Affiliation:
1Department of Life Sciences, University of Coimbra, 3000-456 Coimbra, Portugal
2Marine Resources, Conservation and Technology-Marine Algae Lab, CFE-Centre for Functional Ecology: Science for People & Planet, 3000-456 Coimbra, Portugal
Email: leonel.pereira@uc.pt
ORCID: http://orcid.org/0000-0002-6819-0619
Affiliation:
3MARE-Marine and Environmental Sciences Centre/ARNET-Aquatic Research Network, University of Coimbra, 3000-456 Coimbra, Portugal
4Biomedical Laboratory Sciences, Polytechnic Institute of Coimbra, Coimbra Health School, 3045-043 Coimbra, Portugal
ORCID: https://orcid.org/0000-0002-0157-6648
Explor Drug Sci. 2023;1:475–496 DOI: https://doi.org/10.37349/eds.2023.00032
Received: June 17, 2023 Accepted: September 29, 2023 Published: December 28, 2023
Academic Editor: Juergen Reichardt, James Cook University, Australia
Seaweeds, also known as marine algae, have gained attention as a promising source of bioactive compounds with potential applications in drug discovery. This review explores the emerging field of seaweed-based drug discovery and highlights the diverse range of bioactive compounds found in seaweeds, including polysaccharides, phlorotannins, pigments, and peptides. These compounds exhibit various pharmacological activities such as antioxidant, anti-inflammatory, antimicrobial, antiviral, and anticancer effects. Seaweeds have demonstrated particular promise in the areas of cancer research, with certain species showing potent antitumor properties. Additionally, their anti-inflammatory, antimicrobial, and neuroprotective potential has captured scientific interest in the treatment of chronic diseases and neurodegenerative disorders. However, challenges related to compound identification, extraction methods, scalability of seaweed cultivation, and understanding the mechanisms of action still need to be addressed. As researchers employ advanced technologies and dive deeper into the chemical composition of seaweeds, the untapped potential of these marine organisms in drug discovery awaits further exploration and holds significant promise for future therapeutic advancements.
Seaweed, or macroscopic marine algae, has long been appreciated for its ecological importance in marine ecosystems and its extensive use in food, cosmetics, and other industrial applications [1]. However, recent research has shed light on the untapped potential of seaweeds as a valuable source of bioactive compounds with significant therapeutic properties [2]. The oceans cover more than 70% of the Earth’s surface and harbor an extraordinary diversity of marine organisms, including seaweeds, making them a promising and largely unexplored resource for drug discovery [3].
The development of new drugs is of paramount importance in addressing the increasing challenges posed by various diseases, including cancer, infectious diseases, and neurodegenerative disorders [4]. The exploration of new sources for drug discovery is crucial, considering the limitations of current therapeutic options. Seaweed represents a rich reservoir of natural compounds that have evolved to survive in the challenging marine environment, offering a vast array of chemical diversity that can be exploited for novel drug development [5].
The primary objective of this manuscript is to provide an in-depth exploration of the potential of seaweed in drug discovery [6]. The goal is to clarify the chemical makeup of seaweed while emphasizing the wide range of bioactive compounds present in these organisms. Furthermore, the discussion revolves around the methodologies utilized in extracting, isolating, and characterizing these compounds. Additionally, an exploration is conducted into the pharmacological activities displayed by compounds derived from seaweed, along with their potential applications across diverse therapeutic domains. Subsequently, attention is given to the obstacles and prospective pathways ahead within the realm of seaweed-based drug discovery [7].
Seaweeds, as a broad group of multicellular marine algae, encompass a wide range of species that inhabit marine environments worldwide. They exhibit a remarkable array of shapes, sizes, and colors, contributing to the visual richness and ecological importance of coastal ecosystems. Seaweeds can be broadly classified into three main types based on their pigmentation and photosynthetic pigments: brown algae (Ochrophyta, Phaeophyceae), red algae (Rhodophyta), and green algae (Chlorophyta) [1, 8].
Brown algae are one of the most visually striking seaweeds, characterized by their distinct brown to olive-green coloration [8]. They are typically found in temperate and colder waters, including rocky shores and kelp forests. Brown algae are known for their large size and complex thallus structure, which may include holdfasts, stipes, and blades. Examples of brown algae include Laminaria (Figure 1a), Saccharina (kombu) (Figure 1b), Undaria (wakame) (Figure 1c), Fucus (bladderwrack) (Figure 1d), and Sargassum species [9]. Brown algae are particularly rich in unique bioactive compounds such as fucoidans, alginates, and phlorotannins, which have demonstrated various pharmacological activities, including antioxidant, anti-inflammatory, and anticancer properties [10].

