Affiliation:
Institute of Clinical Physiology, National Research Council, 56124 Pisa, Italy
Email: francesca-gorini@cnr.it
ORCID: https://orcid.org/0000-0002-4619-6227
Affiliation:
Institute of Clinical Physiology, National Research Council, 56124 Pisa, Italy
Email: alessandro.tonacci@cnr.it
ORCID: https://orcid.org/0000-0001-8335-5541
Explor Cardiol. 2023;1:114–140 DOI: https://doi.org/10.37349/ec.2023.00012
Received: July 25, 2023 Accepted: August 24, 2023 Published: November 13, 2023
Academic Editor: Karina Wierzbowska-Drabik, Medical University of Lodz, Poland
The article belongs to the special issue Environmental Cardiology
Congenital heart defects (CHD) represent the most frequent congenital anomalies among newborns, as well as the leading cause of spontaneous abortion, stillbirth, neonatal and infant death. CHD have been recognized as multifactorial diseases, with environmental contaminants as potential contributors to the etiopathogenesis of CHD. Toxic elements, such as arsenic (As), cadmium (Cd), lead (Pb), and mercury (Hg) are known to be associated with adverse reproductive outcomes and certain congenital anomalies, however their association with the risk for CHD remains inconsistent. This review summarizes the updated evidence on the CHD-associated risk related to exposure to As, Cd, Hg, Pb during pregnancy, reporting the main findings from epidemiological and experimental studies and the underlying molecular mechanisms. Additionally, being diet the major source of these elements in the general population, after having identified the main vectors of toxic metals in food, possible remediation strategies to reduce diet-related risks are also described. Among these, a novel, consumer-centered approach in developing new foods is discussed, considering not only the nutritional characteristics of edible compounds foods are made up of, but also their organoleptic features, making the food even more appealing to the consumer. Overall, current data support the association of maternal exposure to As and Pb with increased risk for CHD, although significant associations have only been observed for total and/or specific subgroups. On the other hand, the evidence of association for Cd and Hg exposure in pregnancy with CHD in the offspring remains, yet, quite speculative. Further large prospective cohort studies and insights into the molecular and biomolecular processes of these relationships are warranted to further explore and/or verify these findings.
Congenital heart defects (CHD), which account for nearly one-third of all major congenital anomalies and 3.3 million patients worldwide as of 2019, are defined as structural abnormalities of the heart and/or great vessels present at birth [1–3].
According to a recent systematic review and meta-analysis of 260 studies, the global birth prevalence of CHD increased by 10% every 5 years between 1970 and 2017, reaching a maximum of 9.4 per 1,000 live births in the years 2010–2017 [4]. Notably, over 90% of this increase is possibly due to improved diagnostics and, consequently, increased detection of milder lesions [1, 4]. On the other hand, although the global all-ages death rate due to CHD declined by 60.4% from 1990 to 2019 because of advances in interventional cardiology and congenital heart surgery, CHD still represent the leading cause of spontaneous abortion, stillbirth, neonatal and infant death [2, 5, 6]. Of note, both the prevalence and mortality of CHD are greatly heterogeneous throughout the world. As such, the highest CHD prevalence rates are mainly observed in developing countries, whereas wealthier nations experience a significant reduction in terms of CHD-related mortality [7].
The etiopathogenesis of CHD is complex and poorly understood, and only approximately 30% of cases can be attributable to chromosomal or single gene disorders, or other known causes (e.g., maternal diabetes, maternal viral infections) [8–10]. Conversely, in most cases, their cause is largely unknown and is suggested to be multifactorial, i.e., genetic, epigenetic, and environmental factors all influence and interact in the development of non-syndromal forms of CHD [11, 12]. Given the substantial medical and economic impact of CHD, it is of critical importance to explore modifiable determinants such as environmental factors in this regard [13].
Among environmental pollutants, certain metals [e.g., arsenic (As), cadmium (Cd), lead (Pb), mercury (Hg)] represent a serious concern to human health and, as being able to cross the placental barrier, they can act as developmental toxicants [14, 15]. Indeed, they have been associated with increased risk for preterm birth, low birth weight, small-for-gestational age, preeclampsia, impaired neurodevelopment, and autonomic dysfunction [16–21]. However, the current evidence for a risk association between toxic metals exposure and CHD remains inconclusive due to the limited number of studies conducted, especially those using biological matrices [22].
