Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Affiliation:
Department of Translational Research, College of Osteopathic Medicine of the Pacific, Western University of Health Sciences, Pomona, CA 91766, USA
Email: vrai@westernu.edu
ORCID: https://orcid.org/0000-0001-6286-2341
Explor Endocr Metab Dis. 2025;2:101447 DOI: https://doi.org/10.37349/eemd.2025.101447
Received: July 25, 2025 Accepted: November 06, 2025 Published: November 10, 2025
Academic Editor: Andreas Barthel, Universitätsklinikum Carl Gustav Carus Dresden, Germany
The article belongs to the special issue The Fountain of Youth: Decoding the Hormonal Regulation of Aging
Hormonal dysregulation plays a central role in aging, exerting a profound, multidimensional impact on quality of life. This narrative review examines the impact of hormonal changes in understanding how age-dependent alterations in key hormonal axes, specifically those related to insulin, IGF-1, cortisol, thyroid hormones, parathyroid hormone, and sex hormones, affect metabolic, musculoskeletal, cognitive, and reproductive health. Altered hormone secretion and receptor sensitivity contribute to conditions such as type 2 diabetes mellitus, osteoporosis, sarcopenia, cognitive decline, and disrupted sleep patterns. Age-related shifts in thyroid and parathyroid function, including decreased T3 conversion and elevated PTH, further compound these physiological changes. These hormonal imbalances manifest as a multidimensional burden on quality of life, encompassing physical, cognitive, and psychosocial domains, which are particularly pronounced in postmenopausal women. Emerging therapies targeting GH secretion, myostatin inhibition, heat shock proteins, and IGF-1 offer promising avenues for mitigating age-associated symptoms and improving quality of life.
Aging is a complex biological process characterized by gradual physiological, cellular, and molecular alterations that affect nearly all organ systems. Among the most profound and systemic changes are those involving the endocrine system, where the synthesis, secretion, and regulatory control of hormones undergo significant modifications [1]. Hormones are essential for maintaining homeostatic balance, orchestrating metabolic processes, supporting cognitive and emotional functioning, and sustaining musculoskeletal integrity [2]. With advancing age, there is a progressive decline in the circulating levels of several key hormones, including insulin, insulin-like growth factor-1 (IGF-1), cortisol, thyroid hormones, parathyroid hormone/parathormone (PTH), and others involved in metabolic and endocrine regulation [3].
The cumulative impact of hormonal imbalance with aging plays a critical role in the development of various aging syndromes, including frailty, sarcopenia, osteoporosis, cognitive impairment, and affective disorders [4]. These conditions are strongly associated with decreased functional capacity, greater healthcare utilization, and loss of independence; factors that substantially diminish the quality of life in the elderly. Notably, endocrine changes do not occur in isolation; rather, they interact dynamically with lifestyle factors, comorbidities, and environmental influences to shape the aging process [5]. This is exemplified by the interplay between hormonal factors, such as cortisol and thyroid hormones, and lifestyle behaviors, including diet and physical activity, which collectively modulate key metabolic processes such as glucose homeostasis, lipid storage, and the preservation of skeletal muscle mass [6]. A comprehensive understanding of these endocrine alterations is essential for designing targeted interventions that support healthy aging and enhance quality of life in the elderly population.
In addition to their role in functional decline and the development of aging syndromes, age-related hormonal alterations play a pivotal role in the onset and progression of chronic diseases that further impact quality of life. These hormonal changes can critically influence overall health and quality of life in older adults. Changes in endocrine and metabolic function with aging are particularly significant in the pathogenesis of type 2 diabetes mellitus (T2DM) and thyroid disorders [7]. T2DM, a complex metabolic disorder marked by chronic hyperglycemia, primarily results from insulin resistance and, over time, impaired β-cell function [8]. These metabolic disturbances are often exacerbated by dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis and proinflammatory signaling, which collectively impair insulin action and glucose homeostasis [9]. Similarly, thyroid disorders, including both hypothyroidism and hyperthyroidism, frequently arise from dysfunction of the hypothalamic-pituitary-thyroid (HPT) axis, autoimmune mechanisms, and age-associated changes in thyroid hormone production and regulation [10]. These endocrine imbalances not only disrupt systemic metabolic stability but also interact with genetic susceptibility, environmental exposures, and lifestyle factors to facilitate disease development and progression, ultimately exerting profound effects on physical, cognitive, and emotional aspects of quality of life in the aging population. This narrative review aims to discuss the mechanisms by which hormonal changes associated with aging affect metabolic regulation, endocrine health, and overall quality of life.