Brown seaweeds (Ochrophyta, Phaeophyceae). a. Laminaria digitata; b. Saccharina latissima; c. Undaria pinnatifida; d. Fucus vesiculosus (F. vesiculosus). Scale bar: 1 cm
Red algae are characterized by their red or purplish coloration due to the presence of pigments called phycoerythrin and phycocyanin [11]. They are commonly found in both tropical and temperate marine environments, ranging from intertidal zones to deep-sea habitats. Red algae display several growth forms, including filamentous, foliose, and coralline structures [12]. Examples of red algae include Palmaria palmata (Dulse) (Figure 2a), Porphyra/Pyropia (Nori) (Figure 2b), and coralline algae [13]. Red algae are renowned for their rich content of bioactive polysaccharides, such as carrageenans and agar, which possess anticoagulant, antiviral, and immune-modulating properties. Additionally, red algae contain polyphenols, terpenoids, and other secondary metabolites with potential therapeutic applications [14].

Red seaweeds (Rhodophyta). a. Palmaria palmata; b. Porphyra umbilicalis. Scale bar: 1 cm
Green algae encompass a diverse group of seaweeds that exhibit green pigmentation due to the presence of chlorophylls [15]. They are found in a wide range of marine habitats, including intertidal zones, coral reefs, and freshwater environments. Green algae can exhibit varied forms, including unicellular, filamentous, sheet-like, or colonial structures. Examples of green algae with seaweed morphology include Ulva lactuca (U. lactuca, sea lettuce) (Figure 3a), and Codium tomentosum (Figure 3b) [16]. Green algae possess a unique biochemical composition, including bioactive polysaccharides, polyphenols, and terpenoids, which have demonstrated antioxidant, antimicrobial, and anti-inflammatory activities. Some green algae also produce bioactive peptides with potential therapeutic properties [17].
The diverse characteristics and biochemical compositions of seaweeds contribute to their immense potential in drug discovery [18]. Each type of seaweed harbors a distinct repertoire of bioactive compounds, which have been harnessed for various pharmacological applications. Exploring the rich diversity of seaweed species and their unique chemical profiles holds great promise for the discovery of novel drugs and therapeutics [19, 20].
Seaweed exhibits a remarkable chemical diversity, owing to its ability to synthesize a wide range of bioactive compounds [21]. This section focuses on the major chemical classes found in seaweed, including polysaccharides, polyphenols, terpenoids, and peptides. It discusses their structural characteristics, biological functions, and potential applications in drug discovery.
Seaweed is rich in polysaccharides, which are complex carbohydrates composed of long chains of sugar molecules. The primary polysaccharides found in seaweed include agar (Rhodophyta) (Figure 4), carrageenan (Rhodophyta) (Figure 5), porphyran (Rhodophyta) (Figure 6), alginic acid (Phaeophyceae) (Figure 7), laminaran (Phaeophyceae) (Figure 8), fucoidan (Phaeophyceae) (Figure 9), and ulvan (Chlorophyta) (Figure 10) [22]. These polysaccharides have unique structural characteristics and exhibit various bioactive properties. For example, agar is commonly used as a gelling agent in the food industry [23], while carrageenan is used as a stabilizer and thickening agent [24]. Alginate has applications in wound healing and drug delivery systems, and fucoidan possesses antioxidant, anticoagulant, and anti-inflammatory properties [25]. These properties make them suitable for various drug delivery applications, including controlled release systems and mucoadhesive formulations. The seaweed polysaccharides are bioactive molecules with unique chemical structures that contribute to their remarkable properties [17].
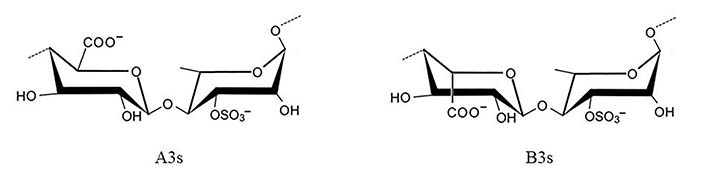
Idealized structure of the chemical units of ulvan. A3s: type A ulvanobiuronic acid 3-sulfate; B3s: type B ulvanobiuronic acid 3-sulfate
Agar (E 406) (Figure 4) is a heterogeneous mixture of agarose and agaropectin. Agarose forms a rigid gel, while agaropectin contributes to its flexibility. These properties enable agar’s utilization in mucoadhesive formulations and as carriers for controlled drug release [17].
