Affiliation:
1Department of Food Science and Technology, Olusegun Agagu University of Science and Technology, Okitipupa 350105, Nigeria
Email: oa.oladeji@oaustech.edu.ng
ORCID: https://orcid.org/0000-0001-6191-4584
Affiliation:
2Department of Food Science and Technology, Federal University of Technology, Akure 340252, Nigeria
ORCID: https://orcid.org/0000-0002-5499-1888
Explor Foods Foodomics. 2025;3:101089 DOI: https://doi.org/10.37349/eff.2025.101089
Received: January 23, 2025 Accepted: June 23, 2025 Published: July 25, 2025
Academic Editor: Sandra R. S. Ferreira, Federal University of Santa Catarina, Brazil
Aim: The study evaluated the influence of different wall materials on the bioactive compounds in encapsulated Justicia carnea leaves extract.
Methods: Combinations of gelatin with maltodextrin or starch, and gum arabic with maltodextrin or starch were prepared in ratios of 1:3 to create four types of wall matrices. Each combination was dissolved in water to obtain 20% w/v solutions. J. carnea leaves were extracted, concentrated, and the resulting extract added to the wall material at a ratio of 1:2 and homogenized. Encapsulation was achieved through homogenization at 12,000 rpm for 30 min, followed by freeze drying. The resulting microcapsules were characterized using a scanning electron microscope (SEM) and differential scanning calorimeter (DSC). Physicochemical properties, pigment concentrations, and micronutrient compositions of the microcapsules were also evaluated using standard methods.
Results: Carotenoids, chlorophyll, and anthocyanin were significantly higher (P ≤ 0.05) in the sample containing starch and gelatin [gelatin + starch + core (DGES)] as the wall matrix compared to other samples. Vitamins E and D, calcium (Ca), and manganese (Mn) in sample gum arabic + starch + core (CGS; blend of gum arabic starch) and sample DGES were not significantly different from each other. DGES exhibited significantly lower (60.19%) solubility than others (60.48–70.86%) and the highest (76.72%) encapsulation efficiency. SEM analysis revealed smooth surfaces and mostly polyhedral shapes, with particle sizes ranging from 10.534–14.159 μm across all samples. DSC analysis revealed that the particles are endothermic and amorphous in nature, except for the CGS sample, which became semi-crystalline at about 203.2°C.
Conclusions: The study showed that a composite wall material comprising starch and gelatin demonstrates enhanced effectiveness in the encapsulation of J. carnea leaves bioactive compounds.
The leaves of Brazilian plume (Justicia carnea) are utilized in traditional medicine to treat various ailments in developing countries. The leaves are commonly employed for managing nutritional anemia, a condition affecting over 2 million people globally [1], particularly in Nigeria. The leaves of J. carnea contain an array of natural antioxidants and bioactive constituents, which contribute to the diverse pharmacological activities attributed to the plant [2]. These compounds include phenolics, flavonoids, pigments (such as carotenoids, anthocyanins, and chlorophyll), saponins, tannins, and phytates, alongside essential minerals such as calcium, iron, zinc, magnesium, and vitamins A, B-complex, C, and E [3, 4]. Specifically, the leaf extract is reported to demonstrate high levels of vitamin C (232.32 mg/100 g) and iron (8.61 mg/kg) [1, 3]. Processing techniques, both thermal and non-thermal, can significantly impact the stability and bioavailability of these bioactive compounds, necessitating the need for encapsulation.
Encapsulation involves the formation of a thin layer protection around bioactive components of the food matrix to prevent environmental and enzymatic degradation, preserve bioactivity, and control the release of the substance at the active site [5]. Encapsulation can be achieved on a nano scale (1–100 nm) or micro scale (1–1,000 μm). The thin layer formed is referred to as the cell wall/wall matrix or nano/micro carrier, while the bioactive substance entrapped is the core matrix. Encapsulation, either micro or nano encapsulation, significantly enhances the nutritional value and functionality of food products. It protects bioactive compounds from degradation due to environmental factors such as heat, light, and oxidation, thereby improving their stability and shelf life [6, 7].