Aging is characterized by a complex and dynamic shift in hormonal regulation that influences virtually all physiological systems. These endocrine alterations encompass both reductions and elevations in specific hormonal signals, collectively contributing to changes in metabolic function, stress responsiveness, tissue maintenance, and the overall physiological capacity of organ systems [11]. Notable among the declining hormones are the sex steroids, estrogens, progesterone, and testosterone, which exhibit significant age-related reductions (Figure 1). In women, the transition to menopause typically results in an abrupt decline in estrogen and progesterone levels around the fifth decade of life [12]. In contrast, men undergo a more gradual decrease in testosterone, beginning as early as the third or fourth decade [13]. The decline in dehydroepiandrosterone (DHEA) levels is also associated with obesity and its related complications [14]. For example, obesity is associated with increased leptin production from fat tissue, but also with leptin resistance, meaning the body doesn’t respond to the hormone’s signal to reduce appetite and increase energy expenditure (Figure 1).
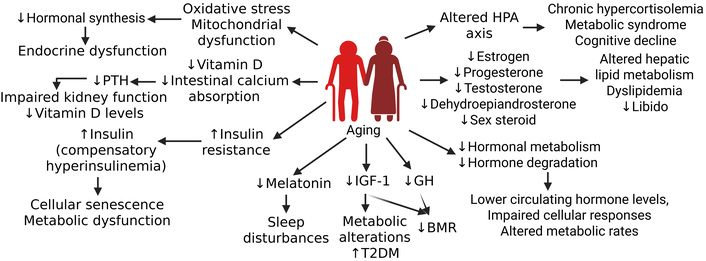
Effects of aging on hormone synthesis, metabolism, and its effects on physiology, cell function, and behavior. Aging results in decreased hormone synthesis, metabolism, and degradation. Decreased hormones in the body cause altered metabolism, metabolic syndrome, sleep disturbance, cellular senescence, and other metabolic alterations in the body. BMR: basal metabolic rate; GH: growth hormone; HPA axis: hypothalamic-pituitary-adrenal axis; IGF-1: insulin-like growth factor 1; PTH: parathyroid hormone; T2DM: type 2 diabetes mellitus. Created in BioRender. Rai, V. (2025) https://BioRender.com/jiuxure
Growth hormone (GH), IGF-1, and melatonin also decrease with age, which results in metabolic and disrupted sleep symptoms [14, 15]. Hormones that tend to increase or become dysregulated with advancing age include PTH, cortisol, and insulin. Elevated PTH levels are commonly attributed to age-related declines in vitamin D and impaired intestinal calcium absorption, which stimulate compensatory PTH secretion [16] (Figure 1). Although basal cortisol concentrations may remain stable or show mild elevation, circadian amplitude diminishes with age, leading to a blunted nocturnal nadir and morning peak, which results in prolonged tissue exposure to glucocorticoids [17]. Furthermore, insulin resistance increases with age, marked by post-receptor defects in insulin signaling and reduced peripheral glucose disposal; this often coincides with compensatory hyperinsulinemia [18] (Figure 1). Prolonged elevation of insulin not only perpetuates resistance but may actively contribute to cellular senescence and metabolic dysfunction. Other notable endocrine alterations associated with aging include changes in hormone metabolism, as the efficiency of hormonal degradation and clearance declines over time [19]. Additionally, age-related increases in oxidative stress and mitochondrial dysfunction can further disrupt hormone synthesis and impair the integrity of signaling pathways, thereby contributing to broader dysregulation of endocrine function [20] (Figure 1).