Carrageenan (E 407) is a sulfated polysaccharide classified into three main types: kappa, iota, and lambda, based on the number and position of sulfate groups. Its ability to form gels and provide viscosity makes it valuable in designing sustained-release formulations and enhancing drug stability [17, 18].
Porphyran is a sulfated polysaccharide isolated from algae of the order Bangiales, phylum Rhodophyta, especially from the genus Porphyra/Pyropia. The chemical structure of porphyran contains a linear backbone of alternating 3-linked β-D-galactose and 4-linked α-L-galactose-6-sulfate or 3,6-anhydro-α-L-galactose units (Figure 6) [17].
Alginic acid (E 400) is a linear copolymer composed of β-D-mannuronic acid (M) and α-L-guluronic acid (G) units (Figure 7). The relative proportions of M and G units influence their properties, such as gel-forming ability and biocompatibility. This versatility makes alginic acid suitable for controlled release systems and wound healing applications [17].
Laminaran (Laminarin) (Figure 8), is extracted from Phaeophyceae (brown algae), mainly from the Laminaria genus. It is a small glucan, present in either soluble or insoluble form. The first form is characterized by complete solubility in cold water, while the other is only soluble in hot water. This polysaccharide is composed of D-glucose with β-(1,3) linkages, with β-(1,6) intra-chain branching [17, 18].
Early work on fucoidan structure showed that it contained primarily (1→2) linked 4-O-sulfated fucopyranose residues (Figure 9) [17]. However, 3-linked fucose with 4-sulfated groups was subsequently reported to be present on some of the fucose residues. Additionally, it was determined to contain branches every 2–3 fucose residues of fucoidan extracted from F. vesiculosus and Ascophyllum nodosum (Phaeophyceae) contained a predominant disaccharide motif with sulfate at the 2-position of the 3-linked fucose, and sulfate groups on the 2- and 3-positions of the 4-linked fucose [17].
Ulvan represents 8–29% of the algae’s dry weight and is produced by species belonging to the phylum Chlorophyta (green algae), mostly belonging to the class Ulvophyceae. It is mainly made up of disaccharide repeating sequences composed of sulfated rhamnose, glucuronic acid, iduronic acid, or xylose [17]. The two major repeating disaccharides are aldobiuronic acids designated as A3s and B3s (Figure 10).
Seaweed contains a wide range of polyphenolic compounds, which are secondary metabolites responsible for the characteristic flavors, colors, and antioxidant properties of many plants. The polyphenols in seaweed include phlorotannins (Figure 11), flavonoids (Figure 12), and phenolic acids [26]. These compounds have been studied for their potential health benefits, including anti-inflammatory, antimicrobial, and anticancer activities. Additionally, polyphenols from seaweed have shown promise as natural antioxidants in various industries, such as food and cosmetics [27].
Seaweed synthesizes various terpenoid compounds, which are derived from the basic building block of isoprene units. These compounds contribute to the distinct aromas and flavors of seaweed [28]. Terpenoids found in seaweed include halogenated monoterpenes (Figure 13), diterpenes, and sesquiterpenes. Some terpenoids have demonstrated antimicrobial, antifungal, and antiviral activities. They also play a role in the defense mechanisms of seaweed against predators and pathogens [29, 30].
Seaweed produces a wide range of peptides, short chains of amino acids, with diverse structures and functions [31]. Peptides derived from seaweed exhibit various bioactivities, such as antioxidant, antihypertensive, and anticancer properties. Some peptides have been explored for their potential in drug development and as functional food ingredients [32, 33].
The chemical composition of seaweed is highly influenced by factors such as species, geographical location, and environmental conditions. Different species of seaweed contain varying amounts of these chemical classes, leading to differences in their bioactive properties [34]. The study of seaweed’s chemical composition and bioactive compounds has opened up avenues for drug discovery, functional foods, and biotechnological applications [35].
