Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
ORCID: http://orcid.org/0000-0002-5074-9197
Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Affiliation:
1Department of Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Affiliation:
2Department of Internal Medicine, General, Emergency and Post-Acute, Division of Metabolic Internal Medicine, Civil Hospital of Baggiovara, Azienda Ospedaliero-Universitaria di Modena, 41126 Modena, Italy
Email: pietro.andreone@unimore.it
ORCID: http://orcid.org/0000-0002-4794-9809
Explor Drug Sci. 2024;2:1–5 DOI: https://doi.org/10.37349/eds.2024.00033
Received: February 11, 2023 Accepted: August 21, 2023 Published: January 22, 2024
Academic Editor: Fernando Albericio, University of KwaZulu-Natal, South Africa; University of Barcelona, Spain
The article belongs to the special issue Innovative Therapeutics in Hepato-Gastroenterology
Primary biliary cholangitis (PBC) is an autoimmune cholangiopathy that affects mainly women and, if untreated, can evolve into biliary cirrhosis. Its prevalence varies worldwide, depending on race, and accounts for 22.27 cases/100,000 habitants in Europe. To establish the diagnosis of PBC according to the European Association for the Study of the Liver (EASL) guidelines, two criteria must be satisfied among alkaline phosphatase (ALP) alterations, autoantibody positivity, and histologic abnormalities. Early treatment is effective in prolonging survival. Current guidelines do not suggest hepatic biopsy in patients with autoantibody positivity without cholestasis alterations. However, many patients with these characteristics have been diagnosed with PBC disease only histologically, mainly patients with normal ALP and elevated gamma-glutamyl transferase (GGT), whose normalization has been used as a marker for the follow-up. In contrast, this is the case of a patient with autoantibody positivity and both ALP and GGT within the range, diagnosed for PBC by histology. The manuscript wants to propose the re-evaluation of the role of liver biopsy in PBC diagnosis and the need for a serological or histological biomarker in the follow-up of patients without cholestatic alterations.
Primary biliary cholangitis (PBC) is an autoimmune chronic cholestatic disease that affects mostly women and, when untreated, can lead to biliary cirrhosis [1]. Its prevalence varies worldwide, from a few to 60 cases/100,000 habitants, depending on race [2]. In Europe, a recent epidemiological review concluded for a prevalence of 22.27/100,000 subjects and an incidence of 1.87 cases/100,000 per year [3].
According to the current European Association for the Study of the Liver (EASL) guidelines, the diagnosis of PBC is established when two of the following criteria are satisfied: serological alteration of alkaline phosphatase (ALP), positive antimitochondrial antibodies (AMA)/PBC-specific antinuclear antibodies (ANA) and hepatic biopsy compatible with PBC [4]. There is high evidence that early treatment with ursodeoxycholic acid (UDCA) in PBC prolongs survival [5].
The liver histologic study is not recommended in the case of autoantibody positivity only [4], however, this case demonstrates that, escaping the diagnostic algorithm and performing hepatic biopsy in these types of patients, some diagnoses would be earlier and some treatments would not be started too late.
A 50-year-old woman presented to the hepatological office for the occasional finding of ANA (1:320, nuclear dots pattern) and AMA positivity, without symptoms of PBC. Familiar history was negative for hepatic diseases. In the past, she suffered from hand arthritis and oral lichen planus, now resolved. Her hepatic and cholestatic biochemical profile had never been altered.
The liver biochemical profile tested showed: aspartate aminotransferase (AST) 41 U/L [upper limit of normal (ULN) 37 U/L], alanine aminotransferase (ALT) 37 U/L (ULN 40 U/L), gamma-glutamyl transferase (GGT) 34 U/L (ULN 50 U/L), ALP 109 U/L (ULN 280 U/L), total bilirubin 5 mg/L (ULN 10 mg/L), biliary acids 30 mg/L (ULN 60 mg/L), total cholesterol 2,020 mg/L (ULN 2,000 mg/L); immunoglobulin M (IgM) were not dosed. Abdominal ultrasound excluded hepatosplenomegaly. Hepatic profile was normal and elastometry documented the absence of fibrosis (5.2 kPa).
It was performed hepatic biopsy, which showed moderate periportal fibrosis, moderate-severe portal chronic inflammation with granulomas (Figure 1), plasma cells, eosinophils, and lymphocytic infiltration of biliary ducts with metaplasia. It concluded for evidence of “florid duct lesions” (Figure 2), compatible with active PBC. UDCA therapy was started.
