Affiliation:
Department of Veterinary Medicine, Çaycuma Food and Agriculture Vocational School, Zonguldak Bülent Ecevit University, Zonguldak 67900, Turkey
Email: serkan.sugecti@hotmail.com
ORCID: https://orcid.org/0000-0003-3412-2367
Explor Foods Foodomics. 2026;4:1010111 DOI: https://doi.org/10.37349/eff.2026.1010111
Received: September 02, 2025 Accepted: January 20, 2026 Published: January 29, 2026
Academic Editor: Olga Pardo, University of Valencia, Spain
The article belongs to the special issue Food Contaminants: Analysis, Occurrence and Risk Assessment
Aim: Food contaminants such as acrylamide, 3-monochloropropane-1,2-diol (3-MCPD), glycidyl stearate, deoxynivalenol, hydroxymethylfurfural, and zearalenone represent significant toxicological concerns in humans due to their potential genotoxic, hepatotoxic, and carcinogenic properties. This study aimed to investigate the molecular interactions of these contaminants with cytochrome P450 2E1 (CYP2E1), a key enzyme in xenobiotic metabolism, using an in silico approach.
Methods: Molecular docking simulations were performed to assess the binding affinities and interaction profiles of selected food contaminants with the active site of human CYP2E1. The docking scores and binding poses were analyzed to predict possible metabolic outcomes and risks associated with exposure.
Results: Docking analysis revealed variable binding affinities among the tested contaminants. Glycidyl stearate, zearalenone, and deoxynivalenol demonstrated stronger binding interactions (higher docking scores) compared to acrylamide and 3-MCPD, suggesting higher potential for CYP2E1-mediated metabolism. Quantitative results have been added: glycidyl stearate, deoxynivalenol, and zearalenone showed the stronger binding energies (−6.4, −7.2, and –7.9 kcal/mol), while acrylamide and 3-MCPD were weaker (−3.7 and −4.1 kcal/mol). Hydroxymethylfurfural showed an intermediate binding affinity (ΔG = –5.3 kcal/mol), suggesting a moderate potential for CYP2E1-mediated metabolism. Differential binding patterns highlighted possible metabolic activation or detoxification pathways.
Conclusions: The results indicate that CYP2E1 plays an important role in mediating the biochemical responses to multiple food contaminants. Stronger interactions with certain contaminants suggest a higher risk of metabolic activation, which may contribute to their toxic effects. This study demonstrates the utility of molecular docking for predicting human biochemical responses and supports its use as a complementary tool in food safety risk assessment.
Food safety is a global concern, as chemical contaminants formed during food production, processing, and storage pose significant risks to human health [1, 2]. In addition to pesticide residues and environmental pollutants, thermal processing of foods often generates hazardous compounds such as acrylamide, 3-monochloropropane-1,2-diol (3-MCPD), glycidyl esters, and hydroxymethylfurfural. These compounds are frequently detected in heat-treated foods, including baked goods, fried products, coffee, and edible oils, raising concerns about chronic dietary exposure and long-term toxicological consequences [3, 4]. Understanding the biochemical interactions of these contaminants is therefore critical for advancing food toxicology and public health protection.
Among the wide spectrum of contaminants, acrylamide, 3-MCPD, and glycidyl esters are of particular interest due to their carcinogenic and genotoxic potential, while hydroxymethylfurfural has been associated with hepatotoxicity and oxidative stress [5, 6]. Acrylamide is formed via the Maillard reaction between reducing sugars and amino acids at high temperatures, whereas 3-MCPD and glycidyl esters primarily occur during the refining of edible oils [7]. Due to their prevalence and toxicological profiles, these contaminants have been classified as potential human health hazards, prompting regulatory agencies and researchers to investigate their biological impacts more deeply. Glycidyl stearate is a derivative of glycidyl esters formed during high-temperature processing of refined vegetable oils; upon digestion, it releases glycidol, a compound with recognized genotoxic potential [8]. Deoxynivalenol, a trichothecene mycotoxin produced by Fusarium species, is commonly found in cereal-based products and exerts its toxicity mainly through inhibition of protein synthesis, leading to gastrointestinal disturbances and immunomodulatory effects [9, 10]. Zearalenone, another Fusarium-derived mycotoxin, is frequently detected in maize and its derivatives, and is of particular concern due to its estrogenic properties and endocrine-disrupting activity [11, 12]. Despite their distinct chemical origins, these compounds share the ability to enter the food chain and elicit biochemical responses in humans, including oxidative stress, enzyme inhibition, and disruption of hormonal balance, underscoring their toxicological relevance in food safety research.
