cytokines induced a significant upregulation of proteins involved with angiogenesis and resolution of inflammation [38]. Of note, priming MSCs with pro-inflammatory molecules, such as IFN-γ and TNF-α elicits an immunomodulatory phenotype by these cells characterized by an upregulation secretion of chemokines such as CXCR3, CCR5, CXCL9, CXCL10, and CXCL12, which attract immune cells via chemotaxis; anti-inflammatory cytokines (e.g., IL-10), and many other soluble immunosuppressive factors (e.g., IDO, COX-2, PGE-2, TGF-β) [2, 42].
Notably, these findings have been used to potentiate the immunomodulatory effect of MSCs before their in vivo administration [58]. However, a limitation of this strategy is that some pro-inflammatory signals, for example, IFN-γ, also upregulates the expression of genes associated with programmed cell death, reflecting a negative impact on MSC survival and proliferation after implantation [36]. Likewise, only cytokine-activated MSCs, but not non-activated MSCs, are effective in the reduction of an exacerbated inflammatory response through the release of anti-inflammatory factors [59].
MSC-based therapies typically rely on the ex vivo expansion of cells under ambient oxygen and high glucose levels that favour mitochondrial respiration for energy generation [5]. However, upon transplantation, donated MSCs are exposed to a hypoxic microenvironment which significantly impacts stem cell biology, particularly its metabolism. Given the microenvironment of hypoxia usually created at the site of tissue injury, the culture of MSCs under hypoxic conditions has been gaining substantial interest to understand how such conditions can influence the regenerative properties of MSCs.
Since the therapeutic functions of these cells can be impacted by physical conditions, several in vitro studies have shown higher viability and proliferative rate of MSCs cultured under hypoxia (1–10% O2) in comparison to normoxia (± 21% O2) [39, 45, 60]. Additionally, hypoxic conditions induce distinct changes in the biological activities of MSCs, influencing their metabolism, secretion of cytokines, growth factors, and EVs. For instance, the pre-incubation of MSCs for 2 days or more in 1% oxygen reduced serum deprivation-mediated cell death, while expressing significantly low levels of cytochrome c and heme oxygenase 1 compared to controls [60]. The consumption of glucose and secretion of lactate at a slower rate in comparison to controls, possibly promoted cell survival, as glucose remained available for longer periods of time. Most importantly, MSCs showed enhanced survival in vivo after intramuscular injection into immune-deficient mice. These findings suggest that the preincubation of MSCs under hypoxia induces key metabolic changes that yield higher retention after transplantation. The role of metabolism in regulating the function of MSCs has opened new avenues to improve the effectiveness of stem cell-based therapies for tissue regeneration. As a consequence, strategies to mitigate the metabolic shock experienced by MSCs after transplantation have become an attractive alternative to alleviate stem cell death while improving the antioxidant, cytoprotective defense, and immunomodulatory effects of MSCs [42].
Despite metabolic changes, the co-culture of MSCs and human umbilical vein endothelial cells (HUVECs) under normoxic (21% O2) vs. hypoxic conditions (5% O2) showed that hypoxia significantly increased the tube formation capacity of these cells compared to normoxia [45]. This result was correlated with the secretion of more functionally potent MSCs-derived EVs cultured under hypoxia, indicating that the angiogenic potential of MSC is facilitated by EVs release rather than by soluble factors under hypoxic compared to normoxic conditions.
Similar results were reported from MSCs cultured in serum-free media under hypoxia [6]. The release of anti-inflammatory EVs was also accompanied by an increased hypoxia-inducible factor 1-alpha (HIF-1-alpha). To corroborate these results, pre-treated MSCs cultured under hypoxic conditions demonstrated greater therapeutic effects after implantation in mice with liver cirrhosis [61]. The regenerative properties were correlated with local secretion of prostaglandin E synthase, macrophage polarization to M2 phenotype, and reduced hepatocyte cell death.