Overall, it is known that a non-negligible source of these potential toxicants for humans is represented by food. Indeed, anthropogenic activities account for the relevant accumulation of contaminants, including toxic metals, into agricultural soils, then heading to food crops and, subsequently, to the diet of people [23]. Understanding which are the main toxicants potentially involved in this process, their sources, their possible mechanisms of action in the body would have manifold consequences. At first, it can provide insights into the etiopathological mechanisms for CHD. Second, it could increase the awareness among the citizenship in the sense of enhanced sensibility towards healthy, safe and, possibly, sustainable food choices. Finally, in agriculture, such knowledge would lead to developing best practice to avoid, or eventually limit, the accumulation of these elements in edible substances, aiming at making safer food available for the global community, without sacrificing environmental sustainability of the whole production chain.
In this review, we therefore summarized the updated evidence on the CHD-associated risk related to exposure to toxic metals (i.e., As, Cd, Hg, Pb) during pregnancy, also reporting the possible underlying molecular mechanisms explaining these relationships. Furthermore, in this framework, we attempted at identifying the major toxic metals sources in the environment, focusing on edible compounds, in turn being one of the main vectors of these contaminants into the human body, and hypothesizing remediation strategies to reduce the related risks for the community at large.
In non-occupationally exposed individuals, diet and drinking water are the most common sources of exposure to toxic metals [24]. Exposure assessment can be performed through an ecological design, thus measuring metal concentration in environmental matrices (e.g., private wells for exposure to As via drinking water) and assigning maternal exposure based on geocoded residence and proximity to the environmental source [24]. Therefore, if exposure during pregnancy cannot be estimated on personal but on settlement level, and any observed association is limited to the ecologic unit of analysis (resulting in ecologic fault), this study design enables to include a large sample of liveborn cases [24, 25]. Alternatively, human source measurement is a direct method of maternal exposure based on appropriate bio-indicators [22]. Blood and urine samples have been the most widely used matrices for biomonitoring exposure to toxic metals in occupational and environmental toxicology [26]. Blood represents the ideal matrix, due to its contact with the whole organism and its equilibrium with organs and tissues where metals can be stored [27]. On the other hand, compared to blood and urine, which reflect recent exposure, hair and nails are considered more reliable, long-lasting matrices, as they better reflect the average level of metals over a longer period [28]. Hair also presents some limitations, such as the potential for external contamination not easily removable through washing procedures, as well as significant variations in metal content between various subgroups according to age, gender, hair color, hair care, smoking habits, and racial/ethnic factors [29]. Nails can also be used as bioindicators for metal toxicity due to the ease collection, transport, and storage [28]. Besides, although data on the heavy metal concentration of tooth dentine is scarce, teeth (dentine, enamel, or whole-teeth) offer interesting advantages as suitable long-term bio-indicators of environmental heavy metal exposures [30]. Indeed, the uptake of metals may occur during the formation and mineralization processes of tooth dentine, by replacing the mineral tooth compounds; therefore, metals can be stored over many years [30].
Exposure to As, a metalloid with ubiquitous distribution throughout the Earth’s crust [31], is considered as a major public health concern [32]. In particular, the inorganic forms of As are more toxic when compared to the organic As [33]. Although it has been estimated that between 94 million and 220 million people are at risk of exposure to high As concentrations in groundwater [34], diet still represents the primary source for As in nearly 1 billion people worldwide, with fish and seafood as main contributors for people not exposed through occupation or drinking water [35, 36]. In contrast to the predominance of inorganic As in water and many terrestrial foods, organic As species such as arsenobetaine and different arsenosugars are the most common forms in seafood [33, 37]. On the other hand, the main contributors to the dietary exposure to inorganic As are rice, rice-based products, other grains and grain-based products across the different age classes, with vegetables and fish being prevalent in adults [38].