Encapsulation allows the controlled release of the bioactive ingredients in food matrices, thereby extending bioavailability and effectiveness upon consumption. It also helps to mitigate challenges associated with the direct incorporation of bioactive compounds, such as undesirable effects on sensory characteristics [6–8]. Encapsulation has been used to fortify bread with bioactive substances without adversely affecting its physicochemical and textural properties [8]. Plant bioactive compounds are substances found in plants that have beneficial effects on human health. The bioactive compounds often possess therapeutic properties and act as antioxidant and anti-inflammatory agents. Pigments such as carotenoids, anthocyanins, betalains, and chlorophylls are examples of such bioactive compounds [9, 10]. Some micronutrients (e.g., Fe, Se, Zn, vitamins B6, B12, C, D, and E) also exhibit biological activities, including antioxidant functions and contributions to the proper functioning of the immune system [11, 12]. Since these substances are sensitive to environmental degradation, incorporating them into food products through encapsulation helps minimize their degradation and loss during food processing.
Various wall materials have been explored for food encapsulation purposes, including chitosan, starches, alginates, proteins, lipids, maltodextrin, gum arabic, gelatin, and combinations thereof. For example, a combination of maltodextrin and whey protein isolate has been reported to produce microcapsules with a high blueness index, whereas a blend of gum arabic and whey protein isolate yielded the highest microencapsulation efficiency (91.19%) for phycocyanin encapsulation [13]. Similarly, maltodextrin/alginate mixtures have been shown to yield more homogeneous microcapsules with higher oil-encapsulation efficiency (EE; 90%) and enhanced stability at elevated temperatures compared to β-glucan/alginate mixtures [14]. The choice of wall material is, therefore, critical to determine the properties of the microcapsules.
Currently, there are limited or no studies on the encapsulation of J. carnea leaf bioactive compounds. Therefore, this study aims to evaluate the influence of different blends of cell wall materials on the bioactive substances in encapsulated J. carnea leaf extract for potential use in food fortification. The wall materials were selected on the basis of their common usage in food encapsulation, availability, and cost effectiveness.
About 1 kg of J. carnea leaves used in this study were collected on the premises of Olusegun Agagu University of Science and Technology (OAUSTECH), Okitipupa (6°33’N 4°43’E), Ondo State, Nigeria. The leaves were rinsed with distilled water, drained and blended with 2 L of distilled water using blender (Kenwood model). The blended leaves were manually shaken vigorously for 2 h and filtered using double-layered muslin cloth to obtain the J. carnea leaf extract. The filtrate, J. carnea leaf extract, was concentrated using a rotary evaporator (Searchtech Instruments, RE-52A) at 40°–45°C.
Four types of wall materials were used in two combinations each at a ratio (1:3 w/w) to make 20% w/v of each mixture following the methods of Awolu et al. [15] and Tatasciore et al. [16] with slight modifications. Ten grams each of gum arabic and gelatin were dissolved separately in 200 mL warm distilled water, mixed and continuously stirred for 1 h at 40°C using a magnetic stirrer (SCILOGEX, MS-H280-Pro). Exactly 30 g each of maltodextrin and modified corn starch were added separately to each of the dissolved gum arabic and gelatin by continuous stirring (400 rpm) on a magnetic stirrer at 40°C for 60 min to obtain four types of cell wall material solutions, i.e. maltodextrin and gum arabic [gum arabic + maltodextrin + core (AGM)], gelatin and maltodextrin [gelatin + maltodextrin + core (BGEM)], gum arabic and modified starch [gum arabic + starch + core (CGS)], gelatin and modified starch [gelatin + starch + core (DGES)]. Approximately 20 g of the core (concentrated J. carnea leaf extract) was added to the prepared cell wall materials at a ratio of 1:2 w/v and mixed vigorously until the mixture dissolved. The mixture of the core and wall materials was homogenized using a high-speed homogenizer (GEN 700 Cole Parmer) at 12,000 rpm for 30 min. Thereafter, the homogenized microcapsules were freeze-dried at –40°C for 24 h in a freeze dryer (Vaco 2-E Zirbus Technology, Germany). After freeze-drying, the J. carnea extract microcapsules were pulverized with a blender and packed in an airtight container.
The sample formulation as described above is shown in Table 1.