Cytochrome P450 2E1 (CYP2E1) is a member of the cytochrome P450 superfamily, known for its role in xenobiotic metabolism, particularly in the bioactivation of low molecular weight toxicants such as ethanol, benzene, and nitrosamines [13]. CYP2E1 catalyzes oxidation reactions that can convert relatively inert molecules into reactive intermediates, often resulting in oxidative stress and cellular damage. Given its substrate specificity toward small hydrophobic molecules, CYP2E1 is considered a key enzyme in mediating the toxicological effects of several foodborne contaminants [14, 15]. Its activity and binding interactions thus represent a critical molecular target for assessing dietary chemical exposure.
In silico approaches, including molecular docking and molecular dynamics simulations, provide powerful tools for predicting the binding affinities and interaction profiles of contaminants with metabolizing enzymes [16]. These computational methods allow researchers to explore binding modes, estimate free energies of interaction, and prioritize compounds for further in vitro and in vivo toxicological evaluations [17]. In the context of food toxicology, in silico studies can efficiently complement experimental data, reduce laboratory workload, and identify potential molecular mechanisms underlying contaminant toxicity [18, 19].
In contrast to most previous studies, which examined a single food contaminant or a single class of compounds, the present work comparatively investigates process-induced contaminants (acrylamide, 3-MCPD, glycidyl esters) alongside naturally occurring mycotoxins (deoxynivalenol, zearalenone) within the same enzymatic framework (CYP2E1). This comparative approach provides a broader mechanistic perspective and highlights structural features that may influence metabolic activation, thereby advancing current knowledge in the molecular toxicology of food contaminants. The present study aims to investigate the binding interactions of selected food processing contaminants, including acrylamide, 3-MCPD, glycidyl esters, and hydroxymethylfurfural, with CYP2E1 using molecular docking analysis. By comparing the binding energies and interaction patterns of small polar versus hydrophobic contaminants, we sought to elucidate potential differences in metabolic activation pathways. The outcomes of this study are expected to contribute to a better understanding of the molecular toxicology of food contaminants and provide a basis for future risk assessment and regulatory considerations.
The chemical structures of selected food processing contaminants such as acrylamide (PubChem CID: 6579), 3-MCPD (PubChem CID: 7290), glycidyl stearate (PubChem CID: 62642), deoxynivalenol (PubChem CID: 40024), zearalenone (PubChem CID: 5281576), hydroxymethylfurfural (PubChem CID: 237332) were retrieved in SMILES format from the PubChem database [20].
The three-dimensional crystal structure of human CYP2E1 was obtained from the Protein Data Bank (PDB ID: 3E6I). Prior to docking, the protein structure was prepared using AutoDockTools (v1.5.7) [21]. Preparation steps included removal of water molecules and heteroatoms, addition of polar hydrogens, and assignment of Gasteiger charges. The protein structure was then saved in the PDBQT format required for docking simulations.
Molecular docking simulations were carried out using AutoDock Vina [22]. The active site of CYP2E1 was defined based on the co-crystallized ligand position in the PDB structure. For each ligand, ten docking runs were performed, and the best binding pose was selected based on the lowest binding free energy (ΔG, kcal/mol) and consistency of binding orientation. Docking setup was validated by re-docking the co-crystallized ligand, successfully reproducing the experimentally observed binding orientation and confirming the reliability of the docking predictions. The grid box dimensions were optimized individually for each ligand.