It is worth noting that while seaweed offers significant potential in various fields, further research is necessary to fully explore and understand the complex chemical composition and bioactivity of seaweed species [36].
Seaweeds have long been recognized for their nutritional value, but recent research has shed light on their rich reservoir of bioactive compounds. These compounds, such as polysaccharides, phlorotannins, pigments, and peptides, have exhibited a wide array of biological activities, including antioxidant, anti-inflammatory, antimicrobial, antiviral, and anticancer properties [37, 38]. These compounds encompass a range of chemical classes, which contribute to the remarkable bioactivity exhibited by seaweeds [39].
Seaweeds are well-known for their abundant polysaccharide content, which plays a crucial role in their structural integrity. Polysaccharides derived from seaweeds, such as fucoidans, laminarans, carrageenans, and ulvans (Figures 4–10), have garnered significant attention due to their potential therapeutic applications [40]. These polysaccharides possess a wide range of biological activities, including antioxidant, immunomodulatory, anticoagulant, and antitumor properties. They have shown promise in various fields, such as cardiovascular health, cancer prevention, and immune system regulation [41, 42].
Phlorotannins are a unique class of polyphenolic compounds that are exclusive to brown seaweeds. These compounds are derived from the polymerization of phloroglucinol units and exhibit diverse chemical structures and degrees of polymerization [43]. Phlorotannins have demonstrated notable antioxidant, anti-inflammatory, and antimicrobial activities. They also exhibit potential for inhibiting enzymes involved in the progression of diseases and have shown promise as photoprotective agents and as potential therapeutics for neurodegenerative disorders and skin-related conditions [44, 45].
Seaweeds display a vibrant range of pigments, including chlorophylls (Figure 14), carotenoids (Figure 15), and phycoerythrobilin (Figure 16), which contribute to their distinct coloration [46]. These pigments possess antioxidant properties and have demonstrated potential as photoprotective agents. Additionally, some seaweed pigments, such as fucoxanthin (Figure 17) and astaxanthin (Figure 18), have exhibited anti-inflammatory, anticancer, and antiviral activities. Fucoxanthin, in particular, has attracted significant interest due to its potential as an anti-obesity and anti-diabetic agent [47, 48].
Macroalgae contains a diverse array of bioactive peptides, which are short chains of amino acids with specific biological activities. These peptides are produced by enzymatic hydrolysis of proteins present in seaweeds or are synthesized as defense mechanisms against environmental stressors [49, 50]. Seaweed peptides have demonstrated antioxidant, antimicrobial, antihypertensive, and anticancer activities. They have shown potential as functional ingredients in nutraceuticals and cosmeceuticals [51].
The bioactive compounds found in seaweeds hold tremendous potential for various therapeutic applications. Their antioxidant properties help combat oxidative stress and associated diseases [52]. Anti-inflammatory activities make them valuable in managing inflammatory conditions [53]. Antimicrobial and antiviral properties offer possibilities for the development of new antimicrobial agents and antiviral drugs. Furthermore, the anticancer properties of seaweed-derived compounds have shown promise in cancer prevention, treatment, and supportive care [54].
The exploration and utilization of these bioactive compounds from seaweeds open up exciting avenues for drug discovery, functional foods, and nutraceuticals. Continued research and understanding of the mechanisms of action of these compounds will further enhance their potential applications in various fields of medicine and biotechnology [55].
Seaweeds have shown great promise in the fight against cancer. Some species of red algae (Rhodophyta), such as Gracilaria (Figure 19a), Gelidium (Figure 19b), Gigartina (Figure 19c), Pterocladiella (Figure 19d), Porphyra (Figure 19e), Jania squamata (Figure 19f), Asparagopsis (Figure 19g), and Jania (Figure 19h), contain sulfated polysaccharides that exhibit potent antitumor effects [56–60]. These compounds have demonstrated the ability to inhibit tumor growth, induce apoptosis (cell death) in cancer cells, and suppress angiogenesis (formation of new blood vessels to nourish tumors) [61].

Red seaweeds (Rhodophyta). a. Gracilaria gracilis; b. Gelidium corneum; c. Gigartina pistillata; d. Pterocladiella capillacea; e. Porphyra umbilicalis; f. Jania squamata; g. Asparagopsis armata; h. Jania rubens. Scale bar: 1 cm
In the realm of biomedical research, the potential of seaweeds in the battle against cancer has ignited significant interest and excitement. Among the diverse array of marine organisms, certain species of red algae (Rhodophyta) have emerged as particularly promising contenders in the pursuit of novel antitumor agents. This diverse group encompasses a range of genera, each has its own unique properties that hold the key to unlocking new dimensions in cancer treatment [14].
