Affiliation:
Departamento de Clínica, Patologia e Cirurgia Odontológicas, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte CEP 31270-901, Brazil
ORCID: https://orcid.org/0000-0002-5602-228X
Affiliation:
Departamento de Clínica, Patologia e Cirurgia Odontológicas, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte CEP 31270-901, Brazil
ORCID: https://orcid.org/0000-0002-9179-0145
Affiliation:
Departamento de Clínica, Patologia e Cirurgia Odontológicas, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte CEP 31270-901, Brazil
ORCID: https://orcid.org/0000-0001-7820-4749
Affiliation:
Departamento de Clínica, Patologia e Cirurgia Odontológicas, Faculty of Dentistry, Federal University of Minas Gerais, Belo Horizonte CEP 31270-901, Brazil
Email: vegneer2003@yahoo.com.br
ORCID: https://orcid.org/0000-0002-5370-4867
Explor Drug Sci. 2023;1:312–321 DOI: https://doi.org/10.37349/eds.2023.00022
Received: March 04, 2023 Accepted: June 07, 2023 Published: October 9, 2023
Academic Editor: Fernando Albericio, Universities of KwaZulu-Natal, South Africa; Universidad de Barcelona, Spain
Green propolis is collected by Apis mellifera from the flowers and buds of Baccharis dracunculifolia. It has several chemical compounds that confer anti-inflammatory, antimicrobial, healing, and antioxidant biological activities. To report a series of clinical cases in the treatment of oral mucositis (OM) in patients with cancer undergoing radiotherapy in the head and neck region. Rapid treatment of OM means restoring quality of life to patients and lowering the cost of cancer treatment for public health. There male patients with oral carcinoma undergoing radiotherapy treatment were followed between August 2018 and April 2019. The patients presented themselves to the clinics in the Faculty of Dentistry of Federal University of Minas Gerais with erythematous and ulcerated coalescing lesions with purulent fibrin pseudomembranes in the oral mucosa, classified as grade IV OM according to the World Health Organization. The patients complained about the inability to eat, drink, and speak, which caused the radiotherapy interruption. After completing the clinical forms, anamnesis, and proper oral hygiene of each patient, a mucoadherent gel containing 5% propolis was prescribed for daily use, with a 3 time-a-day application every 8 h. After 7 days of use, there was an 80% lesion reduction, with total remission after 15 days of its application. The rapid response with total remission of lesions seems to be related to the chemical composition of propolis. Clinical and cellphone monitoring of patients, weekly and daily, respectively, were essential for successful treatment. The patients were monitored for one year, being encouraged to make constant use of the gel to control hyposalivation caused by changes in the salivary glands during radiotherapy.
Radiotherapy has been an effective method in the treatment of cancer in the head and neck region [1, 2]. However, this therapy type causes orofacial comorbidities during and after treatment, such as oral mucositis (OM), saliva change, dental caries, osteoradionecrosis, and radiodermatitis in more than 80% of cases [3, 4]. OM is a painful pathology that generates nutritional impacts, decreases the quality of life, changes cancer therapy, and increases economic costs for public health [5]. Radiotherapy in the head and neck region usually causes changes in the function of the salivary glands and cell death in the oral mucosa and oropharynx, evidencing hyposalivation (dry mouth) and consequently, OM [6, 7].
The most recent studies on OM mainly used the classification proposed by the World Health Organization (WHO) [8]. The development of OM is considered in four stages: vascular, epithelial, ulcerative, and repair. The first phase is due to the release of cytokines as a tumor necrosis factor that induces an increase in subepithelial vascularization. Soon after, in the second phase, an unwanted effect occurs in the cells with the inhibition of cellular DNA synthesis, being directly linked to epithelial renewal. The ulcerative phase is the one that harms the patient’s quality of life since there is a greater susceptibility to infections due to the breakdown of the mucosal barrier [9].
OM is the most common comorbidity observed during chemoradiotherapy in virtually all patients with oral squamous cell carcinoma (SCC), being classified by the WHO from grade zero to grade V, according to the observed clinical severity [10–12]. The complex origin of OM involving local and systemic predisposing factors makes it difficult to adopt treatments that are at the same time effective, easy to use, and inexpensive for patients. In this context, products containing medicinal plant extracts have been proposed for the treatment of OM [13]. Several forms of treatment for OM have been proposed, considering the anti-inflammatory and antimicrobial properties of drugs, since the breakdown of the mucosal barrier is the gateway to colonization by fungi such as Candida albicans and bacteria from the oral microbiome [14].