Although the preconditioning of MSCs under hypoxic conditions significantly impacts their angiogenic and immunomodulatory potential before implantation, the use of MSC-secretome has also emerged as another kind of MSCs-based therapy. Currently, MSCs-free therapy, more specifically MSC-secretome therapy, presents key advantages compared to direct MSC transplantation, including: (i) decrease the risk of immune reactions and carcinogenesis; (ii) facility to be manufactured in a large scale; (iii) safety has been confirmed in vivo and in some clinical trials [35].
In contrast to the cell-only regenerative medicine paradigm, biomaterials have been extremely useful to recreate biomimetic 3D microenvironments resembling key properties of the regenerative process while also directing tissue-specific repair. Basically, a critical mode of action by which biomaterials can promote tissue repair is by influencing the differentiation of stem cells and modulation of the immune response. To this end, several strategies have combined numerous components of tissue engineering tools (e.g., cells, bioactive molecules, biomaterials, and technologies) [62]. In this sense, various biomaterials with tunable biophysical and biochemical characteristics have been designed to facilitate and improve the attachment, migration, proliferation, and differentiation of MSCs into tissue-specific cell types, while modulating the immune response in favour of a pro-regenerative state [63, 64].
Basic approaches have focused on the interaction between biomaterials and cells, namely stem cells and immune cells, using several types of monoculture or co-culture systems [20, 65–67]. The use of biomaterials as a 3D platform can both preserve the tissue architecture and provide a 3D biomimetic milieu for MSCs, which enhances their paracrine functions, including their immunomodulatory potential [68]. The dimensionality, physical, topographical, and biochemical cues, and nano/microstructure can be used to improve the regenerative properties and immunomodulatory capacity of MSCs [64, 68–70]. For example, the use of liquefied capsules containing MSCs and macrophages has been proposed as a promising immunomodulatory platform, which favours the osteogenic potential of MSCs and macrophage polarization toward a regenerative profile, through the up-regulation and release of anti-inflammatory molecules [20]. Despite the direct effect of biomaterials on MSCs’ regenerative potential, the use of biomaterials has been extremely useful to elucidate the main upstream effector immune cells and their functions throughout the whole regeneration process [71].
In this line, studies have suggested that biomaterials can play a major role in influencing the polarization of both macrophages and T-helper cells, which have significant crosstalk during tissue healing [72, 73]. Therefore, among immune cells, macrophages are the dominant effector cells, playing a prominent role in tissue repair through the polarization from M1 to M2 phenotype. This can be illustrated by a study showing that T-helper cells are necessary for macrophage polarization to an M2 phenotype seeded in decellularized ECM scaffolds [65]. Translating for in vivo models, titanium implants of varying wettability (rough, and rough-hydrophilic) placed in the femur of mice showed that surface modifications applied to titanium implants polarized the adaptive immune response towards a type 2 helper cells (Th2), characteristic of a pro-wound healing phenotype, with increased recruitment of MSCs and macrophages, both in vitro and in vivo [71].
Remarkably, these kinds of approaches exemplify the use of biomaterials as a key tool for the development of promising exploratory platforms with several applications, such as (i) investigation of the involvement and crosstalk of many cellular components during tissue repair; (ii) combination of different biomaterials, properties, and technologies to design strategies with high biological complexity and regenerative capacity; (iii) synchronization of cells with biochemical and physical cues in a regulated manner able to guide and improve new tissue formation, both in vitro and in vivo; (iv) design of effective immunomodulatory strategies; and (v) personalized therapies for tissue regeneration [62].
In this perspective, the design of bioengineering microenvironments which recapitulate crucial characteristics of the different regeneration phases holds great promise to provide new treatment concepts for replacement, regeneration, and tissue healing. However, in vitro, strategies that diversify the cellular components to understand the host responses under different stimuli need to be intensively explored.
In summary, despite all the approaches presented here have supported the use of MSCs as “multi-talented cells” for regenerative therapies with a broad clinical application (Figure 2), most of the overwhelmingly positive results seen in preclinical studies have not yet translated into clinical [74]. Maybe the use of these cells in the clinic should consider the conjugation of MSCs-parameters such as tissue source, number of cells, activated vs. nonactivated-MSCs, study design (preconditioning/non-preconditioning, cell-based therapy, cell-free therapy, absence/presence of biomaterials) with individual parameters, namely age, gender, patient health conditions, and the stage of the regenerative process [41]. As a consequence, the conjunction of multiple parameters and standard protocols may facilitate the development of new therapeutic tools based on the use of MSCs or MSCs-derived products that positively influence the immune response while improving tissue regeneration in a more personalized way.