Gracilaria, for instance, has been found to contain polysaccharides and sulfated compounds with notable immune-modulating properties. These compounds possess the ability to stimulate the immune system, which in turn can lead to enhanced recognition and destruction of cancer cells by the body’s defenses [17, 57].
Gelidium, another prominent red algae genus, has showcased antioxidant and anti-inflammatory properties. Such attributes are valuable in counteracting the oxidative stress and chronic inflammation often associated with cancer development and progression. By mitigating these factors, Gelidium compounds have the potential to hinder tumor growth and metastasis [59].
The genus Gigartina has been lauded for its fucoidan content, a sulfated polysaccharide that has demonstrated a wide spectrum of beneficial effects. Fucoidan’s antitumor potential stems from its ability to interfere with various stages of cancer progression, including inhibiting angiogenesis, inducing apoptosis (programmed cell death), and modulating immune responses [41].
Porphyra, often known as nori and a staple in sushi, has revealed anticancer bioactive peptides that can interfere with tumor cell signaling pathways, contributing to the suppression of cancer cell growth and proliferation [17, 57].
Asparagopsis and Jania hold potential due to their bioactive compounds, which have demonstrated cytotoxic effects on various cancer cell lines. These compounds could serve as the basis for the development of targeted therapies [14].
Saccharina japonica (S. japonica, brown macroalga), commonly known as kombu or brown kelp, emerges as a standout species with extracts that exhibit remarkable antitumor activity. The allure of these extracts lies in their diverse array of bioactive compounds, each holding the potential to revolutionize cancer treatment [57].
Fucoxanthin (Figure 17), a carotenoid pigment abundantly present in S. japonica, has captured the interest of researchers due to its multifaceted benefits. This compound, responsible for the distinctive brown hue of the algae, has shown potent anticancer effects. Studies have demonstrated fucoxanthin’s ability to suppress tumor growth by influencing key cellular pathways involved in proliferation and apoptosis. Moreover, fucoxanthin’s impact on angiogenesis, the process through which tumors create new blood vessels to sustain their growth, cannot be understated. By inhibiting angiogenesis, fucoxanthin stymies the tumor’s supply of nutrients and oxygen, effectively thwarting its expansion [13, 15, 46].
Fucoidan (Figure 9), another compelling compound derived from S. japonica, adds another layer of intrigue to its antitumor potential. Fucoidan is a sulfated polysaccharide with an impressive track record in the realm of immunomodulation. By interacting with immune cells and signaling molecules, fucoidan enhances the body’s immune response against cancer cells. This immune-boosting capacity can potentially synergize with conventional cancer therapies, resulting in a more robust defense against the disease. Furthermore, fucoidan’s anti-inflammatory and antioxidant properties contribute to its multifunctional role in suppressing tumor progression [41].
The journey of harnessing the antitumor properties of S. japonica doesn’t end with just these two compounds. Alginic acid (Figure 7), which is extracted from the cell walls of brown algae, is being investigated for its potential in drug delivery systems, allowing for targeted and controlled release of antitumor agents. This speaks to the broader implications of macroalgae research, extending beyond direct compounds to innovative drug delivery strategies [17].
As the scientific community delves deeper into the potential of S. japonica and other macroalgae, it becomes increasingly evident that these marine organisms hold a wealth of untapped potential for cancer therapy. The intricate interplay of fucoxanthin, fucoidan, alginates, and potentially many more compounds within these extracts showcases nature’s complexity and its potential to provide solutions to some of the most challenging medical problems. With ongoing research and exploration, macroalgae-derived extracts could one day become integral components of the antitumor arsenal, revolutionizing the landscape of cancer treatment [18, 57].