Sample formulation
| Sample | Core (J. carnea leaf extract) | Gum arabic | Gelatin | Maltodextrin | Modified corn starch |
|---|---|---|---|---|---|
| AGM | 20 g | 10 g | - | 30 g | - |
| BGEM | 20 g | - | 10 g | 30 g | - |
| CGS | 20 g | 10 g | - | - | 30 g |
| DGES | 20 g | - | 10 g | - | 30 g |
-: not included; J. carnea: Justicia carnea; AGM: gum arabic + maltodextrin + core; BGEM: gelatin + maltodextrin + core; CGS: gum arabic + starch + core; DGES: gelatin + starch + core
The physicochemical properties determined are moisture content, solubility, and color. Moisture of the microcapsule was determined by the standard AOAC [17] official method by drying approximately 2 g sample in a hot air oven at 105°C until constant weight was obtained. The color of the samples was measured using a CIE colorimetric method described by Falade and Okafor [18]. The colorimeter (Colour Tec PCM™ Colour Tec Associates, Inc., Clinton, NJ, USA) was standardized using a white color. The samples were measured in triplicate and the L* (lightness), a* (red vs. green), b* (yellow vs. blue) color values were recorded. The method described by Falade and Okafor [18] was used for the solubility of the samples. One gram of sample was mixed with 10 mL of distilled water in a centrifuge tube and stirred for 1 h. The samples were centrifuged for 15 min at 3,000 rpm and decanted into Petri dish. The supernatant in the Petri dish was weighed and dried at 105°C for 1 h. The solubility of the samples was calculated by weight difference and expressed in percentage.
EE was determined as % according to the method described by González-Ortega et al. [19] using phenolic content and calculated according to Equation 1. Approximately 20 mg of the microcapsule sample was weighed into a centrifuge tube, 1.4 mL of 98% ethanol was added, vortexed for 10 min and centrifuged for 2 min at 1,500 rpm. The supernatant ethanol was collected and the residue was suspended in 1.4 mL of water by vortexing. The suspension was centrifuged and the supernatant was collected. Both fractions were analyzed for phenolic content. Surface or non-encapsulated phenolics are the phenolic content of the ethanol fraction, while encapsulated phenolics are the phenolic content of the water fraction.
Pigments evaluated include carotenoids, chlorophylls, and anthocyanins. Anthocyanins were extracted and determined as described by Liu et al. [20]. Approximately 2 g of the freeze-dried microcapsules was extracted with 0.1 mol/L HCl and 95% ethanol (1:1) in a water bath at 60°C for 1 h, centrifuged at 500 rpm/min for 15 min. Approximately 2 mL of the supernatant was added to 2 mL KCl (pH 1.0) and sodium acetate (pH 4.0) separately and measured at 520 nm and 700 nm, respectively. Anthocyanin was calculated using Equation 2:
where An means anthocyanin; A means difference in absorbance value at 520 nm and 700 nm; V means total volume of extract; n means dilution factor; M means the total molecular mass of cyanidin-3-glucoside chloride (449.2 g/mol);
Carotenoid and chlorophyll were extracted by homogenously dissolving 0.5 g of the freeze-dried microcapsules in 10 mL of 95% ethanol and allowed to stand for 5 min. The extract was filtered into a brown bottle using filter paper and the absorbance value of the filtrate was measured at 665 nm, 649 nm, and 470 nm with 95% ethanol used as a blank. Chlorophyll and carotenoid were determined using Equations 3 and 4, where Ca is chlorophyll a (mg/g) = 13.95A665 nm – 6.88A649 nm; Cb is chlorophyll b (mg/g) = 24.96A649 nm – 7.32A665 nm; V means total volume of extract (mL); n means dilution factor; m means sample weight (g).
The mineral composition of the J. carnea microcapsules was analyzed from the extract obtained when 5 g of the sample was digested with 10 mL of 5 N concentrated HCl. The mixture was placed on a water bath and evaporated almost to dryness. The solution was cooled and filtered into a 100 mL standard flask and diluted to volume with distilled water. Atomic absorption spectrophotometer (Bosch, Searchtech instrument, model 752N) was used to analyze the minerals separately after acid digestion of the sample, as described in AOAC [17].