Exposure to As, especially inorganic As, has been associated with several adverse health effects, including developmental, cancer, and cardiovascular outcomes [39]. In addition to being considered as a human toxicant and carcinogen, significant positive associations have been reported between maternal occupational As exposure and cleft palate [40] and between maternal total dietary As exposure and cleft lip (with or without cleft palate), as well as cloacal exstrophy [39, 40]. Furthermore, As can act as a potent teratogen able to induce neural tube defects (NTD) or interfere with folic acid metabolism and gene expression related to NTD development [41–44].
So far, a few studies have evaluated the potential impact of As on CHD occurrence in humans. A recent systematic review and meta-analysis (a total of 13 studies, of which 1 cohort, 10 case-control, 2 semi-ecologic) assessed the effect of heavy metal exposure on CHD. Regarding As (6 studies included, of which 4 using water as environmental source measurement and 2 using the hair as human source measurement), it was significantly associated with total CHD and septal defects, with an odds ratio (OR) = 2.12 (95% confidence interval (CI) = 1.21–3.71) and OR = 1.82 (95% CI = 1.23–2.71), respectively, while no associations were found with conotruncal defects (CTD) and left ventricular outflow tract obstruction (LVOTO) [22]. Of note, a high level of heterogeneity was observed across studies mainly due to different measurement methods, with human source measures showing a stronger association with CHD compared to environmental source environment [22]. In the nationwide cohort study by Richter et al. [45] including 1,042,4013 Danish liveborn children of whom 1% had CHD diagnosis, the authors found an increasing risk for CHD in the offspring with maternal exposure to higher As levels in drinking water. Specifically, the OR was 1.42 (95% CI = 1.24–1.63) for levels of As above 5.0 μg/L, and even maternal exposure to As in drinking water at low concentrations (0.5–0.9 μg/L) determined an OR of 1.13 (95% CI = 1.08–1.19). Similar results were observed for congenital septal defects, while no dose-response effects were shown for severe CHD and valvular heart defects [45]. If this study firstly provided evidence of an association between As and CHD at exposure levels well below the provisional guideline value of 10 μg/L [34], the lack of individual data did not allow to accurately estimate maternal exposure [45]. Another recent study investigated associations between maternal dietary As exposure (using self-reported dietary assessments and As concentration estimates in food items) during the year before pregnancy and CHD in mothers of 10,446 unaffected control children and 6,483 affected children who took part in the US National Birth Defects Prevention Study [46]. Significantly positive associations were only reported between the middle and high tertiles of dietary exposure to total As and perimembranous ventricular septal defects [OR = 1.2 (95% CI = 1.0–1.5) and OR = 1.6 (95% CI = 1.3–1.9), respectively]. Nonetheless, the paucity of information on inorganic As concentration on food items and the possibility of recall bias in self-report of diet might have underestimated the maternal As exposure, overall [46]. In contrast, a multi-center case-control study conducted in 7 cities and one county represented by 8 Chinese provinces, and including 303 CHD cases and 303 controls, found no significant association of maternal plasma As concentration with the risk for fetal CHD [13]. Overall, the inconsistence of results might depend on differences in the study design, sample size and methods of exposure assessment [13].
Epigenetic mechanisms—all modifications to the genome that confer heritable changes in gene function independently of nucleotide sequence—are suspected to increase the risk of NTD, and epigenetic dysregulation has also been suggested as the mechanistic link between As exposure and health outcomes [47–49].
In particular, prenatal As exposure can be associated with adverse effects in newborns and increased risks of disease development later in life through DNA methylation changes [50–52], histone post-translational modifications [48, 53, 54], alterations in noncoding microRNA (miRNA) expression [55, 56]. Na and co-authors [57] have demonstrated that folic acid supplementation during the periconception period might play a protective role in heart development against the toxic effects of As in fetal rats, which, in the presence of folic acid, exhibited a lower level of H3K9 acetylation in fetal rat cardiomyocytes than those exposed to As alone.