In the intricate realm of cellular biology, a nuanced understanding of antioxidant systems is essential to grasp the delicate balance that sustains life. While the term “antioxidant” often conjures thoughts of external supplements and chemical interventions, the truth runs much deeper. Within a cell, a remarkable interplay of intricate mechanisms constantly mitigates the oxidative stress posed by reactive oxygen species (ROS). Far from being reliant solely on exogenous chemical antioxidants, cells possess an array of intrinsic defense mechanisms. Enzymes like catalase and superoxide dismutase (SOD) stand as sentinels against oxidative damage, demonstrating an efficiency that dwarfs that of their chemical counterparts. This discourse aims to unveil the intrinsic prowess of ROS-detoxifying enzymes, shedding light on the remarkable orchestration within cells that underscores their vitality.
The bioactive compounds found in seaweeds, particularly the phlorotannins, have displayed significant anti-inflammatory and antioxidant properties [62]. These properties make them potential candidates for developing drugs to treat chronic inflammatory conditions, such as rheumatoid arthritis, as well as diseases associated with oxidative stress [63].
The brown seaweed F. vesiculosus (Bladderwrack) (Figure 1d), commonly found in the Atlantic and Pacific oceans, contains various bioactive compounds, including phlorotannins, fucoidans, and polyphenols [64]. Studies have shown that extracts from F. vesiculosus possess anti-inflammatory properties by inhibiting the production of pro-inflammatory cytokines and enzymes. Additionally, it exhibits antioxidant activity by scavenging free radicals and protecting against oxidative stress [26, 44, 65, 66].
U. lactuca (sea lettuce) (Figure 3a) is a green seaweed that is commonly found along coastlines worldwide. It contains various bioactive compounds, including polyphenols, flavonoids, and polysaccharides [67–69]. Extracts from U. lactuca have been shown to possess anti-inflammatory properties by inhibiting the production of inflammatory mediators and reducing the activity of inflammatory enzymes. Its antioxidant effects are attributed to the presence of phenolic compounds that scavenge free radicals and protect against oxidative stress [52].
Porphyra/Pyropia [several species (spp.), Nori] (Figure 2b and Figure 19e) is a red seaweed that is extensively consumed in Asian cuisines. It contains a range of bioactive compounds, including phycobiliproteins, polysaccharides, and polyphenols [70]. Research has indicated that Porphyra/Pyropia spp. exhibits anti-inflammatory effects by suppressing the production of inflammatory cytokines and inhibiting the activation of inflammatory pathways. It also displays antioxidant activity by scavenging free radicals and enhancing the activity of antioxidant enzymes [17, 71].
Seaweeds possess natural defense mechanisms against microbial pathogens in their harsh marine environments. Compounds derived from seaweeds have demonstrated antimicrobial activity against various bacteria, fungi, and viruses.
Extracts from brown algae, such as Fucus (Figure 1d) and Sargassum, have shown potential as antibacterial agents, while red algae, such as Laurencia and Gracilaria (Figure 19a), have exhibited antiviral properties [30, 54].
Spp. of Sargassum, a brown algae genus, have exhibited antimicrobial properties. For example, Sargassum muticum [72, 73] and Sargassum polycystum [74] have demonstrated antibacterial effects against pathogens like Vibrio parahaemolyticus and Salmonella typhi. The active compounds responsible for these activities include fucoidan and polyphenols [75].
Ulva (Chlorophyta) species have been studied for their antimicrobial properties [29]. Extracts from Ulva spp. have shown antibacterial effects against pathogens such as Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli). These antimicrobial properties are attributed to the presence of sulfated polysaccharides and other bioactive compounds [76].
U. lactuca (Figure 3a) is one of the most well-known and widely studied for its antimicrobial activity. Extracts from U. lactuca have shown antibacterial effects against various pathogenic bacteria, including S. aureus, E. coli, and Salmonella typhi. The antimicrobial activity is attributed to the presence of bioactive compounds like lectins, peptides, and sulfated polysaccharides [77, 78]. Ulva fasciata, one of the synonyms of U. lactuca, has also been investigated for its antimicrobial properties. Extracts from this species have demonstrated antibacterial effects against gram-positive and gram-negative bacteria, such as Bacillus subtilis (B. subtilis) and Pseudomonas aeruginosa. The antimicrobial activity is associated with the presence of compounds like ulvan, a sulfated polysaccharide [79].
Ulva intestinalis (U. intestinalis) (Figure 20a), another species of Ulva, has exhibited antimicrobial activity against pathogenic bacteria. Extracts from U. intestinalis have shown inhibitory effects against bacteria such as S. aureus and Vibrio parahaemolyticus. The antimicrobial activity is attributed to the presence of compounds like lectins, peptides, and phenolic compounds [80, 81].