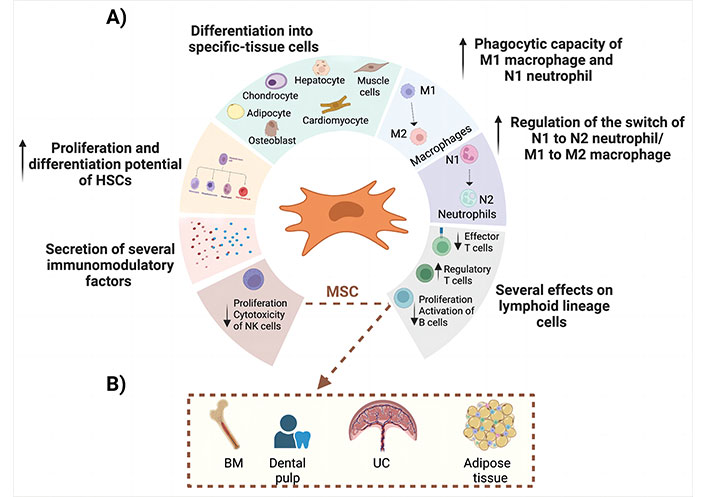
Representation of the main regenerative properties of the MSC. A) This panel shows the most important effects of MSCs during tissue regeneration; B) MSC can be isolated from BM aspirate, dental pulp, UC tissue, and adipose tissue
HSCs are multipotent primitive cells that give rise to all types of blood and immune cells in a process called hematopoiesis [12]. Hematopoiesis is a complex and highly orchestrated process by which a rare population of multipotent HSCs differentiate into functional blood and immune cells. In a hierarchical proliferation and differentiation process, self-renewing HSCs first differentiate into long-term HSCs (LT-HSCs), short-term HSCs (ST-HSCs), and MPPs. The MPPs further differentiate into committed myeloid progenitors (CMPs) or common lymphoid progenitors (CLPs) according to the body’s need or under specific stimulation [75, 76]. In this sense, HSCs-derived blood cells are originated from two main lineages:
(i) Myeloid-lineage cells. To originate these cells, CMPs can be converted into mature types of myeloid cells, such as platelets, neutrophils, monocytes, macrophages, eosinophils, basophils, erythrocytes, and DCs.
(ii) Lymphoid-lineage cells. From CLPs, B cells, T cells, and NK cells are originated in a lymphopoiesis process.
HSCs and their progenitors can be found in several organs and tissues, namely BM, UC blood (UCB), and PB (Figure 3). However, BM is the main responsible for the homeostasis of blood cells production through the balance between HSCs self-renewal and differentiation. Due to this balance, HSCs generate appropriate HSC-derived cell types which are required by the immune system and PB in response to physiological or pathological conditions [77].
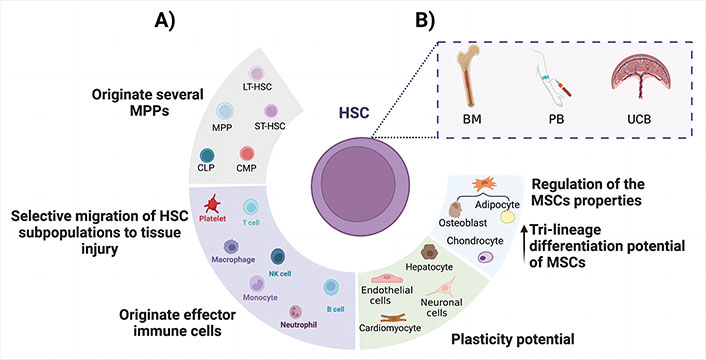
Overview of the main regenerative properties of HSC. A) Schematic representation of the regenerative properties of HSCs during tissue repair; B) HSC can be isolated from either BM aspirate, PB, and UCB
HSCs and HSCs-derived progenitors are categorized according to the presence or absence of specific cell membrane markers, namely CD34, CD38, CD90, CD45, CD49, CD123, CD135, CD7, CD10 [12, 78, 79]. These markers have been used to perform multi-parametric panels for each HSC-derived subpopulation [76, 80]. This categorization has been useful for the standardization of protocols for specific hematopoietic pool isolation, purification, and transplantation. Notably, the transplantation of HSCs is the oldest and most refined technique of regenerative medicine. Despite the potential of HSCs for the treatment of several blood disorders, HSCs, and their progenitors can also act through a variety of mechanisms to promote repair and tissue regeneration of several non-hematopoietic tissues, particularly for tissues injured through degeneration, ischemia, and inflammation [12, 81]. In this sense, HSCs may contribute to the regeneration of damaged tissues through the following main mechanisms:
(i) Capacity to originate several MPPs.