In the developing heart, the transforming growth factor beta (TGFβ) family of ligands and receptors are pivotal for developmental cardiac epithelial to mesenchymal transition (EMT) and differentiation into coronary smooth muscle cells [58]. Exposure to arsenite [As(III)] resulted in a dose-dependent decrease in the expression of EMT genes including TGFβ2, TGFβ receptor-3, Snail, and Has-2 in immortalized murine epicardial cells [58]. As(III) exposure has also been reported to block nuclear accumulation of Smad2/3 (effectors regulating cell differentiation and extracellular matrix remodeling) in response to TGFβ2 [58, 59]. Monomethylarsonous acid [MMA(III)], the most toxic metabolite of As(III), caused a significant blockage in epicardial cellular transformation and invasion at doses 10 times lower than As(III) by downregulating EMT genes (TGFβ ligands, TβRIII, Has2, CD44, Snail1, TBX18, and MMP2) [60]. Additionally, MMA(III) disrupted Smad2/3 activation at a dose 20 times lower than As(III) does [60].
Also, oxidative stress, defined as the imbalance between the production of reactive oxygen species (ROS) and antioxidant defenses, is a relevant pathophysiological mechanism in the development of many cardiovascular disorders [61, 62]. Despite the heterogeneity of biomarkers and subgroups of diseases analyzed and the conflicting results between studies, the disruption of redox signaling pathways could have a causative role in CHD [62, 63–66]. Importantly, one of the possible mechanisms for As cardiotoxicity involves the generation of intracellular ROS, both via the mitochondrial electron transport chain and biotransformation of As and its detoxification and production of metabolites in cells [67–69]. ROS may affect signaling pathways, which lead to activation or inhibition of transcription factors, or cause direct oxidative damage to molecules [68, 69]. Alteration in miRNA, which regulate gene expression and are known to be involved in diverse cell functions, such as proliferation, apoptosis, differentiation, and tumorigenesis, is another modification closely related with intracellular ROS levels and, conversely, the levels of ROS can be regulated by miRNA [68, 70, 71]. As(III) treatment can downregulate the specificity protein 1 (Sp1), which is involved in a variety of biological processes, including embryo development, cell growth, differentiation, and carcinogenesis and acts as a transcriptional activator of growth/differentiation factor 1 (GDF1) that is related to the formation of the left-right axis of developing heart [72, 73]. Indeed, as shown in mammalian cells, As suppresses GDF1 expression through the ROS-dependent downregulation of Sp1 [73]. As is also an activator of nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB), which is suggested to involve generation of ROS and, at the same time, of nuclear factor-erythroid 2-related factor 2 (Nrf2), a master transcription factor in the antioxidant system, whose expression is tightly regulated in the cardiac muscle tissue in physiological conditions [68, 74, 75].
Cd can be found in nature at low concentrations and is also present in the environment as a pollutant deriving from agricultural and industrial sources (e.g., combustion of fossil fuels, mining waste, leachate generation from landfill sites) [76]. In the general population, the major routes of exposure to Cd are cigarette smoking (one cigarette contains from 0.5 μg to 1 μg Cd) and, in no-smokers, diet based on certain foods if grown on contaminated soil [77, 78]. Wheat, rice, and potatoes provide 40–60% of the overall dietary Cd intake and high concentrations of Cd are also present in shellfish, crustaceans, mollusks [79, 80]. Therefore, not only during pregnancy, diet is the main source of Cd exposure, but vegetarians and shellfish consumers might be exposed to higher Cd intake than omnivores do [76, 81]. The mean dietary exposure across European countries was estimated ranging from 1.9 μg/kg to 3.0 μg/kg bodyweight (bw) per week, with vegetarians reaching an average weekly exposure of up to 5.4 µg/kg bw per week [82]. Due to the long half-life of Cd in the human body (20–30 years), the European Food Safety Authority (EFSA)’s Panel on contaminants in the food chain set a tolerable weekly intake (TWI) for Cd of 2.5 µg/kg bw [82, 83].
A range of epidemiological evidence has shown that even low-level environmental exposure to this metal poses a risk for health in the general population, leading to damage to the kidneys, liver, skeletal system, and cardiovascular system (e.g., heart disease, stroke), as well as to deterioration of sight and hearing [76, 80, 84]. Even moderate or high-level exposure to Cd during pregnancy can result in serious health issues for the fetus, including malformations (i.e., NTD and cleft lip and palate), decreased fetal growth rate, low birth weight [85–88].