Green seaweeds (Chlorophyta). a. U. intestinalis; b. Ulva rigida (U. Rigida); c. Halimeda tuna. Scale bar: 1 cm
U. rigida (Figure 20b) has been studied for its antimicrobial properties. Extracts from U. rigida have demonstrated antibacterial effects against bacteria such as E. coli and Pseudomonas aeruginosa. The antimicrobial activity is associated with the presence of compounds like peptides and sulfated polysaccharides [82, 83].
These are some examples of Ulva species that have been investigated for their antimicrobial properties. The antimicrobial activity of Ulva spp. is often attributed to a combination of bioactive compounds present in their cell walls, including lectins, peptides, polysaccharides, and phenolic compounds. These compounds can disrupt bacterial cell membranes, interfere with enzymatic processes, and inhibit bacterial growth [84].
It’s worth noting that the antimicrobial activity of Ulva spp. can vary depending on factors such as species, geographical location, season, and extraction methods. Ongoing research aims to further explore the bioactive compounds in Ulva spp. and their potential applications in the development of new antimicrobial agents [85].
Padina species, a brown algae genus, have demonstrated antimicrobial activity. Extracts from Padina spp. have exhibited inhibitory effects against bacteria such as B. subtilis, E. coli, Klebsiella pneumonia, S. aureus, and Staphylococcus epidermidis (S. epidermidis). The antimicrobial activity is associated with the presence of compounds like flavonoids and polyphenols [86].
Halimeda species, a genus of green algae, have shown antimicrobial properties. Extracts from Halimeda spp. (Figure 20c) have exhibited antibacterial effects against various pathogens, including S. aureus and Streptococcus mutans [87, 88]. The antimicrobial activity is attributed to the presence of compounds like halimedatetraacetate [89].
Dictyota species, a brown algae genus, have been investigated for their antimicrobial activity. Extracts from Dictyota spp. have shown inhibitory effects against some bacteria. The antimicrobial activity is associated with the presence of compounds like diterpenes and sterols [90].
Dictyota ciliolata (D. ciliolata) (Figure 21a) is another species within the Dictyota genus that has shown antimicrobial activity. Extracts from D. ciliolata have exhibited inhibitory effects against bacteria such as S. aureus and Salmonella typhi. The antimicrobial activity is associated with the presence of compounds like diterpenes and polyphenols [90].

Some macroalgae provided with compounds with bioactivities. a. D. ciliolata (Phaeophyceae); b. D. dichotoma (Phaeophyceae); c. C. crispus (Rhodophyta); d. Padina pavonica (Phaeophyceae). Scale bar: 1 cm
Dictyota dichotoma (D. dichotoma) (Figure 21b), also known as “brown fan weed”, is a species that has shown antimicrobial activity. Extracts from D. dichotoma have exhibited inhibitory effects against bacteria such as E. coli, S. aureus, and Vibrio cholerae (V. cholerae). The antimicrobial activity is associated with the presence of compounds like diterpenes and sterols [91–93].
Dictyota flabellata (D. flabellata) has also been studied for its antimicrobial properties. Extracts from D. flabellata have demonstrated antibacterial effects against various pathogens, including S. aureus, E. coli, Bacillus cereus (B. cereus), B. subtilis, and S. epidermidis [91]. The antimicrobial activity is attributed to the presence of compounds like diterpenes and polyphenols [94].
These are a few examples of Dictyota that have been investigated for their antimicrobial properties. The antimicrobial activity of Dictyota spp. is often attributed to the presence of bioactive compounds like diterpenes, polyphenols, and sterols. These compounds can disrupt bacterial cell membranes, inhibit enzymatic processes, and exhibit bactericidal or bacteriostatic effects [92].
It’s important to note that the antimicrobial activity of Dictyota species may vary depending on factors such as species, geographical location, and extraction methods. Further research is ongoing to explore the bioactive compounds present in different Dictyota spp. and to elucidate their potential applications in the development of new antimicrobial agents [93].
Chondrus crispus (C. crispus, Rhodophyta) (Figure 21c) has demonstrated antimicrobial and antiviral properties. Extracts from C. crispus have shown antibacterial effects against pathogens like Pseudomonas aeruginosa and antiviral effects against herpes simplex virus (HSV) and human papilloma virus (HPV) [95, 96].