From HSC to mature cells, there are several intermediate progenitor cells, namely LT-HSCs, ST-HSCs, MPP, CMPs, and CLPs, which can display both functional multipotent and lineage-committed properties simultaneously or separately prior to complete maturation [12, 75, 79]. For example, HSPCs found at the PB are mostly ST-HSCs. These cells may directly contribute to recovering damaged tissues, being considered as an optimal curative cell source in regenerative medicine [75].
(ii) Differentiation of key blood/immune cells involved with tissue repair.
Several HSCs-derived cells participate in the regeneration and repair after a tissue injury. In fact, inflammatory responses are often accompanied by blood system changes, including overproduction of myeloid cells, namely platelets, neutrophils, monocytes, and macrophages, the main effector cells involved with damaged tissue repair [79, 82]. Besides, the recruitment of lymphocytes and NK cells is also necessary [13].
(iii) Transdifferentiation into non-hematological lineage cells.
Transdifferentiation or “plasticity” refers to the ability of adult stem cells to acquire mature cell phenotype distinct from their tissue of origin. In this sense, some works have explored and demonstrated that HSCs also have the capacity to transdifferentiate into cells of non-hematological lineages, more specifically endothelial cells, cardiomyocytes, neuronal cells, and hepatocytes, under restrictive conditions [78, 83–86].
(iv) Regulation of MSCs properties.
Taking into consideration that MSCs are another type of adult stem cell which resides in the BM, it is well recognized that MSCs and HSCs function synergistically in specialized microenvironments of BM [87, 88]. While MSCs regulate the functions, the self-renewal properties of HSCs as well as the hematopoiesis process, HSCs regulate not only the proliferation of MSCs but also stimulate their tri-lineage differentiation potential both in vitro and in vivo under specific conditions [30, 89]. This synergic crosstalk involves both direct cell-to-cell interaction and stemness signals from MSCs to HSCs and from HSCs to MSCs (Figure 3). This intrinsic relationship has been explored through the analysis of the combined effect of HSC transplantation (HSCT) with MSC [90, 91] or by their combined effect in several studies to evaluate its therapeutic potential to regenerate damaged tissues [87, 92, 93].
The recruitment and mobilization of HSCs, HSCs-derived progenitors, as well as several effector mature hematopoietic cells, namely neutrophils, monocytes, macrophages, and lymphocytes, are indispensable for the regeneration of several non-hematological tissues [81, 94]. While the role of mature and differentiated blood and immune cells has been well defined along the different phases of the regenerative process, an understanding of the roles of HSCs and their progenitors during tissue repair is necessary to clarify how these cells influence such a process. This knowledge could elucidate the best way to apply HSCs and their MPPs in tissue regeneration strategies. In this line, some approaches have been established to explore the role of HSPCs during tissue regeneration and repair:
Normally, HSPCs reside in specialized niches in the BM microenvironment, in which these cells usually stay in a quiescent state [12, 95, 96]. However, in some cases, such as infection or inflammation, there is a selective mobilization and migration of rare populations of HSPCs to injured regions [81, 94]. In this line, there is the necessity to clarify the molecular mechanisms by which specific HSC subpopulations home to the tissue of interest, and what is the exact role of these cells at the injured tissue.