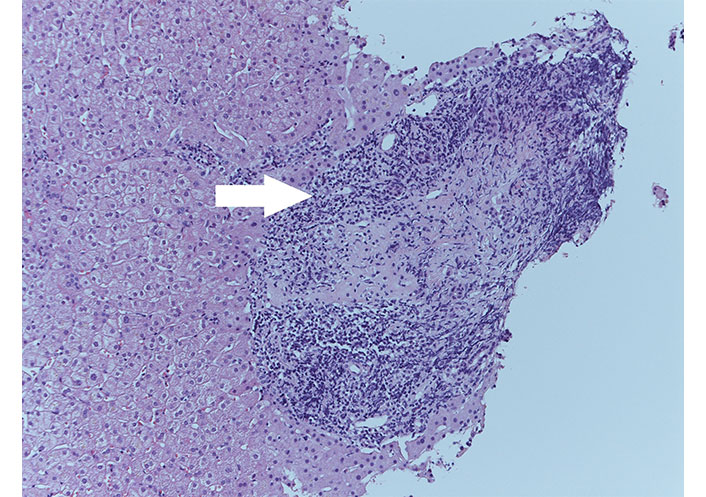
Florid duct lesions (white arrow) with irregular bile duct surrounded by inflammatory infiltrate, HE stain, ×20
At six months and sixteen months follow-up visits, no symptoms of PBC were reported by the patient, biochemical liver profile maintained within ULN [at six months: AST 27 U/L (ULN 37 U/L), ALT 23 U/L (ULN 40 U/L), GGT 21 U/L (ULN 50 U/L), ALP 111 U/L (ULN 280 U/L); at sixteen months: AST 29 U/L (ULN 37 U/L), ALT 25 U/L (ULN 40 U/L), GGT 17 U/L (ULN 50 U/L), ALP 133 U/L (ULN 280 U/L )] and abdominal ultrasound were unvaried, with elastometry 4 kPa and 5.5 kPa, respectively. Hepatic biopsy was not repeated and therapy was confirmed.
EASL guidelines want for diagnosis of PBC two of three criteria among serological alteration of ALP, positive AMA/PBC-specific ANA and liver histology compatible with PBC and they do not suggest hepatic biopsy in the case of autoantibody positivity only [4]. Mitchison et al. [6], in 1986, showed that 24/29 (82.8%) AMA-positive subjects with normal liver function tests and no symptoms of disease, had hepatic biopsy compatible with PBC. Some patients with similar characteristics have been then described by Berdichevski et al. [7], and two cases of liver biliary cirrhosis in AMA positivity without cholestasis biochemical signs by Brostoff et al. [8] and Sultan et al. [9]. All observations have been confirmed more recently by Sun et al. [10] and Terziroli Beretta-Piccoli et al. [11]. Authors also proposed some criteria in order to establish the need for liver biopsy in cases of autoantibody positivity only, such as ALP > 0.475 × ULN [10], IgM > 0.773 × ULN (ULN 2,800 mg/L) [12], and GGT as a new biomarker when normal baseline ALP is present [11]. GGT has already been introduced in the last Asian Pacific Association for the Study of the Liver (APASL) diagnostic algorithm [13]. Applying these threshold values, however, the patient would not have been selected for biopsy because IgM were not dosed, GGT was in range and ALP was < 0.475 × ULN.
EASL guidelines recommend annual biochemical follow-up of these patients until they maybe will develop symptoms or ALP will increase after years since autoantibody detection, as observed by Mitchison et al. [6]. Also, Metcalf et al. [14], following 29 AMA-positive subjects for a median of 17.3 years, showed that 76% developed symptoms and 83% cholestatic alterations for PBC but no patient developed clinically apparent portal hypertension or cirrhosis. Another study revealed that 16.6% of 66 AMA-positive patients with normal ALP developed PBC within 5 years [15].
Some authors sustain this is a neglected aspect of PBC [16, 17]: the patient, as well as many others with the same biochemical characteristics, maybe would have waited years to develop symptoms and cholestatic alterations of PBC and to fulfill the diagnostic criteria of the EASL guidelines, consequently with the late start of therapy. At that point, however, the disease would have progressed and the liver could already have been damaged.
PBC registry can be a solution for collecting these types of PBC patients [18].
In conclusion, guidelines are not adequate for all PBC patients, mainly those who do not show biochemical alterations. It is necessary to find some other biomarkers or re-evaluate threshold values: more studies are necessary to explain these emerging aspects of PBC and eventually correct diagnostic algorithm, re-evaluate the role of hepatic biopsy, clarify if these patients need treatment, and which serological or histological markers could be used for the follow-up.
ALP: alkaline phosphatase
ALT: alanine aminotransferase
AMA: antimitochondrial antibodies
ANA: antinuclear antibodies
AST: aspartate aminotransferase
EASL: European Association for the Study of the Liver
GGT: gamma-glutamyl transferase
IgM: immunoglobulin M
PBC: primary biliary cholangitis
ULN: upper limit of normal
MB: Writing—original draft, Conceptualization. EB and PA: Conceptualization. CC, ER, and FZ: Data curation. PS: Resources. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
In accordance with the Constitutional Regulation of the Ethics Council of the region where the Azienda Ospedaliero-Universitaria di Modena is located (REGOLAMENTO COSTITUTIVO DEL COMITATO ETICO DELL’AREA VASTA EMILIA NORD), ethical approval is not required for cases of less than 5 persons. The study complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2024.
Copyright: © The Author(s) 2024. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 4252
Download: 24
Times Cited: 0
Davide Scalabrini ... Pietro Andreone
Elena Franchi ... Pietro Andreone