Laurencia spp. (Rhodophyta) have shown antiviral activity against various viruses. Laurencia species have been found to inhibit the replication of HSV and dengue virus [97]. The antiviral effects are attributed to the presence of sulfated polysaccharides, such as carrageenan and agar, which interfere with viral attachment and entry [98].
These are just a few additional examples of seaweed species that have been studied for their antimicrobial and antiviral activities. Seaweeds are incredibly diverse, and many more species hold potential for their bioactive compounds. Ongoing research continues to explore the vast array of seaweed species for their medicinal and pharmaceutical applications [57, 99].
Preliminary studies suggest that certain compounds derived from seaweeds have neuroprotective effects [100]. These compounds may help combat neurodegenerative disorders, such as Alzheimer’s and Parkinson’s diseases, by protecting neurons from oxidative stress, reducing inflammation, and promoting neuronal growth and repair [101].
Gracilaria edulis and Gracilariopsis longissima (also known as Gracilaria verrucosa, Rhodophyta) extracts have shown neuroprotective effects in studies [101–103]. The presence of bioactive compounds like polysaccharides, phycobiliproteins, and antioxidants contributes to their neuroprotective potential by combating oxidative stress and inflammation [104].
Sargassum fulvellum and Sargassum polycystum (Phaeophyceae) extracts have demonstrated neuroprotective effects. These extracts contain bioactive compounds such as phlorotannins, fucoidans, and polyphenols, which exhibit antioxidant, anti-inflammatory, and neurogenic properties that may support brain health and protect against neurodegenerative conditions [96, 105].
Palisada perforata (also known as Laurencia papillosa) and Laurencia obtusa (Rhodophyta) extracts have shown neuroprotective potential in studies. The presence of bioactive compounds like diterpenes, bromophenols, and polyphenols contributes to their neuroprotective effects by exhibiting antioxidant and anti-inflammatory properties that can help protect neurons from oxidative stress and inflammation-related damage [106, 107].
Extracts from Padina pavonica (Phaeophyceae) (Figure 21d) have demonstrated neuroprotective effects. The presence of bioactive compounds like polyphenols, flavonoids, and polysaccharides contributes to their neuroprotective properties by reducing oxidative stress, inflammation, and neuronal apoptosis [108].
The compounds that F. vesiculosus (Figure 1d) and Fucus serratus (F. serratus, Phaeophyceae) produce have shown neuroprotective potential in several studies. The presence of bioactive compounds like fucoidans, phlorotannins, and antioxidants contributes to their neuroprotective effects by attenuating oxidative stress, inflammation, and neurodegenerative processes [7, 109].
These marine algae species represent a fraction of the diverse range of marine algae with potential neuroprotective properties. Continued research in this field will help further uncover the specific mechanisms and therapeutic potential of these algae in promoting brain health and protecting against neurodegenerative diseases [101, 106].
While seaweed-derived compounds hold immense promise, several challenges need to be addressed to harness their full potential in drug discovery [110]. These include the identification and isolation of specific bioactive compounds, optimizing extraction methods, ensuring scalability and sustainability of seaweed cultivation, and understanding the mechanisms of action and potential side effects of these compounds [111].
Researchers are also exploring the use of advanced technologies, such as metabolomics and genomics, to unravel the complex chemical compositions of seaweeds and identify novel compounds with therapeutic potential [112].
Seaweeds, once considered mere marine plants, have emerged as a valuable source of bioactive compounds with diverse therapeutic potential [113]. Their unique biochemical composition, coupled with their abundance and sustainability, make them an attractive avenue for drug discovery. As scientists continue to explore the hidden depths of our oceans, the pharmaceutical world eagerly awaits the groundbreaking discoveries that may emerge from these unassuming marine algae. The future of drug discovery may very well lie beneath the waves [114].
B. subtilis: Bacillus subtilis
C. crispus: Chondrus crispus
D. ciliolata: Dictyota ciliolata
D. dichotoma: Dictyota dichotoma
E. coli: Escherichia coli
F. vesiculosus: Fucus vesiculosus
S. aureus: Staphylococcus aureus
S. japonica: Saccharina japonica
spp.: several species
U. intestinalis: Ulva intestinalis
U. lactuca: Ulva lactuca
U. rigida: Ulva rigida
LP and AV: Conceptualization, Writing—original draft, Writing—review & editing. All authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