Aging is associated with significant hormonal alterations that critically influence metabolic homeostasis. A decline in basal metabolic rate is partly attributable to the reduction of lean body mass and decreased secretion of GH and IGF-1 [21]. The prevalence of insulin resistance increases with age, linked to dysregulated adipokines, including leptin and adiponectin, alongside reduced physical activity and augmented visceral adiposity [22]. Moreover, age-related declines in sex steroids, particularly estrogen and testosterone, contribute to the development of dyslipidemia through disruptions in hepatic lipid metabolism [23] (Figure 1). These endocrine changes further influence the development of sarcopenia and a redistribution of adipose tissue, as diminished anabolic signaling mediated by GH, testosterone, and estrogen results in skeletal muscle atrophy concomitants with increased central fat deposition.
Aging induces significant alterations in the HPA axis, resulting in dysregulated stress hormone dynamics and downstream physiological consequences (Figure 1). A fundamental consequence of aging is the reduced sensitivity of target tissues to hormonal stimuli, which may result from alterations in receptor density, binding affinity, or impairments in downstream intracellular signaling pathways [24]. Another prominent change is the flattening of the diurnal cortisol rhythm, characterized by elevated evening and nocturnal cortisol levels, which have been associated with hippocampal atrophy, impaired memory function, and suppression of immune responses [25]. Additionally, diminished negative feedback sensitivity within the HPA axis may prolong stress hormone exposure, contributing to chronic hypercortisolemia, metabolic syndrome, and cognitive decline [26] (Figure 1). Aging is also accompanied by reduced responsiveness to catecholamines, which impairs cardiovascular and metabolic adaptability to stress, exacerbating vulnerability to both acute and chronic stressors [27]. These cumulative alterations in HPA axis regulation with age not only disrupt homeostatic balance but also heighten susceptibility to stress-related pathologies and age-associated functional decline.
As aging progresses, associations can be made with alterations in glucose metabolism [28]. This consists of a changing balance of hormones, including insulin, IGF-1, and cortisol, collectively disrupting metabolic equilibrium. This then contributes to the increased pervasiveness among older adults, with nearly half of all individuals with T2DM being over the age of 65 years [29].
Insulin has a central role in glucose regulation and uptake. Peripheral insulin resistance occurs as target tissues such as muscle, liver, and adipose tissue develop decreased sensitivity to insulin. For example, as skeletal muscle ages, features such as decreased mitochondrial function, increased inflammation, decreased enzyme activities, decreased muscle mass, and an over-activated renin-angiotensin system occur, contributing to resistance to insulin [30] (Figure 2). While in adipose tissue, age-dependent changes in distribution, adipogenesis, browning characteristics, and lipotoxicity lead to resistance [31]. Overall, insulin resistance can be attributed to a combination of several factors, such as inflammation, oxidative stress, decreased physical activity, or altered adipokine expression (Figure 2). This is compensated by the body, resulting in hyperinsulinemia and a possible acceleration in aging, further compounding the effects [32]. Eventually, β-cell function may be impaired due to the previously stated factors of aging and insulin resistance, leading to a decline in insulin production and T2DM.
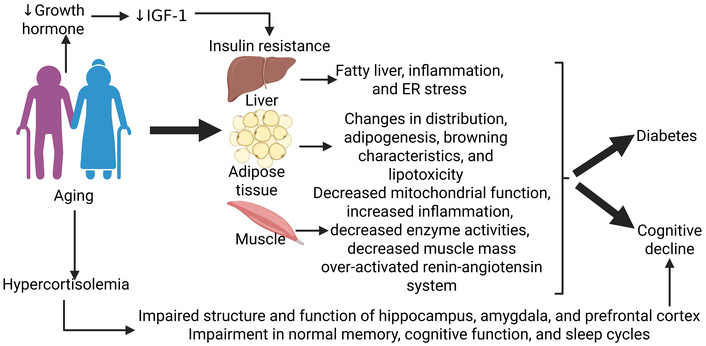
Aging associated insulin resistance and diabetes. Insulin resistance with aging due to multiple contributors results in development of diabetes and altered metabolism may result in cognitive decline. IGF-1: insulin-like growth factor-1. Created in BioRender. Rai, V. (2025) https://BioRender.com/35e29u1
IGF-1 is an anabolic hormone mainly secreted by the liver and transported to other tissues where it aids in growth, development, and metabolism [33]. A decreased secretion of GH, followed by a decreased IGF-1 over time, is detected in individuals over the age of 60 years, when they are in very low levels, a phenomenon known as “somatopause”. This results in insulin resistance due to a decrease in insulin sensitivity and, therefore, less glucose being transported to peripheral tissues [34] (Figure 2). In addition to IGF-1, insulin-like growth factor binding proteins (IGFBPs) further modulate cell signaling. They work to inhibit or even enhance IGF-1 receptors as well as modulate cell survival, migration, metabolism, and other cellular events [34]. These IGFBPs then further regulate bioavailability and receptor interaction, causing dysregulation to exacerbate the effects of declining IGF-1 levels, demonstrating the relationship between aging and decreased suppression of serum IGFBP-1 by insulin [35]. Reduced IGF-1 signaling also contributes to conditions such as sarcopenia and decreased metabolic flexibility, further compounding the effects of aging [36].