The vitamins evaluated in microencapsulated J. carnea leaf extract include vitamin B1, B2, B3, C, D, and E. AOAC method was used for vitamin D determination with wavelength of 265 nm for UV detection. Vitamin B1 (thiamine), B2 (riboflavin), were determined according to fluorometric method described in AOAC [17], while vitamin C by indophenol methods, and vitamin B3 and E were determined, all according to AOAC [17].
Scanning electron microscope (SEM) was used for granule morphology studies. A thin layer of starch granules was mounted on an aluminum specimen holder by double-sided tape. The specimen holder was loaded in a Polaron SC 7610 sputter coater (Fisons Instrument, UK). It was coated with gold palladium to a thickness of about 30 nm. The specimen holder was then transferred to an XL-20 series (Philips) SEM (JEOL, JSM 7600F), and samples were examined at 10 kV [21]. Particle size was determined from SEM image using IMAGE J software (Fiji, ImageJ2) [22].
Thermal analysis of the powders was carried out by using a differential scanning calorimeter (DSC; Nanjing DAZHAN Therma analyzer) as reported by González-Ortega et al. [19]. Approximately 5–7 mg of J. carnea microcapsule was weighed, placed into DSC aluminum pans (50 μL, PerkinElmer) and hermetically sealed with pierced aluminum lids to allow evaporation of residual water upon heating scan measurement. Samples were scanned from 30°C to approximately 200°C above the glass transition temperature at 5°C/min heating rate and cooled at 10°C/min cooling rate to the initial temperature.
The results were expressed as mean ± SD of triplicate (n = 3) samples. Data were analyzed with one-way analysis of variance (ANOVA) using SPSS version 16.0. (SPSS Inc. 2007) Statistical significance among group means was determined at P ≤ 0.05 using Duncan’s post hoc test.
The physicochemical properties and bioactive compounds in freeze-dried microcapsules of J. carnea leaf extract are presented in Table 2. Moisture content and solubility of the microcapsules are 4.56% to 7.89% and 60.19% to 70.86% respectively. Sample AGM had the lowest moisture content and the highest solubility, while sample DGES, with the highest moisture content, had the lowest solubility. Moisture content and the solubility of the microcapsules were significantly (P ≤ 0.05) greater than each other. The color of the microcapsules presented as a*, b*, and L* in Table 2 has positive values for all the samples. The values are 0.03–1.20, 2.35–3.59, 34.22–44.84 for a*, b*, and L* respectively. Sample encapsulated with a combination of maltodextrin and gelatin (BGEM) has the highest L*, showing the sample had a brighter color than others, while a combination of maltodextrin and gum arabic was also brighter than those containing starch or gelatin without maltodextrin. Sample DGES had the highest (76.72%) EE and sample BGEM (32%) the least. Samples containing starch and gelatin in the wall material possess higher EE than those containing maltodextrin. Carotenoids, chlorophyll, and anthocyanin were significantly higher (P ≤ 0.05) in the sample containing starch and gelatin (DGES) as the wall matrix than other samples. This implied that a combination of starch and gelatin preserves the pigment better than other wall material combinations.