Molecular docking analysis revealed distinct interaction profiles of selected food contaminants with the active site of CYP2E1 (Table 1). 3-MCPD exhibited a modest binding score (ΔG = –4.1 kcal/mol), interacting via hydrogen bonds with ARG126, ILE114, and ARG100, while additional alkyl contacts with ILE115 and ILE114 contributed to its binding orientation. However, an unfavorable acceptor–acceptor interaction was observed with VAL436, which may further explain its relatively low docking energy (Figure 1). Similarly, acrylamide showed relatively weak binding affinity (ΔG = –3.7 kcal/mol), stabilized mainly by conventional hydrogen bonds with THR307 and THR304, along with a Pi-alkyl interaction involving PHE430 (Figure 2).
The docking results of food contaminants on CYP2E1.
| Protein | Food contaminants | Amino acid | Interactions | ΔG (kcal/mol) |
|---|---|---|---|---|
| CYP2E1 | 3-MCPD | ARG126, ILE114, ARG100 | Conventional hydrogen bonds | –4.1 |
| ILE115, ILE114 | Alkyl | |||
| VAL436 | Unfavorable acceptor–acceptor | |||
| Acrylamide | THR307, THR304 | Conventional hydrogen bonds | –3.7 | |
| PHE430 | Pi-alkyl | |||
| Glycidyl stearate | GLU358, THR303 | Conventional hydrogen bonds | –6.4 | |
| VAL364, CYS437, LEU368, ILE115, ALA299 | Alkyl | |||
| PHE207, PHE478 | Pi-alkyl | |||
| PHE298 | Pi-sigma | |||
| Deoxynivalenol | THR58, TYR398, CYS480, ASP394 | Conventional hydrogen bonds | –7.2 | |
| TYR398 | Pi-alkyl | |||
| ILE476 | Alkyl | |||
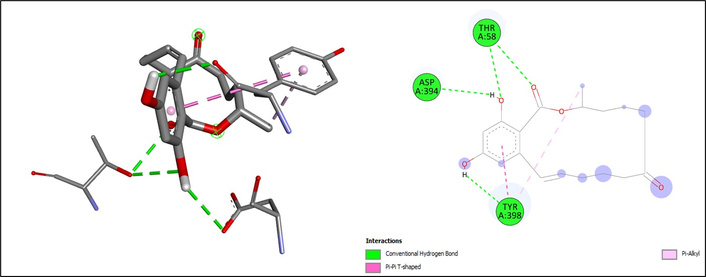
| Zearalenone | ASP394, THR58, TYR398 | Conventional hydrogen bonds | –7.9 | |
| TYR398 | Pi-alkyl | |||
| TYR398 | Pi-Pi T-shaped | |||
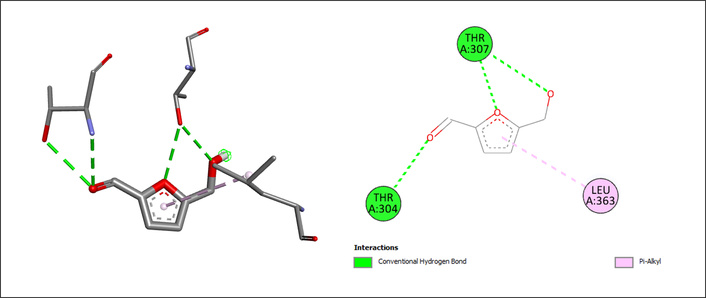
| Hydroxymethylfurfural | THR304, THR307 | Conventional hydrogen bonds | –5.3 | |
| LEU363 | Pi-alkyl |
CYP2E1: cytochrome P450 2E1; 3-MCPD: 3-monochloropropane-1,2-diol.