The widespread contamination of Cd in natural environments by human activities creates a serious threat to the public health since chronic, even at low levels, exposure to Cd is a potential risk factor for damage to many organs and systems, including the developing heart [80]. In fact, the teratogenic effects of Cd on cardiovascular system have been recently shown. In a multicenter case-control study performed in China, Jin and co-authors [89] reported that hair Cd concentrations in mothers of children with CHD (n = 339) were significantly higher than those measured in mothers of control children (n = 333), and that the group with the highest Cd levels (25.85 ng/g) displayed an increased risk of CHD (OR = 1.96, 95% CI = 1.24–3.09), in particular CTD (OR = 2.81, 95% CI = 1.40–5.64), in the offspring. Furthermore, Cd and As could have an interactive effect, enhancing the risk of CHD compared to Cd or As alone [89]. In contrast, the Chinese hospital-based study by Ou et al. [14], including 112 and 109 case and control mothers, respectively, did not find any significant association between maternal blood Cd levels and CHD (or any of the analyzed subgroups) both in single-element and multi-element models. When compared to previous findings, the different results observed might depend on the smaller sample size and blood collection from participants in mid- and late-pregnancy, after the critical period for cardiac development, which occurs in the 3rd–8th week of gestation [14]. Therefore, Li et al. [22], in a small meta-analysis based on three studies, of which two were cited above, reported a weak risk association with a pooled OR of 1.30 (95% CI = 0.93–1.82), with no association with any of the subtypes (CTD, LVOTO, right ventricular outflow tract obstruction, septal defects) considered, despite the acceptable evidence of heterogeneity among studies. In agreement with these results, no association was found between Cd concentrations in maternal plasma and risk of CHD in the multicenter case-control study by Wang et al. [13], which assessed prenatal exposure to selected heavy metals and their association with CHD risk. A previous study carried out on 51 chromosomally normal newborns diagnosed with CTD and 72 healthy newborns, showed that Cd concentrations measured in 1st-day meconium specimens of the former were significantly higher than those of the controls [90]. Of interest, the authors observed significant and positive associations between Cd and trace essential elements: Cd and iron (r = 0.485, P = 0.001), Cd and zinc (Zn; r = 0.386, P = 0.001), and Cd and copper (r = 0.329, P = 0.001) [90].
The ability of Cd to interfere with the cardiac function has also been observed in zebrafish (Danio rerio), which may be used as a model organism for toxicological studies with Cd [91]. Indeed, following Cd exposure (between 24 h and 96 h post-fertilization), heart rate in larvae increased with concentration, this being significant even at the lowest Cd concentration used (0.01 µmol/L) [91]. An enlargement of pericardium and ventricle was also noticed in many of the 10 μmol/L Cd-treated larvae [91]. Consistently, offspring of mice born to Cd-exposed mothers were characterized by an increase in heart weights at birth and susceptibility to hypertension during adulthood, which might be caused by perturbed trace element [e.g., sodium, iron, selenium (Se)] homeostasis and function induced by prenatal Cd exposure [84], thus deficiency of essential biometals can increase gastrointestinal absorption and the accumulation of Cd in the body [92, 93].
Two hypotheses have been proposed to explain the effects of Cd on cardiac tissue structure and integrity, as well as on the cardiac conduction system [93, 94]. The first one is related to the pro-oxidative Cd action and the consequent alteration of cellular redox balance [94]. Although Cd is not able to directly induce ROS (e.g., hydroxyl and superoxide anion, hydrogen peroxide), it could stimulate their production indirectly, by multiple mechanisms—i.e., the inhibition of mitochondrial electron transport chain, the displacement of redox-active metals, the depletion of antioxidants, and the inactivation of antioxidant enzymes [95, 96]. This element may also induce the release of transition metals such as iron and copper from metallothionein, ferritin or ceruloplasmin, increasing their availability in cells, which results in the generation of ROS [93, 95]. Furthermore, Cd may interact with sulfhydryl (SH) groups, present in many proteins and in reduced glutathione (GSH), which is the main intracellular antioxidant, so that decreased cellular GSH concentration results in disturbance of the cell redox state and alteration of the antioxidant system [83]. Induction of oxidative stress damages the key macromolecules (proteins, DNA, and lipids), intracellular organelles, and cellular membranes, thereby interfering with cell proliferation and causing inflammation, apoptosis, and genomic instability [80, 97, 98]. The second mechanism involved in the Cd-induced cardiotoxicity is based on the interaction of Cd with contraction and acceleration systems [94]. Therefore, Cd can be transported into endothelial cells of vessel walls, causing disruption of endothelial integrity and Cd-mediated endothelial cell death, which is speculated as being the pivotal process leading to atherosclerosis and contributing to vascular inflammation, too [99].