Another key hormone in the interplay between diabetes and aging is cortisol. Cortisol is the main glucocorticoid released from the zona fasciculata of the adrenal cortex and is regulated by the HPA axis. In addition to its role in stress, immune function, and the inflammatory response, it is also vital in regulating metabolism [37]. With aging, there is a general increase in mean daily serum cortisol levels. The excess of glucocorticoids, or hypercortisolemia, in the elderly population can significantly affect the structure and function of areas of the brain, including the hippocampus, amygdala, and prefrontal cortex, with consequent impairment in normal memory, cognitive function, and sleep cycles [38] (Figure 2). However, there are also specific effects that contribute to insulin resistance. Glucocorticoids promote gluconeogenesis by inducing expression of gluconeogenic genes in the liver and by suppressing glucose uptake in skeletal muscle and adipocytes [inhibiting translocation of glucose transporter type 4 (GLUT 4) to the cell surface], which induces insulin resistance [39]. Loss of regulation to this intricately regulated mechanism can lead to diabetes as well as cortisol excess disorders (such as Cushing syndrome) or cortisol insufficiency (such as Addison’s disease).
The combined impact of all these hormonal shifts has a significant impact on aging adults. Specifically, the elderly population with diabetes suffers from shifts in quality of life and body function. Chronic hyperglycemia leads to damage and failure of both macrovascular and microvascular systems as seen in various organs, especially the heart, blood vessels, eyes, kidneys, and nerves [40]. Older age diabetic patients often experience overall functional decline, increased frailty and disability, and reduced mobility and independence [41]. Chronic inflammation and vascular damage due to insulin resistance also contribute to cognitive impairment and associated symptoms, as previously mentioned. All of these, coupled with the typical symptoms of diabetes, including but not limited to polyuria, polydipsia, polyphagia, blurred vision, and slow wound healing, reduce daily functioning and overall quality of life. For example, functional decline leads to decreased sensorimotor function, musculoskeletal deficits, and pharmacological complications that increase dependency, increase risk of falls, and compromise quality of life [42].
Like those involved in aging and diabetes, thyroid and PTHs are also significantly altered throughout the aging process. The thyroid and parathyroid glands affect the majority of systems in the body, including metabolism, growth, muscle function, thermoregulation, and bone health, all of which can be negatively impacted due to aging [43]. The major hormones involved are the thyroid hormones (T3 and T4) and PTH. The thyroid gland produces approximately 90% inactive thyroid hormone (T4) and 10% active thyroid hormone (T3). T4 is converted into T3 by deiodinase enzymes in peripheral tissues such as the liver and kidneys [44]. With aging, the efficiency of this conversion declines due to reduced deiodination, resulting in altered levels of active and inactive thyroid hormones, including increased levels of the inactive reverse T3 (rT3) [45]. This age-related reduction in deiodinase activity, combined with tissue-specific variation in enzyme expression, leads to diverse effects on thyroid hormone availability and action in various organs. Thyroid hormones, particularly T3, affect organs and tissues throughout the body, ultimately leading to increased metabolic rate and protein synthesis. This results in increasing cardiac output and heart rate, basal metabolic rate and oxygen consumption, wakefulness and alertness, and dysregulation of normal reproductive function in both men and women [44]. With varying thyroid hormones with aging, the prevalence of thyroid dysfunction also increases [46].