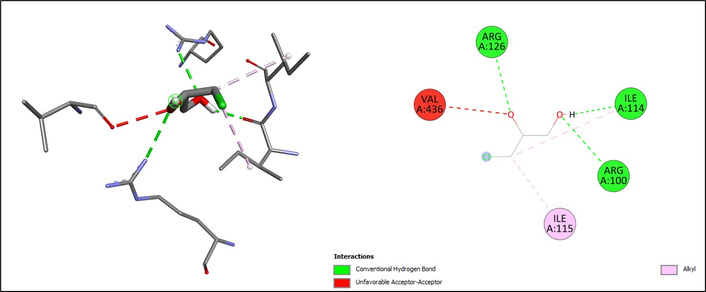
The docking results of 3-MCPD on CYP2E1. The chemical structure of 3-MCPD was retrieved from the PubChem database (PubChem CID: 7290) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1; 3-MCPD: 3-monochloropropane-1,2-diol.
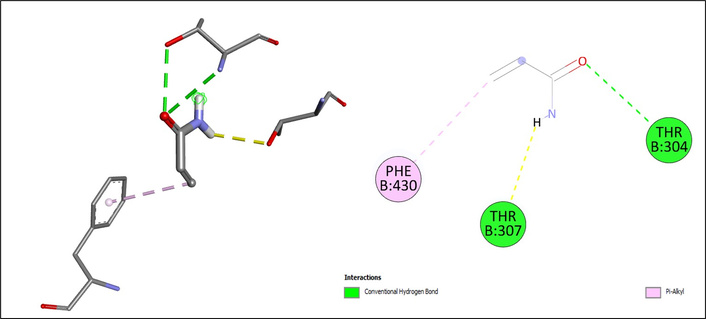
The docking results of acrylamide on CYP2E1. The chemical structure of acrylamide was retrieved from the PubChem database (PubChem CID: 6579) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1.
In contrast, glycidyl stearate displayed a stronger affinity (ΔG = –6.4 kcal/mol), supported by multiple hydrophobic and aromatic interactions. The ligand formed hydrogen bonds with GLU358 and THR303, as well as extensive alkyl contacts with VAL364, CYS437, LEU368, ILE115, and ALA299. Moreover, Pi-alkyl and Pi-sigma interactions involving PHE207, PHE478, and PHE298 highlighted its favorable stabilization within the binding cavity (Figure 3).
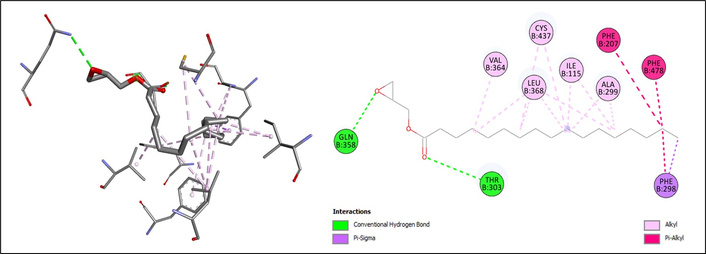
The docking results of glycidyl stearate on CYP2E1. The chemical structure of glycidyl stearate was retrieved from the PubChem database (PubChem CID: 62642) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1.
Among the tested compounds, mycotoxins demonstrated the highest binding affinities. Deoxynivalenol exhibited a binding energy of –7.2 kcal/mol, forming hydrogen bonds with THR58, TYR398, CYS480, and ASP394, together with hydrophobic interactions at TYR398 (Pi-alkyl) and ILE476 (alkyl) (Figure 4). Zearalenone showed the strongest binding (ΔG = –7.9 kcal/mol), characterized by hydrogen bonding with ASP394, THR58, and TYR398, as well as Pi-alkyl and Pi-Pi T-shaped interactions at TYR398, suggesting a highly stable orientation in the catalytic pocket (Figure 5).