Pb, a naturally occurring toxic metal detected in the Earth’s crust, is a cumulative toxicant, which has been estimated to account for 21.7 million years lost to disability and death worldwide due to its long-term effects on health [100]. Extensive environmental contamination from Pb owing to its widespread use in human activities (e.g., mining, smelting, manufacturing, and recycling activities), represents a significant public health concern in many regions of the world [100]. While many commercial and industrial uses of Pb have been phased out, more than three-quarters of the global Pb consumption comes from the production of Pb-acid batteries for motor vehicles [100, 101]. Hence, while exposure to Pb has largely dropped in the last 30 years, there is no known safe blood Pb concentration and the current blood Pb reference value of 3.5 µg/dL does not protect children from the reduction in learning capacity, ability to pay attention, and academic achievement [102, 103]. Human exposure to inorganic Pb, the most predominant form in the environment, occurs primarily through food and water, with estimated Pb dietary exposure ranging from 0.36 to 1.24 for adults in 19 European countries, and up to 2.43 µg/kg bw per day in high consumers in Europe [104]. Cereals, vegetables, and tap water represent the most important contributors to Pb exposure in the general European population [104].
Being characterized by long half-life (30 days and between 10 years and 30 years in the blood and bone, respectively), chronic toxicity to Pb is a major concern especially for the nervous system and kidneys [105]. Pb exposure also causes anaemia, hypertension, immunotoxicity, and toxicity on the reproductive and skeletal systems [100, 105]. The metal stored in the bones may be released into the blood during pregnancy, exposing the fetus via the placenta, therefore increasing the risk of premature birth and low birth weight in the offspring [100, 102, 104, 106]. Additionally, prenatal Pb exposure was associated with an excess risk of oral clefts and musculoskeletal anomalies [107] and of NTD, although with somewhat inconsistent results [107, 108].
Regarding the risk of CHD associated to maternal Pb exposure, the meta-analysis (including five studies) by Li et al. [22] revealed a significant association with CHD, CTD, and LVOTO outcomes, with ORs of 2.30 (95% CI = 1.37–3.85), 2.34 (95% CI = 0.91–6.02), and 1.93 (95% CI = 1.10–3.36), respectively. Conversely, no significant associations were found between Pb exposure and septal defects [22]. Additionally, there was high heterogeneity among studies due to different measurement sources [22]. A case-control study performed on a total of 246 Iranian mothers (146 mothers with CHD children and 100 age-matched mothers with healthy children) showed that mothers’ blood Pb concentration in the group of children with CHD was significantly higher than that in the control group (P = 0.03), although the small size and the difference in group sizes could affect results [109]. These findings were further supported by a multicenter case-control study conducted in China, which revealed an increased risk for CHD associated with maternal plasma levels of Pb (OR = 2.74, 95% CI = 1.00–7.57). Finally, a significantly higher meconium Pb concentration was found in newborns with CTD compared to that measured in the meconium of healthy newborns [90]. As the composition of meconium plausibly reflects the nutritional history of the fetus, we could also speculate that toxic metal levels in this material are the result of the fetus exposure during the gestational period [90]. Also, the study evidenced positive and significant correlations between Pb and Cd (r = 0.618, P = 0.001), Pb and iron (r = 0.368, P = 0.001), Pb and Zn (r = 0.245, P = 0.005), Pb and copper (r = 0.291, P = 0.001) [90].