The use of natural products for the treatment of diseases that affect human beings was one of the first forms of medicine in the world [15]. Propolis is a natural product resulting from the mixture of chemical components collected from plants with the saliva of the bees. Brazilian green propolis has different botanical and regional origins: Baccharis dracunculifolia, Vernonia rubriramea (savannah region), and Mimosa tenuiflora (dry region), and has a complex chemical constitution of chalcones, flavonoids, triterpenes, prenylated phenylpropanoids, chlorogenic acids, and condensed tannins [16–19]. The biological properties, quality, and origin of propolis have been proven through several methods, including high performance liquid chromatography (HPLC) and gas chromatograph-mass spectrometer (GC-MS), by identifying its chemical components [16, 17]. Several works have proven the potent anti-inflammatory action of one of the compounds of propolis, artepillin C (3,5-diisopentenyl-4-hydroxycinnamic acid), and also its various antioxidant, antibiotic, and cytotoxic actions, which act synergistically among its more than 400 components [19]. The biological activities of propolis seem to be associated with the synergism between its chemical compounds, which give it anti-inflammatory, antimicrobial, healing, immunoprotective, anesthetic, and antioxidant characteristics [20–26]. The main chemical components found in Brazilian green propolis are shown in Table 1. The objective of this work is to report a series of three clinical cases of cancer patients diagnosed with grade IV OM and treated with mucoadherent gel content of 5% green propolis.
Main chemical components of Brazilian green propolis extract
| No | Formula | Compound | Unit (mg) |
|---|---|---|---|
| 1 | C9H8O3 | p-Coumaric acid | 3.56 |
| 2 | C25H24O12 | Dicaffeoylquinic acid | 6.64 |
| 3 | C15H10O7 | Quercetin | 1.38 |
| 4 | C15H12O5 | Pinobanksin | 13.92 |
| 5 | C15H10O6 | Kaempferol | 11.40 |
| 6 | C14H14O4 | Caffeic acid isoprenyl ester | 17.47 |
| 7 | C15H12O4 | Pinocembrin | 19.00 |
| 8 | C15H10O5 | Galangin | 9.75 |
| 9 | C16H12O6 | Kaempferide | 11.60 |
| 10 | C19H24O3 | Artepillin C | 82.96 |
| 11 | C16H14O5 | Sakuranetin | 5.57 |
| 12 | C15H10O4 | Chrysin | 3.51 |
| 13 | C16H12O7 | Isorhamnetin | 0.91 |
| 14 | C17H14O6 | Pinobanksinr-3-O-acetate | 20.68 |
| 15 | C9H8O2 | Cinnamic acid | 1.55 |
Unit (mg) means mg chemical components of per 1g of green propolis
For this present study, all 3 patients were treated at the “Treatment of Irradiated Patients in the Head and Neck Region” Extension Project, at the Faculty of Dentistry of the Federal University of Minas Gerais. The patients signed the free and informed consent form authorizing the disclosure of the photos and medical records for possible studies and were attended in the period between August 2018 and April 2019. Information such as age, phenotype, dates of consultations, prescriptions, medical and dental history, hospital of origin, performed procedures, and current status of the disease was collected from the patients’ medical records. The criteria for inclusion of the three patients in this study were having histopathologically confirmed oral SCC, having undergone head and neck radiation therapy, and having a clinical diagnosis of grade IV OM in the first clinical evaluation. As an exclusion criterion was considered when the patient did not accept the use of the propolis-proposed treatment.
The study with the mucoadhesive propolis gel was previously approved by the Human Research Ethics Committee of Federal University of Minas Gerais (No.0249.0.203.000-10) in Noronha et al. [22]. The propolis mucoadherent gel (OncOralcare®, lot reference number GD030717.DV, manufactured on July 2017 and expiry date on July 2019) was produced by Néctar Pharmaceutica Ltda (Belo Horizonte, Brazil), according to required standards by the Brazilian National Health Surveillance Agency (ANVISA), working license (MS 0.48631.1), under ISO 9001: 2000-good manufaturing practices (resolution No. 275/2002), National Institute of Metrology (certificate No. 54448-A-2009-AQ-BRA-INMETRO), Det Norske Veritas (certificate No. 54507-2009-nb) certified company (Figure 1). Green propolis chemical components were identified for scientific studies by HPLC and reverse phase [27–30]. The gel final composition was composed of Brazilian green propolis (5%), propylene glycol, hydroxypropyl methylcellulose, polysorbate 20, and purified water. The final concentration of 5% is justified by the in vitro experiments against microorganisms and previous clinical trial studies [24, 27]. Patients applying it three times a day every 8 h were instructed to use a portion equivalent to a coffee spoon (10 g). It was recommended to apply the gel to the entire mucosa with finger wearing latex gloves provided to the patient or companion [22]. Patients were encouraged to apply the gel at night before going to bed, that is, sleeping with the gel inside their mouth.