It is well recognised that platelets are the first line of defence after a tissue trauma or injury. These cells rapidly adhere and aggregate upon exposure to subendothelial components such as collagen and von Willebrand factor [97]. Evidence has demonstrated that platelets release specific cytokines for the recruitment of adult stem cells, namely MSCs and HSPCs, to site of vascular injury [81]. This again confirms the indispensable role of both stem cells for an effective resolution of the regenerative process [29]. Similarly, Powerski et al. [94] demonstrated that after a musculoskeletal surgery, the total circulating CD133+/CD34+ HSCs significantly increased in the postsurgical period (after 8 h). These cells selectively migrated to the injured site and stimulated tissue repair through differentiation to parenchymal cells and/ or by post-traumatic immunological response.
Another approach using systemic administration of HSCs in murine models showed that the migration of these cells to the hepatic ischemia-reperfusion (IR) injury done via alpha(4)beta(1)/vascular cell adhesion molecule-1 (VCAM-1) pathway [98]. Interestingly, HSCs adhered in sinusoidal capillaries, suggesting sinusoids are the initial point of entry to the injured liver for HSCs and that the alpha(4)beta(1)/VCAM-1 pathway is responsible to drive the hematopoietic migration mechanism. Likewise, White et al. [99] explored the molecular mechanisms governing HSCs recruitment to injured kidney. Both murine models and HSCs were pre-treated before HSC infusion with blocking antibodies, hyaluronidases, or cytokines. CD49d, CD44, VCAM-1, and hyaluronan governed HSC adhesion to the IR-injured kidney. Remarkably, both pre-treatment strategies with keratinocyte-derived chemokine (KC), murine functional homologue of human IL-8 (released after acute renal injury), and stromal cell-derived factor-1α (SDF-1α, released following renal injury) significantly increased HSC adhesion within the injured kidney, whilst SDF-1α also increased numbers continuing to circulate. However, SDF-1α and KC did not increase CD49d or CD44 expression but increased HSC adhesion to VCAM-1 and hyaluronan, respectively. SDF-1α increased CD49d surface clustering, as well as HSC deformability. Increasing HSC adhesive capacity for its endothelial counter-ligands may explain their enhanced renal retention in vivo. Moreover, increasing HSCs’ deformability with SDF-1α treatment could explain the prolonged systemic circulation, while HSCs can also continue to survey the damaged tissue instead of becoming entrapped within non-injured sites. Taken together these results suggest that both autologous and exogenous HSCs are capable to migrate to damaged tissues, even in low amounts. For instance, some works have shown that it is possible to manipulate the mechanisms of HSC mobilization/migration through the activation of specific HSC signaling pathways by using antibodies, growth factors, or cytokines. Currently, the recruitment of these rare cells to injured tissues may also be a new window of opportunities in the medicine regenerative field.
Similar to MSCs, significant interest has emerged to understand how an inflammatory microenvironment influences the fate of HSCs and HSCs-derived progenitors. Notably, it has been demonstrated that the biology and behavior of HSPCs can be directly affected by inflammatory signaling [100]. During inflammation, the hematopoietic equilibrium is disrupted and quiescent HSCs are directly or indirectly activated to generate more mature immune cells, especially cells of the myeloid lineage [95]. Therefore, HSPCs can be activated directly through toll-like receptors (TLRs) or indirectly through cytokine/growth factor receptors to mediate their hematopoietic responses [101].
Recent findings show that HSCs are the first responders to infection and that pro-inflammatory factors released during infection or inflammation, namely TNF-α, granulocyte colony-stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), IL-1, IL-6, IL-8, and IFNs are key regulators of HSCs [82, 100]. As a consequence of such stimuli, downstream signaling cascades initiate the mobilization of HSPCs in order to provide effector immune cells for the infection/inflammation place [101].