In addition to peripheral metabolic changes, central regulation of thyroid hormones also becomes impaired. The hypothalamus may produce less TRH (thyrotropin-releasing hormone), and the pituitary gland may become less responsive to TRH, leading to reduced TSH (thyroid-stimulating hormone) secretion [47]. The bioactivity of TSH itself may decline due to structural changes or reduced receptor binding affinity. Compounding this, the thyroid gland’s sensitivity to TSH may also decrease with age, requiring higher TSH levels to achieve normal thyroid hormone production [48]. TSH levels’ effects are still being studied; however, it is currently believed that they do not naturally rise with age. Nonetheless, they aren’t currently associated with an increased overall mortality risk [49].
Additionally, the number and activity of thyroid hormone receptors (TRs) in target tissues may undergo age-related changes, affecting the cellular response to thyroid hormones. These alterations may affect the genomic and non-genomic actions of thyroid hormones. Non-genomic pathways, those not involving direct gene transcription, are affected by thyroid hormones interacting with cell membrane receptors, affecting cellular processes like angiogenesis and cell proliferation [50]. Thyroid hormones can additionally crosstalk with other signaling pathways such as those involving RXRs, vitamin D receptors, and retinoic acid receptors, influencing gene expression and cellular responses [51].
Aging is also associated with structural and immune-related thyroid changes. Autoimmune thyroid diseases (AITD), such as Hashimoto’s thyroiditis and Graves’ disease, become more common with age, contributing to thyroid dysfunction [52]. This increase is linked to genetic susceptibility, including variations in immune-regulatory genes like CTLA4, and age-related chronic inflammation, which can interfere with thyroid hormone synthesis, signaling, and action [53]. Furthermore, thyroid autoimmunity, development of nodules, and structural changes like goiter become more prevalent in older adults, further contributing to variability in thyroid function [54].
In general, dysfunction can manifest in the form of hyperthyroidism and an increased risk of osteoporosis, cardiac arrhythmias, and heart failure [55] or in the form of hypothyroidism with aging, which is associated with coronary artery disease and neurocognitive impairment [56]. Subclinical hypothyroidism, defined by normal free T4 hormone and increased TSH levels, is due to a decline in the conversion of inactive and free T4 into T3 and resulting changes in thyroid hormone transport and receptor sensitivity [57].
The parathyroid is made up of 4 separate glands, with the main function being the production and secretion of PTH. PTH’s main role is in serum calcium homeostasis, with levels of PTH and serum calcium being inversely proportional [58]. In the kidneys, PTH increases the reabsorption of calcium, blocks phosphate reabsorption, and stimulates vitamin D synthesis, specifically by converting 25-hydroxyvitamin D [25(OH)D] into its active form, 1,25-dihydroxyvitamin D [1,25(OH)2D], also known as calcitriol. While in the bone, PTH inhibits osteoblast activity and stimulates osteoclast activity, resulting in bone breakdown and calcium release. Overall, PTH levels have been shown to increase with age and are often the result of decreased intestinal calcium absorption, reduced dietary calcium intake, and insufficient vitamin D levels in the body [59]. Low levels of vitamin D contribute to the age-related bone loss and an increase in osteoporosis due to the consequential downregulation of its normal negative feedback loop on PTH secretion while trying to maintain calcium homeostasis [60].
Changes in both thyroid hormones and PTH levels have significant physiological consequences. In addition to effects on energy metabolism, alterations in T3 and T4 levels also lead to weight fluctuations, fatigue, and altered thermoregulation [61]. Loss of skeletal muscle strength, function, and mass also occurs with aging in individuals with hypothyroidism and hyperthyroidism [62]. It is still unclear whether the increase in PTH associated with aging has significant consequences or is not noteworthy [63]. Some studies have suggested correlations between PTH levels and mortality rates in aging populations. Either due to factors such as PTH levels and low bone mineral density leading to increased mortality [64], or the elderly having higher levels of PTH with a higher prevalence for falls and fractures [65]. Overall, the combined effects of thyroid and parathyroid changes decrease well-being and quality of life in older individuals. Osteoporosis, sleep disruptions, memory impairments, muscle weakness, and unexplained fatigue are only a few of the reasons that careful monitoring and management are necessary for maintaining quality of life in aging populations.