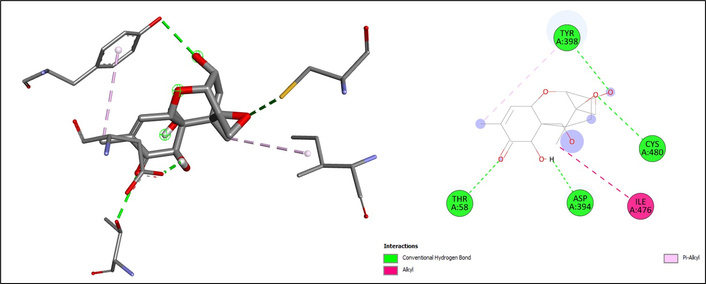
The docking results of deoxynivalenol on CYP2E1. The chemical structure of deoxynivalenol was retrieved from the PubChem database (PubChem CID: 40024) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1.
The docking results of zearalenone on CYP2E1. The chemical structure of zearalenone was retrieved from the PubChem database (PubChem CID: 5281576) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1.
Hydroxymethylfurfural exhibited a docking score of –5.3 kcal/mol, representing an intermediate binding affinity. The ligand formed two conventional hydrogen bonds with THR304 and THR307, and a Pi-alkyl interaction with LEU363, which contributed to its stabilization within the CYP2E1 active site (Figure 6).
The docking results of hydroxymethylfurfural on CYP2E1. The chemical structure of hydroxymethylfurfural was retrieved from the PubChem database (PubChem CID: 237332) and prepared using AutoDockTools (v1.5.7). CYP2E1: cytochrome P450 2E1.
These findings show that CYP2E1 binds food contaminants with varying affinities depending on their structure. Simple molecules such as acrylamide and 3-MCPD exhibited weak interactions, whereas glycidyl esters and mycotoxins showed stronger binding. This pattern highlights the role of CYP2E1 in metabolizing structurally complex contaminants, which may increase the risk of toxic metabolite formation and adverse health effects.
Food contamination remains a major public health concern, as both process-induced toxicants and natural contaminants can enter the food chain and exert harmful effects on humans [23]. Compounds such as acrylamide and 3-MCPD are generated during thermal food processing, while glycidyl esters arise from refined oils, and mycotoxins like deoxynivalenol and zearalenone occur naturally in cereals [24]. These contaminants have been linked to carcinogenicity, endocrine disruption, and oxidative stress. In this study, the molecular interactions of these contaminants with CYP2E1, a key enzyme in xenobiotic metabolism, were investigated to provide insights into their potential bioactivation and contribution to toxic outcomes. In another study, in silico models were employed to prioritize heat-induced food contaminants for mutagenicity and carcinogenicity testing [25]. Recent evidence has also highlighted the pregnane X receptor (PXR) as an alternative molecular target for food contaminants. A study demonstrated that compounds such as bisphenol A, phthalates, chlorpyrifos, and zearalenone can disrupt endocrine signaling through PXR-mediated pathways, as confirmed by binding assays, reporter gene experiments, and molecular docking analyses [26]. In another study, Dellafiora et al. [27] applied computational approaches to investigate the molecular interactions of mycotoxins, highlighting potential toxicological effects. These findings support the relevance of in silico methods in predicting food contaminant bioactivity, although experimental validation remains essential to fully understand their impact on humans. In this study, the molecular interactions of several food contaminants, including acrylamide, 3-MCPD, glycidyl stearate, deoxynivalenol, and zearalenone, with human CYP2E1 were investigated. Docking analyses indicated that small polar compounds, such as acrylamide and 3-MCPD, exhibited relatively weak binding, whereas larger hydrophobic or aromatic compounds, particularly glycidyl stearate and mycotoxins, were found to form stronger affinities and multiple stabilizing interactions within the enzyme’s active site. Residues such as TYR398, ASP394, and GLU358 play a critical role in ligand positioning within the CYP2E1 active site. The observed interactions suggest that ligands forming stable hydrogen bonds with these residues are oriented closer to the heme catalytic center, potentially facilitating metabolic activation reactions. For example, zearalenone’s stabilization via TYR398 and ASP394 may favor oxidative activation, which aligns with its known estrogenic toxicity. Conversely, weaker binding orientations observed for acrylamide and 3-MCPD suggest less favorable positioning for catalytic turnover, consistent with their modest binding affinities. These observations suggest that CYP2E1 may preferentially interact with structurally complex contaminants, potentially affecting their metabolic activation and associated toxic effects. The weak binding of acrylamide to CYP2E1 is consistent with its known biotransformation into glycidamide via CYP2E1-mediated oxidation, a process strongly associated with its genotoxic potential. In contrast, zearalenone’s strong binding affinity is in agreement with its reported endocrine-disrupting activity, which is mediated through metabolic activation and receptor interactions. Likewise, the relatively high affinity of glycidyl stearate supports its recognized genotoxicity following conversion to glycidol. These contextual associations between docking outcomes and established toxicological data underline the relevance of CYP2E1 as a critical mediator of food contaminant toxicity.