Despite inflammatory factors having different effects on HSCs activation and differentiation, studies have shown that such signaling usually activates the differentiation of HSCs towards myelopoiesis rather than lymphopoiesis [95]. In this scope, a murine model which resembles the pathogenesis of human inflammatory bowel disease (IBD) showed that acute gut inflammation expands HSCs and MPPs in the BM while enhancing myelopoiesis via up-regulation of GM-CSF ligands in local tissue and its receptor on HSPCs [79]. At the same time, MPPs migrated to gut-associated lymph nodes to expand, and potentially differentiated into myeloid cells, suggesting a key role of these cells in gut tissue repair. On the contrary, the block of MPP migration exacerbated the colitis symptoms and gut tissue atrophy.
Similarly, studies performed in zebrafish revealed that TNF promotes HSCs survival and myeloid differentiation by activating a specific p65/NF-κB-dependent gene program that primarily prevents hematopoietic necroptosis [102]. However, dysregulation of TNF production has been related to directly inhibiting growth and inducing apoptosis of HSCs, as well as indirectly changing the BM microenvironment.
Additionally, IFN-γ, one of the critical cytokines controlling inflammation, seems to have oppositive effects on regulating HSCs proliferation and differentiation, possibly in a context-dependent manner [101]. While IFN-γ was able to activate HSC proliferation and differentiation during infections in vivo models [77, 103], contrary findings have demonstrated that IFN-γ also acts as a negative regulator of HSCs and their progenitors, directly reducing their proliferative capacity and complicating the restoration of HSC numbers upon viral infection [104]. In fact, not only IFN-γ but also IFN-α have the ability to play dual effects in the hematopoietic system [105]. While these molecules can be beneficial to induce proliferation and differentiation of HSCs, increasing myelopoiesis, and enhancing immune responses in response to acute infections [105], the same molecules are also capable of inducing deleterious long-term effects, namely HSC exhaustion and selection pressure for clonal stem cells during chronic infection [106].
Persistent cytokine stimulation via TNF, IFN, and IL-6 has been correlated with HSC dysfunction and may readily impact the initiation and progression of hematologic malignancies and BM failure [107]. Notably, chronic immune stimulation induces cell stress, DNA damage, and other dysfunctions in the hematopoietic lineage, including somatic mutations and myeloid neoplasms [108].
Overall, these findings suggest that under an inflammatory microenvironment or specific signaling of acute inflammation, HSCs actively cooperate with downstream hematopoietic progenitors, mature cells, and environmental stromal cells as frontline responders to preserve blood homeostasis [79]. However, their ability to respond deftly through self-renewal and differentiation at times brings about detrimental consequences, namely in the case of chronic inflammation. In this sense, a deeper understanding of how different inflammatory microenvironments and signaling factors can affect and influence the HSC biology, in terms of heterogeneity, functionality, adaptability, survival, mutations, and cell death, could clarify in which phase of the regenerative process HSPCs are more appropriate for therapeutic use.
In the last decades, some data have demonstrated the ability of HSCs to transdifferentiate into cells types that do not belong to the hematopoietic lineage tree, such as central nervous, skeletal muscle, endothelial, heart, and liver cells under restrictive and specific conditions [78, 84, 86, 109, 110]. Despite several controversies, the plasticity potential of single HSCs has emerged as a new concept to be explored in the field of HSC-based therapy for regenerative medicine.
For example, the culture of CD34+ cells, also defined as HSPCs, on a surface of fibrin and activated platelets promoted the maturation of CD34+ cells toward a mature endothelial cell phenotype [111]. The endothelial phenotype was then correlated with significant upregulation of CD31, CD105, and VEGF receptor-2 (VEGFR-2) expression, confirming the ability of CD34+ cells to convert into endothelial cells. Although, activated platelets secrete CXCL12, which has been shown to mobilize and activate integrins on the surface of stem cells, including HSCs, the molecular mechanisms by which platelets could induce HSC transdifferentiation needs to be addressed both in vitro and in vivo.