Hormonal changes related to aging can have profound effects on all aspects of a person’s quality of life. For example, a decrease in GH levels due to a progressive decrease in pulsatile GH secretions is associated with muscle weakness and a reduction in lean body mass with an increase in body fat [43]. Specifically talking about increases in body fat, postmenopausal women experience a significant drop in E2 levels, contributing to increased low-density lipoprotein (LDL) levels. This results in higher levels of subcutaneous and visceral body fat contributing to central obesity and metabolic syndrome, a cluster of conditions that increase the risk of heart disease, stroke, and T2DM [12] (Figure 3). As discussed previously, insulin resistance in the elderly and, more so, with diabetics can play a major role in the development of these characteristics. Another change that occurs due to hormonal changes with aging is a more rapid onset of fatigue. Studies have shown that fatigue in the elderly population due to hormonal changes was associated with high TSH levels due to low thyroid activity (low T3 levels). The main symptoms in the elderly population were feeling tired or worn out and having difficulty climbing stairs [66]. This further reinforces the need for additional emphasis on thyroid/parathyroid dysfunction with aging, as mentioned earlier in this article, especially with regard to osteoporosis that may develop. PTH becomes excessively important when dealing with aging, with its relation to bone health, and should be monitored regularly in clinical settings.
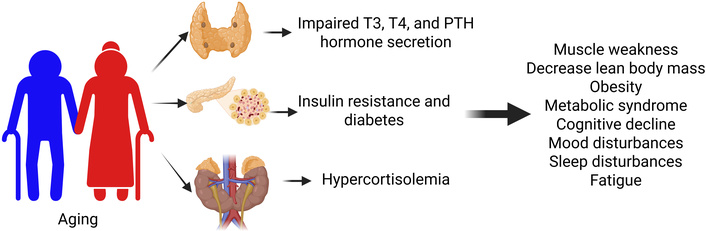
Aging and its effects due to impaired thyroid function, parathyroid function, and insulin resistance. PTH: parathyroid hormone. Created in BioRender. Rai, V. (2025) https://BioRender.com/j2tvtxv
Changes in hormone levels with aging go beyond just the impacts on general health, as they can also affect various mental factors (Figure 3). Hormonal decline and imbalances lead to changes in mental state, mood, and cognition. For example, postmenopausal women compared to premenopausal women reported an increase in forgetfulness and brain fog [67]. Regarding mood, an imbalance in stress and sex hormone responses with aging leads to mood and anxiety disorders. These symptoms are more common in females compared to males due to the drastic drop in hormone levels post menopause [68]. As mentioned earlier, we must be aware of the ages at which men and women start experiencing hormonal changes to most effectively preserve their quality of life with regard to the symptoms presented. Lastly, regarding cognition with aging, it was found that cognitive ability declines, especially with verbal recall, attention, working memory, and processing speed [69]. These findings have largely been because of the decrease in estradiol levels along with decreased nicotinic cholinergic binding sites, which reduces cholinergic acetyl transferases and contributes to cognitive decline [69].
Reduced IGF-1 signaling can impair neurogenesis, the formation of new neurons, particularly in the hippocampus—a region critical for learning and memory. Synaptic plasticity is the ability of synapses to strengthen or weaken over time and is fundamental to memory formation. IGF-1 is a key regulator of synaptic plasticity, and its decline impairs synaptic function, contributing to cognitive deficits. IGF-1 has neuroprotective properties, and its decline leaves neurons more vulnerable to age-related stress and damage. It is a critical factor for neuronal survival and function. IGF-1 helps maintain the proper function of astrocytes, a type of glial cell that provides support to neurons, and a decline in IGF-1 will have adverse effects [70, 71]. Circulating IGF-1 is a protective factor for the cerebrovasculature, and age-related decline in IGF-1 is linked to impaired cerebral blood flow and altered neurovascular coupling. IGF-1 signaling can help to attenuate inflammation in the brain, a common feature of aging and neurodegenerative diseases [72].