Food contaminants have been increasingly recognized as contributors to a variety of human diseases. Process-induced compounds, such as acrylamide, 3-MCPD, and glycidyl esters, as well as naturally occurring mycotoxins like deoxynivalenol and zearalenone, have been associated with carcinogenicity, neurotoxicity, endocrine disruption, and oxidative stress. Chronic exposure to these contaminants through diet may lead to the development or exacerbation of metabolic disorders, liver dysfunction, hormonal imbalances, and disruption of the gut microbiota [28]. Understanding their molecular interactions with xenobiotic-metabolizing enzymes and receptors, such as CYP2E1 and PXR, is therefore crucial for assessing potential health risks and implementing effective food safety measures. In another study, trichothecene mycotoxin deoxynivalenol has been examined through computational docking. The results obtained indicated that flavonoids could be used against the pathological effects of zearalenone [29]. In the present study, in silico studies highlight that both process-induced contaminants and naturally occurring mycotoxins may undergo enzyme-mediated transformations, underlining the value of computational approaches for predicting metabolic fate and potential toxicological outcomes.
In summary, the present study demonstrates that food contaminants exhibit variable binding affinities to human CYP2E1, with small polar compounds showing weak interactions and larger hydrophobic or aromatic molecules, including glycidyl esters and mycotoxins, displaying stronger stabilization within the enzyme’s active site. These interactions suggest that CYP2E1 may play a key role in the metabolic activation of structurally complex contaminants, potentially influencing their toxicological outcomes. The findings highlight the utility of in silico approaches for predicting enzyme–contaminant interactions and provide mechanistic insights that may aid in risk assessment of dietary toxicants. Moreover, chronic exposure to these compounds could contribute to metabolic, hepatic, hormonal, and gut microbiota disturbances, underscoring the importance of monitoring and controlling food contaminant levels to protect human health. Moreover, it should be noted that molecular docking represents an initial step in predicting enzyme–ligand interactions and does not replace molecular dynamics simulations, ΔG calculations, or in vitro validation. While docking provides useful mechanistic hypotheses, further computational and experimental studies are required to confirm the stability and biological relevance of these interactions. In this regard, our work should be considered as a hypothesis-generating framework that can guide the prioritization of food contaminants for subsequent experimental toxicological evaluations.
3-MCPD: 3-monochloropropane-1,2-diol
CYP2E1: cytochrome P450 2E1
PDB: Protein Data Bank
PXR: pregnane X receptor
ΔG: binding free energy
SS: Conceptualization, Investigation, Visualization, Supervision, Resources, Methodology, Writing—original draft, Writing—review & editing. The author read and approved the submitted version.
The author declares that there are no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the author, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2026.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2026. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 372
Download: 37
Times Cited: 0
Olga Pardo, Francesc A. Esteve-Turrillas
Clara Ochoa-Esteso ... María Jesús Lerma-García
Daniel Gallart-Mateu
Gabriel Mustatea ... Elena L. Ungureanu