Physicochemical properties and bioactive compounds in encapsulated J. carnea leaves extract microcapsules
| Properties | AGM | BGEM | CGS | DGES |
|---|---|---|---|---|
| Moisture (%) | 4.56 ± 0.08d | 5.87 ± 0.02c | 7.46 ± 0.04b | 7.89 ± 0.09a |
| Solubility (%) | 70.86 ± 0.06a | 60.85 ± 0.08b | 60.48 ± 0.04c | 60.19 ± 0.04d |
| % EE | 59.87 ± 0.24b | 32.24 ± 0.21d | 38.17 ± 0.18c | 76.72 ± 0.27a |
| Color | ||||
| L* | 35.84 ± 0.13b | 44.84 ± 0.13a | 35.66 ± 0.00b | 34.22 ± 0.19c |
| a* | 0.44 ± 0.02b | 1.20 ± 0.03a | 0.03 ± 0.00c | 0.04 ± 0.02c |
| b* | 2.35 ± 0.02c | 3.59 ± 0.04a | 3.55 ± 0.02a | 2.87 ± 0.02b |
| Pigments | ||||
| Carotenoid | 15.86 ± 0.02c | 15.65 ± 0.01c | 18.75 ± 0.29b | 21.23 ± 0.16a |
| Anthocyanin | 9.61 ± 0.09c | 9.43 ± 0.01d | 12.61 ± 0.06b | 16.57 ± 0.04a |
| Chlorophyll | 2.31 ± 0.04c | 2.14 ± 0.01d | 2.75 ± 0.02b | 3.16 ± 0.03a |
| Betalains | 0.09 ± 0.01b | 0.13 ± 0.01a | 0.08 ± 0.00b | 0.06 ± 0.01b |
Values are the mean ± standard deviation of replicates (n = 3). Values with the same letters along the same row are not significantly different (P ≤ 0.05). L*: lightness; a*: red vs. green; b*: yellow vs. blue; J. carnea: Justicia carnea; AGM: gum arabic + maltodextrin + core; BGEM: gelatin + maltodextrin + core; CGS: gum arabic + starch + core; DGES: gelatin + starch + core; EE: encapsulation efficiency
The effect of the wall materials on the mineral and vitamin composition of freeze-dried J. carnea leaf extract microcapsule is presented in Table 3. Vitamins E and D, Ca, and Mn in sample CGS and DGES were not significantly (P ≤ 0.05) different from each other but significantly (P ≤ 0.05) higher than those present in sample AGM and BGEM. Vitamins B1, B3, and C were higher in sample DGES (1.94, 0.20, and 2.71 μg/100 g) than others (0.87–1.36, 0.05–0.15, and 1.44–2.64 μg/100 g). Among all the minerals, the microcapsules containing a combination of gelatin and starch (DGES) possessed the highest mineral composition than microcapsules of other wall material combinations.
Minerals and vitamin composition of encapsulated J. carnea leaves microcapsules
| Samples | AGM | BGEM | CGS | DGES |
|---|---|---|---|---|
| Minerals (mg/100 g) | ||||
| Zn | 1.07 ± 0.00d | 1.25 ± 0.01c | 1.29 ± 0.00b | 1.59 ± 0.00a |
| Fe | 2.17 ± 0.00c | 2.10 ± 0.00d | 3.05 ± 0.00b | 5.04 ± 0.21a |
| Mn | 1.16 ± 0.23b | 1.15 ± 0.00b | 1.55 ± 0.01a | 1.67 ± 0.00a |
| Ca | 35.55 ± 0.00b | 31.50 ± 0.09c | 51.65 ± 0.28a | 51.96 ± 0.01a |
| Na | 13.54 ± 0.00b | 13.10 ± 0.01d | 13.43 ± 0.02c | 13.68 ± 0.29a |
| Mg | 85.54 ± 0.14c | 82.44 ± 0.00d | 110.14 ± 0.00b | 115.41 ± 0.01a |
| K | 321.04 ± 0.62c | 314.07 ± 0.00d | 342.32 ± 1.27b | 374.35 ± 0.16a |
| Vitamins (μg/100 g) | ||||
| B1 | 0.87 ± 0.02d | 1.13 ± 0.01c | 1.36 ± 0.02b | 1.94 ± 0.02a |
| B2 | 2.08 ± 0.04a | 2.16 ± 0.02a | 1.97 ± 0.01b | 2.08 ± 0.05a |
| B3 | 0.09 ± 0.01c | 0.05 ± 0.01c | 0.15 ± 0.03b | 0.20 ± 0.01a |
| C | 1.52 ± 0.01c | 1.44 ± 0.02d | 2.64 ± 0.01b | 2.71 ± 0.04a |
| D | 0.34 ± 0.03a | 0.25 ± 0.02b | 0.36 ± 0.02a | 0.37 ± 0.03a |
| E | 0.31 ± 0.03b | 0.34 ± 0.02b | 0.44 ± 0.04a | 0.45 ± 0.00a |
Values are the mean ± standard deviation of replicates (n = 3). Values with the same letters along the same row are not significantly different (P ≤ 0.05). J. carnea: Justicia carnea; AGM: gum arabic + maltodextrin + core; BGEM: gelatin + maltodextrin + core; CGS: gum arabic + starch + core; DGES: gelatin + starch + core
The SEM micrograph and DSC curves are shown in Figures 1 and 2, respectively. Characterization of the microcapsules with SEM revealed irregular-shaped (B-type) particles, mostly polyhedral, with a size range of 10.534–14.159 μm for all the samples. The edge dimension of the SEM image is 269 μm at a magnification of 1,000×, which implies 0.1 μm (100 nm). Formation of clusters (agglomeration) is observed mostly in particles of samples containing gum arabic (AGM and CGS). Samples containing gelatin, either with maltodextrin or starch, in the wall materials (BGEM and DGES) are smoother with minimal particles on the surface than samples containing gum arabic with either maltodextrin or starch (AGM and CGS).