The physical aspect of the propolis OncOralcare®—mucoadherent gel content of 5% green propolis
The assessment of the degree of clinically observed OM was based on the WHO classification: grade 0—no change; grade I—soreness/erythema; grade II—erythema and ulcers; grade III—ulcers (liquid diet only); grade IV—food intake not possible [8].
Patient A.D.S., male, melanoderm (dark skinned), 62 years old, presenting grade IV OM (Figure 2) in which oral feeding was no longer possible, given that the medical recommendation was to suspend the radiotherapy treatment. The patient came with a companion, he was unable to speak and already in the initial anorexia phase, with low self-esteem and suffering. The patient was in the fourteenth session when the radiotherapy was interrupted. He signed the free and informed consent form in the first clinical consultation, after completing the clinical form and taking the anamnesis. Adjustment of the oral cavity was performed by removing the fibrinopurulent pseudomembrane with sterile gauze and successive washes with 10% hydrogen peroxide diluted in sterile deionized water, followed by washing with saline solution until the free, hygienized ulcerated mucosa was observed. The mucoadhesive propolis gel was applied with the finger throughout the mucosa, wearing sterile gloves. The patient received a tube containing 100 g of gel for daily use, free of charge. Monitoring was done weekly and through daily contact via cellphone so that the patient did not interrupt or forget to use the gel three times a day, every 8 h. There was regression of approximately 70% of the ulcerations (Figure 3) after 7 days, and with total regression of the lesions after 15 days of using the gel (Figure 4). There was a significant improvement in the quality of life and appearance of the patient who could already speak without the presence of a companion. With the improvement of his clinical condition, it should be highlighted that A.D.S. shaved his mustache and beard, showing up at the clinic with a more groomed and elegant personal appearance on the 7th and 15th day after the application of the gel. The patient was forwarded to the hospital and the oncologist of origin, and radiotherapy was restarted. The constant use of the propolis gel was indicated throughout the radiotherapy period and for six months after the end of the oncological treatment to avoid recurrences, keep the mouth lubricated, and prevent caries and periodontal disease. The patient was followed up for 6 months after the oncological treatment and no mucositis recurrence was observed. However, A.D.S. returned after 1 year, presenting a new ulcerated lesion on the tongue dorsum suggesting SCC recurrence. A biopsy confirmed the diagnosis. The use of propolis gel was suggested throughout the duration of the new cancer treatment, with no signs of OM being observed.
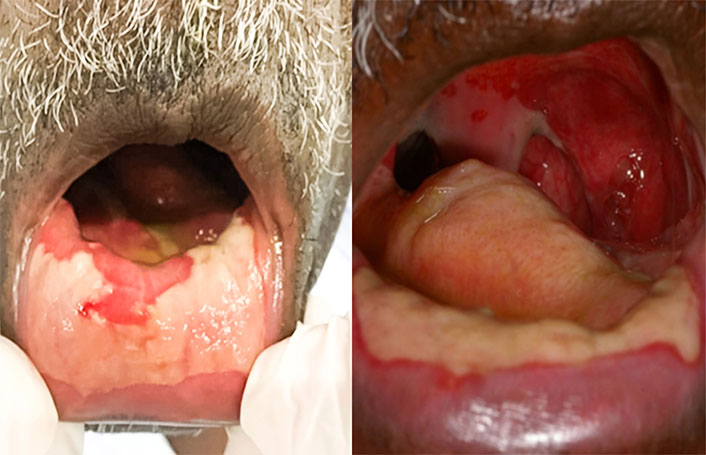
Patient A.D.S.: oral mucosa clinical aspect presenting grade IV OM before gel content propolis application
Patient M.T.O., male, feoderm (brown skinned), 54 years old, was referred by the Hospital Alberto Cavalcanti on October 18, 2018, for the treatment of a case of a grade IV OM induced by radiotherapy for well-differentiated SCC. The patient had a tumor in the oropharynx cervical region and presented trismus and phonetic difficulties. He had a history of smoking and alcoholism, abstaining from use for 7 years. OM made swallowing and speech impossible, concomitant with the presence of trismus and symptoms of hyperalgesia throughout the oral cavity, leading to the interruption of radiotherapy at the sixteen sections and the patient’s sudden weight loss. He was accompanied by his wife who answered the questions about his clinical history and presented the necessary documentation and referral by an oncologist. Following the same standard of care given to patient A.D.S., the gel content propolis was applied since his first clinical appointment. The use of the propolis gel was prescribed three times a day, to be spread throughout the oral mucosa and, if possible, to swallow part of the medicine. On October 26, 2018, the patient returned with a lesion regression of approximately 70%, improvement in the case of trismus, phonetics, and resumption of oral feeding. After fourteen days, the patient returned to the clinic and the lesion had completely regressed. The clinical report was issued and forwarded to the hospital and the oncologist for the resumption of radiotherapy.