Hypothyroidism, common in the elderly, can cause a range of cognitive impairments, including slowed thinking and memory loss. The brain requires a significant amount of energy to function. Hypothyroidism reduces the brain’s uptake of glucose, leading to hypometabolism, which is a hallmark of many forms of cognitive decline, including Alzheimer’s disease. Insufficient thyroid hormones can lead to a systemic increase in inflammation and oxidative stress, causing damage to neurons and other brain cells. Thyroid hormones regulate the expression of a vast number of genes, many of which are essential for normal brain function, including the amyloid precursor protein (APP), which is central to Alzheimer’s pathology. Thyroid hormones are critical for mitochondrial function, and their deficiency impairs energy production in brain cells. This mitochondrial dysfunction is a key feature of aging and neurodegenerative diseases. Studies have shown that a specific thyroid hormone-responsive protein (THRP) can induce cell death (necrosis) in neuronal cells, mediating some of the neurotoxic effects of low thyroid hormone. Hypothyroidism is associated with higher levels of atherogenic lipids and hypertension, which increase the risk of stroke. This can contribute to vascular dementia and other forms of cognitive impairment [73–75].
Along with mental and physical changes with hormonal decline in aging, sleep is also affected. Many people notice changes to their sleep patterns once they reach their mid-40s to 50s, with women typically being more symptomatic. In women, sleep disturbances are correlated with a decrease in their estrogen levels after menopause [76] (Figure 3). In men, the sleep cycle changes tend to be related to an imbalance in testosterone and cortisol signaling. The alterations and misalignment of the signaling of those two hormones lead to interrupted sleep [77]. Sleep disturbances in aging are linked to molecular and cellular changes that accelerate the biological aging process, including increased oxidative stress, inflammation, and DNA damage, which leads to accelerated telomere shortening, cellular senescence, and mitochondrial dysfunction. Poor sleep quality impairs the body’s ability to repair itself, leading to a buildup of misfolded proteins and contributing to the risk of age-related diseases like neurodegenerative disorders. Chronic sleep deprivation increases oxidative stress and triggers an inflammatory response, indicated by elevated levels of inflammatory markers like C-reactive protein (CRP) and interleukin-6 (IL-6). Sleep disturbances contribute to DNA damage and promote cellular senescence, or the aging of cells. This is linked to the shortening of telomeres, the protective caps on the ends of chromosomes. Mitochondrial dysfunction may be caused by increased oxidative stress and the activation of mitochondrial stress pathways. Sleep deprivation, particularly in older adults, can activate the DNA damage response and promote the senescence-associated secretory phenotype (SASP), which is a pro-inflammatory profile that further drives cellular aging and increases the risk for chronic diseases. Insufficient sleep has been shown to cause epigenetic alterations, such as a downward tendency in DNA methylation. These changes can play a role in the pathogenesis of diseases associated with sleep apnea and aging [78–81]. Additionally, with a decrease in testosterone levels, it was found that men can experience symptoms such as reduced appetite, decreased sexual function, and impaired regulation of body temperature [82]. Overall, it is important to understand and be aware of the various consequences that may arise due to hormonal changes with aging.
As mentioned, and reinforced throughout this review, hormone imbalances and reduced levels with aging affect multiple aspects of the body. Thus, it is important to discuss strategies to mitigate these effects. T2DM is more common in women and even more prevalent in post-menopausal women due to the lack of protective hormones. Studies have found that menopausal hormonal therapy has been effective in reducing the risk of developing T2DM but should be used in conjunction with lifestyle modifications [83]. As such, it is evident that future treatment options should be expanded to include more individualized hormonal treatment options, while also ensuring that patients include daily physical activity and maintain a healthy diet.
Regarding physical changes that are associated with hormonal decline, such as sarcopenia, muscle wasting, and increased frailty, recent research has shown that certain medication usage should be further explored. For example, myostatin inhibitors have shown results improving muscle mass and physical performance in people with sarcopenia. Ibutamoren mesylate can counteract decreased GH secretion, appetite, and the loss of muscle mass in people with sarcopenia [84]. Although there are no FDA-approved drugs, these results certainly warrant further research to develop better therapeutic strategies benefiting from the physical changes that arise due to hormonal decline.