Furthermore, to evaluate the HSC potential to differentiate into liver cells, an in vitro combination of FGF-4 and HGF was used to stimulate HSCs from UCB for 14 days [86]. After stimuli, the cells demonstrated high expression of genes related to hepatocytes [tryptophan 2,3-dioxygenase (TO), glucose 6-phosphate (G6P), cytokeratin 18 (CK 18) albumin, and α-fetoprotein (AFP)]. According to the authors, this transdifferentiation potential of HSCs into hepatocytes could be an interesting alternative for cell replacement in case of liver diseases. Translating to in vivo models, Zhou et al. [78] used immune-deficient mouse models with liver damage to evaluate both the capacity of HSCs to migrate to the damaged tissue and their subsequent contribution to liver repair. Notably, transplanted human HSCs isolated from BM or UCB did not robustly become hepatocytes, but some hepatic transdifferentiation was documented. However, injected HSCs did home to the injured liver, contributing to liver repair not by hepatocyte transdifferentiation, but by the local release of paracrine factors.
Regarding the hematopoietic capacity to transdifferentiate into cardiomyocytes, it was demonstrated that not only HSCs-derived progenitors but also mature blood cells, namely macrophages, in response to a cocktail growth factors and ILs treatment or in co-culture with cardiac explants demonstrated ability to cardiac differentiation and integration into contractile heart tissue [110]. Considering that the time-course for cardiac differentiation of HSPCs was only 4 days, there is a lack of information about for example the viability and functionality of these differentiated cells over time. Some in vivo experimentation should also be performed to corroborate the in vitro results.
Together, these findings suggest that the plasticity of adult HSCs, even if it is rare, can be correlated with specific microenvironments/tissues or due to restricted stimulation which reprograms the fate of HSCs to differentiate into non-hematological cells. Additionally, these data highlight further and intensive investigation to elucidate specific conditions and mechanisms by which these HSCs and their progeny can directly or indirectly differentiate into non-hematological cells.
Although the regenerative properties of MSCs and HSPCs have been confirmed by several in vitro and in vivo approaches, such as cocultures, direct stem cell transplantation, injection, autologous/exogenous recruitment, and biomolecules secretion, several gaps in the stem cell biology field need to be addressed. These include a deeper understanding of how different inflammatory microenvironments and signaling factors effectively affect and influence mesenchymal and hematopoietic biology, in terms of stem cell heterogeneity, functionality, adaptability, survival, mutations, and cell death with the ability of these stem cells to positively influence the immune response along time. These could clarify which stem cell type or its derived products could be more appropriate for regenerative therapies.
Despite that, several challenges for clinical translation remain. These involve significant efforts to achieve translational requirement parameters (e.g., reproducibility, handling, manufacturing difficulties, safety, effectiveness, and long-term in vivo response). Furthermore, the use of these cells in the clinic should also consider the conjugation of stem cell parameters (e.g., tissue source, number of cells, activated vs. nonactivated, study design (preconditioning/non-preconditioning, cell-based therapy, cell-free therapy, absence/presence of biomaterials) with individual parameters, such as age, gender, and patient health conditions. These parameters should finally be combined with the stage of the regenerative process. In the next future, the conjunction of multiple parameters may facilitate the development of new therapeutic tools based on the use of mesenchymal and hematopoietic-derived cells and products that clearly influence the immune response and improve tissue regeneration in a more personalized way for clinical applications.
3D: three-dimensional
BM: bone marrow
CD: cluster of differentiation
CLPs: common lymphoid progenitors
CMPs: committed myeloid progenitors
CXCL: CXC-chemokine ligand
DCs: dendritic cells
ECM: extracellular matrix
EVs: extracellular vesicles
HSCs: hematopoietic stem cells
HSPCs: hematopoietic stem and progenitor cells
IFN-γ: interferon-γ
IL: interleukin
LT-HSCs: long-term hematopoietic stem cells
MPPs: multipotent progenitors
MSCs: mesenchymal stem cells
NK: natural killer
PB: peripheral blood
SDF-1α: stromal cell-derived factor-1α
ST-HSCs: short-term hematopoietic stem cells
TGF-β: transforming growth factor-β
TNF-α: tumor necrosis factor-α
UC: umbilical cord
UCB: umbilical cord blood
VCAM-1: vascular cell adhesion molecule-1
VEGF: vascular endothelial growth factor
All figures were created using Biorender.com. The authors acknowledge the Biorender’s support.
CSO: Conceptualization, Writing—original draft, Writing—review editing. FKT: Writing—review editing, Validation. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
This work was supported by National Funds from
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