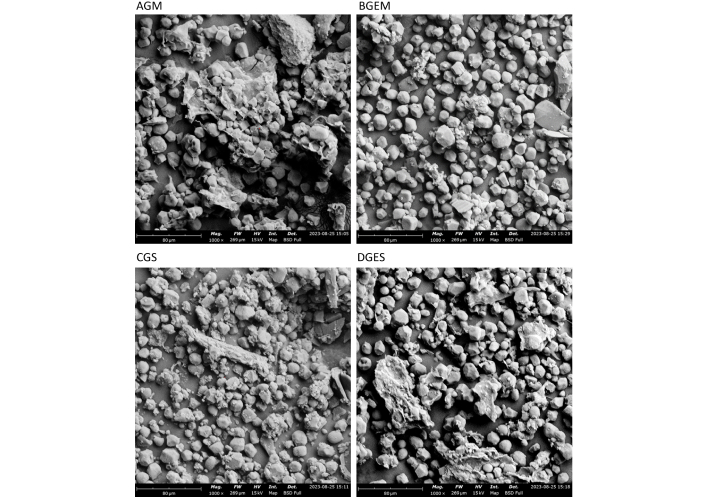
SEM micrographs of microencapsulated Justicia carnea leaf extract. SEM: scanning electron microscope; AGM: gum arabic + maltodextrin + core; BGEM: gelatin + maltodextrin + core; CGS: gum arabic + starch + core; DGES: gelatin + starch + core
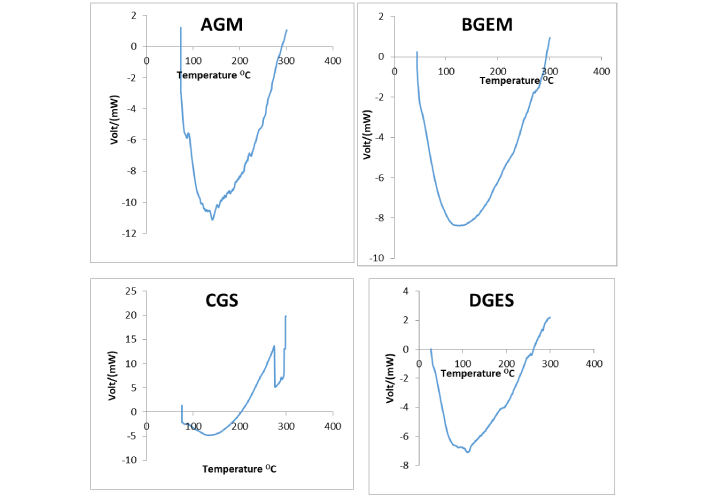
DSC curve showing thermal properties of the encapsulated J. carnea leaf extract. DSC: differential scanning calorimeter; J. carnea: Justicia carnea; AGM: gum arabic + maltodextrin + core; BGEM: gelatin + maltodextrin + core; CGS: gum arabic + starch + core; DGES: gelatin + starch + core
The DSC revealed a transition temperature range of 76.1°C to 87.3°C for all the samples. The particles are endothermic and amorphous in nature, except for sample CGS, which became semi-crystalline at about 203.2°C with unique peaks at the exothermic site. The endothermic peaks observed for the samples are not sharp and are poorly defined.