Another potential target is heat shock proteins (HSPs). HSPs are responsible for dealing with stress in our bodies; however, their effectiveness declines with aging. This results in a chronic stress state that can lead to frailty, tissue dysfunction, and age-related diseases [85]. HSPs become activated when there are aggregates of misfolded protein or surplus protein levels, which then trigger a heat shock response that decreases protein synthesis, promoting proteostasis [86]. With aging, however, the ability to maintain proteostasis diminishes, which can lead to increased amounts of damaged protein aggregates that can lead to diseased states [86]. The protective response of HSPs weakens with age, leading to less refolding of damaged proteins and an increase in proteotoxicity. The heat shock response is regulated by the transcription factor HSF1, which becomes less potent with age, contributing to the reduced HSP induction. Other key transcription factors, like FoxO3a and antioxidant enzymes, also decline. HSPs are regulated by conserved pathways like the insulin/IGF-1 pathway, which is also altered with age and affects longevity [87, 88]. A decline in HSPs contributes to cellular senescence, a state where cells stop dividing but remain metabolically active and can trigger inflammation. Some HSPs, like Hsp27 and Hsp70, protect cells from death pathways; the decline in these HSPs makes cells more vulnerable to damage-induced apoptosis. Aging is associated with mitochondrial dysfunction, which can cause increased oxidative stress that damages proteins and other cellular components. The reduced potency of the heat shock response contributes to aging by permitting the accumulation of damage and increasing genomic instability. The levels of HSPs change with age and correlate with life span, making them potential biomarkers for both aging and individual stress levels [89]. By further exploring this aspect of targeting HSPs, a potential mechanism that can combat the frailty noted with age-related hormonal changes may be investigated.
Lastly, IGF-1 should also be evaluated as a possible target in relation to cognitive decline. IGF-1 plays a protective role in maintaining brain health, and decreased levels of IGF-1 in elderly people contribute to their decline in cognition and mental skills [90]. Additionally, with T2DM, the insulin resistance can also be linked to their IGF-1 levels, further reinforcing the need to delve deeper into this target [52]. Although there are many distinct and unique ways hormonal changes can manifest in patients, depending on the different biological mechanisms they affect, it is encouraging to note the availability of different targets that researchers can further investigate to ultimately counteract the vast number of symptoms.
Aging is accompanied by complex hormonal changes that affect nearly every organ system, leading to a wide range of physical, cognitive, and emotional symptoms. Alterations in insulin, IGF-1, thyroid hormones, cortisol, and sex hormone levels contribute to age-related diseases such as diabetes, osteoporosis, and cognitive decline. These disruptions not only reduce physiological resilience but also diminish quality of life through fatigue, memory loss, mood disturbances, and sleep irregularities. The effects of these hormonal changes vary by individual, with research ongoing as well as research on targeted hormonal therapies. These therapies, including HSP modulation, IGF-1 enhancement, and lifestyle interventions, show promise for improving long-term health outcomes in aging populations.
GH: growth hormone
HPA: hypothalamic-pituitary-adrenal
HSPs: heat shock proteins
IGF-1: insulin-like growth factor-1
IGFBPs: insulin-like growth factor binding proteins
PTH: parathyroid hormone/parathormone
T2DM: type 2 diabetes mellitus
TRH: thyrotropin-releasing hormone
TSH: thyroid-stimulating hormone
CC: Conceptualization, Writing—original draft. SP: Conceptualization, Writing—original draft. SN: Conceptualization, Writing—original draft. VR: Conceptualization, Supervision, Writing—original draft, Project administration, Writing—review & editing. All authors read and approved the submitted version.
As the corresponding author, I declare that this manuscript is original; that the article does not infringe upon any copyright or other proprietary rights of any third party; and that neither the text nor the figures have been reported or published previously. All the authors have no conflict of interest and have read the journal’s authorship statement.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
The authors received no specific funding for this study.
© The Author(s) 2025.
Open Exploration maintains a neutral stance on jurisdictional claims in published institutional affiliations and maps. All opinions expressed in this article are the personal views of the author(s) and do not represent the stance of the editorial team or the publisher.
Copyright: © The Author(s) 2025. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4548
Download: 146
Times Cited: 0
Amar Mann ... Sudarshan Ramachandran
Pravinath Ramachandran ... Geoffrey Hackett
Eugenie Macfarlane ... Markus Joachim Seibel