Patient M.A.S., male, melanoderm (dark skinned), 62 years old, presented clinical signs similar to those observed in patients A.D.S. and M.T.O. Radiotherapy was instituted on September 10, 2018, for the treatment of a fixed neck mass hypopharyngeal SCC. Upon his return on October 26, 2018, the patient presented a case of a grade IV OM with erythematous and ulcerated coalescing lesions with purulent fibrin pseudomembranes with speech difficulty, oral feeding interruption, and consequently, radiotherapy interruption. The standard of clinical care and the therapy of choice was the same used in patients A.D.S. and M.T.O., being prescribed the clinical use of mucoadherent gel content propolis for home use topically spread throughout the mouth three times a day after hygiene and adequacy of the oral cavity. On November 9, the patient returned to the faculty with oral lesion regression of approximately 70%, with only scar tissue being clinically observed, restoring the patient’s quality of life. The comfort reported by the patient when using the mucoadherent gel with the analgesic agent is well-worth mentioning, which in the first days of use allowed him to eat more pleasantly. Total regression of the lesions was observed 14 days after using the gel, as observed in patients A.D.S. and M.T.O.
In order to treat grade IV OM lesions in three patients, mucoadhesive gel content green propolis was used as the therapy of choice due to the anti-inflammatory, antimicrobial, antitumor, immunostimulatory, and healing properties of propolis [27, 31] and also due to the fact that it is a natural product, which does not have side effects that prevent its use and being well accepted by patients. Propolis gel is easy to apply and inexpensive, which is another positive point for its prescription [32, 33]. In this clinical study, patients received the gels free of charge in bottles containing 100 g of the product and it is worth emphasizing the need to adapt already proven treatments in the literature to the reality of each patient. Most patients who seek dental care at Faculty of Dentistry of Federal University of Minas Gerais have low purchasing power and belong to a discriminated social class. Therefore, the biodiversity and the high production of Brazilian green propolis help in the final costs and the gel is inexpensive, making it accessible to patients in general. The therapeutic choice selected was effective and favored nutrition and the consequent improvement in the quality of life of these patients, who were in a situation of physical and psychological frailty. Several clinical studies have reinforced the effectiveness of propolis originating from both Brazil and other countries [6, 8, 34–36], however, there are few clinical cases published on the use of mucoadherent gel for the treatment of grade IV OM. Patients A.D.S., M.T.O., and M.A.S. received treatment for OM for 2 months in a hospital environment, with no regression of the lesions being observed when artificial saliva, antifungal, analgesic, and anti-inflammatory drugs were used. The success in the treatment with the propolis gel was due to biological activities and also, in part, to monitoring and assistance through telephone contact on a daily basis, to encourage use and avoid forgetfulness, as well as face-to-face clinical care once or more times a week, if necessary. Conducting a clinical, double-blind, and randomized study using the same propolis gel is needed for scientific proof of the treatment against grade IV OM.
OM: oral mucositis
SCC: squamous cell carcinoma
WHO: World Health Organization
The authors thank Fernanda Faria Rocha, for technical support in the Laboratory of Microbiology and Biomaterials.
DAS: Investigation, Writing—original draft. PCC: Supervision, Writing—original draft, Writing—review & editing. SFdS: Writing—review & editing. VRS: Conceptualization, Investigation, Project administration, Supervision, Writing—review & editing.
The authors declare that they have no conflicts of interest.
The clinical work was approved by the Human Research Ethics Committee of Federal University of Minas Gerais (No.0249.0.203.000-10) and complies with the Declaration of Helsinki.
Informed consent to participate in the study was obtained from all participants.
Informed consent to publication was obtained from relevant participants.
Data and information were obtained by completing a clinical form, anamnesis, patient history, and care by health professionals, oncologists, and qualified dentists. Data were recorded and archived. Clinical files and records are in the archives of the Faculty of Dentistry of Federal University of Minas Gerais, but are not available, to secure the privacy of patients.
This clinical work was supported by
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 6062
Download: 39
Times Cited: 0