The moisture content of the microcapsule samples was below the 10% threshold recommended for keeping quality of powdery products, indicating that J. carnea leaf microcapsules may exhibit good storage stability. The presence of maltodextrin in the wall materials facilitated a greater reduction in moisture content compared to wall combinations without maltodextrin, which is consistent with the findings of Tatasciore et al. [16] for microcapsules with maltodextrin walls. Moisture content observed for samples AGM and BGEM is comparable to the values reported for probiotic cell microcapsules (4.5%) encapsulated with gum arabic/gelatin [23] and hop extract encapsulated with gum arabic and maltodextrin (5.27%) [16], whereas samples CGS and DGES (containing starch wall) exhibited higher moisture levels. This suggests that the inclusion of starch in the wall materials increases the final moisture content. According to Paula et al. [23], low moisture content prevents the agglomeration of microcapsules and limits molecular mobility, thereby reducing the diffusion rate of external factors that could adversely affect the core.
The lower solubility observed in sample DGES suggests that this sample may be more stable than others in aqueous medium, exhibiting slower, controlled release properties [24]. Conversely, the higher solubility observed in the microcapsules containing maltodextrin-based wall materials could be attributed to the high water solubility of maltodextrin [25]. The solubility values obtained in this study were comparable to those reported for microcapsules of date pit phenolic compounds encapsulated with maltodextrin (60.39%) [26] and papaya seed extract encapsulated with chitosan (61.5–62.7%) [27], except for sample AGM (70.86%). However, the solubility of black cumin seed oil encapsulated with gum arabic and maltodextrin was up to 88.71% [28], higher than the values obtained in the current study (60.85–70.86%). Such variations could be attributed to differences in core-to-wall ratio, wall material composition, core material characteristics, and drying conditions.
Although samples containing starch in the wall materials exhibited lower solubility, maltodextrin-containing walls, with their higher solubility, may facilitate rapid dissolution, which could be leveraged for targeted release under specific conditions (e.g., in the gut, presence of enzyme). However, it should be noted that highly soluble wall material may lead to a burst release of the core, potentially compromising the overall stability of the microcapsules.
The higher brightness observed in microcapsules with maltodextrin walls can be attributed to the white color of maltodextrin. The color, due to positive a* and b* values, indicates that the microcapsules are composed of red and yellow hues, respectively, while the proximity of these values to zero suggests a greyish appearance, with maltodextrin-containing samples appearing brighter. The L*-values of samples containing gum arabic in the wall materials were not significantly different (P ≤ 0.05). In the present study, the coloring power, L* is lower, the b* values were in agreement, and a* values were higher (though less than 1) compared to values reported for hop extract and black cumin seed oil encapsulated with gum arabic and maltodextrin [27, 28]. These differences may be due to variations in the nature of the core and ratio of the wall materials.
The combination of gelatin and maltodextrin resulted in the lowest EE (32.24%), while a blend of gelatin and starch achieves the highest, implying that gelatin and maltodextrin may not be an optimal combination for encapsulating J. carnea leaves extract. The higher EE observed with a blend of gelatin and starch wall may be attributed to a higher molecular weight of starch compared to maltodextrin. Prior studies have shown that EE improves when higher molecular weight carbohydrates are utilized [19, 29]. The EE reported for the sample containing gum arabic and maltodextrin wall (59.87%) was comparable to that reported for hop extract encapsulated with the same wall combination [16], whereas the EE for the gelatin-starch blend, though the highest in this study, was lower than values reported for black cumin seed oil and probiotics cells [23, 28]. Differences in EE could be due to variations in the encapsulation process, including the drying method employed. High EE indicates effective enclosure of the core material within the cell wall.
The sample containing a gelatin-starch blend exhibited the highest concentration of the pigments, except betalain, which was more in the sample containing gelatin-maltodextrin blend. This may be due to the presence of gelatin, a protein-based wall material. Díaz-Montes [30] reported that polysaccharide-based walls may not be optimal for encapsulating chlorophyll and carotenoids, while protein-based walls, either alone or combined with polysaccharides, enhance microcapsule characteristics. Protein-based walls, having amphipathic properties, interact with both hydrophilic (e.g., betalain) and hydrophobic (e.g., chlorophyll and carotenoids) pigments [30]. Wall materials serve as barriers protecting pigments from degradation by external factors such as light, oxygen, and heat.
The highest mineral and vitamin retention was observed in sample DGES, except for vitamin B2, indicating that the combination of gelatin and starch as wall materials is more effective at preserving these nutrients than other combinations. This finding aligns with the report by Bajaj et al. [31] for the microencapsulation of vitamin B12 and D3 using modified starch and maltodextrin. Carbohydrate- and protein-based polymer blends are considered optimal for encapsulating bioactive compounds [32], and encapsulation within such matrices effectively extends the shelf-life of substances susceptible to oxidation and thermal deterioration [33].
SEM characterization revealed smooth surfaces and polyhedral shapes with varying sizes. Samples containing gelatin exhibited smoother surfaces compared to those without gelatin, suggesting that blending gelatin with either maltodextrin or starch improves microcapsule stability and enhances bioavailability of bioactive compounds in J. carnea leaf. Alves et al. [22] also reported smooth surfaces for encapsulated essential oil containing maltodextrin as wall material. According to Bajaj et al. [31], smooth surfaces promote higher retention of encapsulated materials. Moreover, smooth, regular shapes are associated with low gas permeability, thus leading to retention of the core within the microcapsules [28]. The variation in particle size observed is consistent with the report of other researchers [21, 28, 34]. However, the particle sizes obtained in this study were larger than those reported for papaya seed extract and vitamin B and D microcapsules produced via spray drying [27, 31]. Santiworakun et al. [28] reported collapsed, irregular shapes for black cumin oil microcapsules encapsulated with a blend of gum arabic and maltodextrin, contrasting with the regular shapes observed in this study, likely due to differences in the method of encapsulation and drying process.
The endothermic site observed in the DSC curves indicates that the samples absorb heat and undergo melting at the active sites. The poorly defined peaks are an indication that a complex phase transition may be occurring in the sample at the active site. It may also be an indication of an irreversible transition process. According to Michael et al. [35], broad or poorly defined DSC peaks often indicate complex thermal behavior or overlapping transformations, making it challenging to determine characteristic properties of the products. The amorphous nature exhibited by the samples indicates that the wall materials effectively encapsulate the bioactive substances in J. carnea leaves extract. Amorphous nature is reported to be a confirmation that the core is properly encapsulated by the cell wall material [22]. The transition temperatures revealed that the samples are thermally stable and resist structural changes up to approximately 76°C, with the sample containing a blend of gum arabic and maltodextrin exhibiting thermal and structural stability up to 87°C. These findings show that the wall materials can protect the bioactive compounds in J. carnea leaves during food processing temperatures up to 76°C. Low transition temperatures are considered crucial for biological systems [36, 37] such as food systems meant for human consumption.
The study showed that gum arabic can be blended with maltodextrin or starch for encapsulating J. carnea leaves extract. However, the blend of gelatin-starch wall enclosed the highest quantity of most bioactive compounds examined, while the gelatin-maltodextrin blend resulted in the least. Gelatin or gum arabic combined with starch encapsulates bioactive substances in J. carnea leaves more effectively than other wall material combinations. Although blends of gum arabic with maltodextrin encapsulate some bioactive compounds, encapsulation was less effective with gelatin-maltodextrin blends. Overall, the gelatin-starch combination achieved the highest EE and lower solubility in aqueous medium. The use of gelatin-starch composite wall material shows strong potential for the encapsulation of bioactive compounds in J. carnea leaves extract, indicating potential applications in food fortification and pharmaceuticals.
AGM: gum arabic + maltodextrin + core
BGEM: gelatin + maltodextrin + core
CGS: gum arabic + starch + core
DGES: gelatin + starch + core
DSC: differential scanning calorimeter
EE: encapsulation efficiency
J. carnea: Justicia carnea
SEM: scanning electron microscope
The contributions of Oluwatomini I. Falade and Precious I. Agbi during the bench work and analysis are gratefully acknowledged.
OAO: Conceptualization, Methodology, Investigation, Project administration, Supervision, Writing—original draft, Writing—review & editing. OOA: Methodology, Validation, Writing—review & editing. Both authors read and approved the submitted version.
Authors declare that there is no known conflict of interest, either financial or personal relationship, related to this study.
Not applicable.
Not applicable.
Not applicable.
Raw data supporting the figures and the conclusions will be made available upon request to any qualified researchers.
Not applicable.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 2659
Download: 173
Times Cited: 0