Although the teratogenic mechanisms of Pb are not fully known, several possible explanations for Pb toxicity on the embryo’s heart have been hypothesized [109]. One of them involves the generation of ROS that have been shown to play a crucial role in the pathophysiology of cardiac remodeling and heart failure [105, 110]. Notably, besides its effects on body pressure, exposure to high levels of Pb is also associated with increased risks of cardiovascular outcomes including stroke, peripheral arterial disease, coronary heart disease, and cardiovascular functional abnormalities such as left ventricular hypertrophy and alterations in cardiac rhythm [111, 112]. As reported in the previous chapters, oxidative stress can lead to irreversible cell damage and ultimately to death, causing pathological cardiovascular conditions including CHD [113]. Within a case-control study (97 cases with CHD and 201 controls without any abnormalities), Liu and co-authors [113] observed that the activity of two antioxidant enzymes, superoxide dismutase (SOD), and glutathione peroxidase, in the fetal umbilical serum of CHD cases (particularly for CTD or outflow tract malformations) was significantly lower than in the control fetuses, with SOD activity significantly decreasing with increasing Pb concentration in the umbilical serum. Consistently, a previous study on chick embryos demonstrated that the remodeling of the cardiac outflow tract was particularly sensitive to ROS-mediated injury, thus arguing that ROS might contribute to the onset of CHD [114].
Pb may also play a significant role in the formation and development of inflammatory diseases by inducing the expression of cytokines (interleukin-6,8, tumor necrosis factor, interferon-gamma), enhancing the expression and activity of enzymes involved in inflammation (cyclooxygenase-2), upregulating the expression of endothelial nitric oxide (NO) synthase and NO, increasing CD4+ T cell percentages, neutrophil, and monocyte counts and reducing immunoglobulin production, thereby predisposing to a further increase in oxidative stress and the development of vascular endothelial dysfunction [115–117].
In men aged 55 and older, an increase in blood Pb was positively associated with higher plasma concentration of homocysteine, a one-carbon metabolite that is also elevated among women with a history of adverse pregnancy outcomes including CHD [118, 119]. In addition, this relationship was significantly stronger among individuals with estimated dietary intakes of B6 vitamin and folate below (vs. above) the study population medians, suggesting that increasing the intake of the two substances might reduce Pb-associated increases of homocysteine [119].
Finally, DNA methylation could be one of the mechanisms underlying the adverse effects of prenatal Pb on the offspring [120]. Epigenome of the developing fetus could be influenced by maternal cumulative Pb burden, since prenatal low levels of Pb exposure was inversely associated with genomic DNA methylation in umbilical cord blood, especially in female infants [120, 121]. Perinatal exposure to Pb in mice might also promote gender-specific programming of the cardiac epigenome (i.e., methylation at genes related to immune function and metabolism in males, and histone demethylation in females) that persists long after the cessation of exposure [21, 122].
Like other toxic elements, the presence of Hg in the environment derives from both natural causes (volcanic activity, weathering of rocks) and human activities (coal-fired power stations, residential coal burning for heating and cooking, industrial processes, waste incinerators and mines) [123]. Estimates of annual global Hg emissions from anthropogenic sources are approximately 2,220 metric tons per year [124]. These estimates include Hg that is re-emitted from soils and oceans, in turn receiving past atmospheric depositions of Hg, the magnitude of which is 2–3 times larger than the primary anthropogenic emissions. Of note, Hg is the only element to have its own multilateral environmental agreement (i.e., the Minamata Convention on Mercury), which addresses specific human activities that are contributing to widespread Hg pollution, thus highlighting the key role for Hg pollution as an environmental and public health issue [125–127].
The various forms of Hg—elemental (persisting in the environment for long periods of time and capable of traveling long distances), inorganic (mercurous and mercuric salts to which people may be exposed through their occupation) and organic (as methylmercury, to which people may be exposed through their diet, especially seafood where it bioaccumulates) differ in their effects on human health [123, 128]. The Contaminants in the Food Chain (CONTAM) Panel of the EFSA established a TWI for methylmercury of 1.3 μg/kg bw [129]. The 95th percentile dietary exposure is close to or above the TWI for all age groups, while high fish consumers, which could include pregnant women, may exceed the TWI by up to approximately six-fold [129].
While brain is the primary target of elemental Hg, which has a half-life of up to 20 years, inorganic Hg species, being more water soluble, are more easily absorbed by the gastrointestinal tract, where they exert their toxic effects (half-life of approximately 40 days) [130]. Once absorbed by the gastrointestinal tract, methylmercury can then enter the red blood cells and the brain and, due to its low water solubility, is primarily eliminated from the body in an inorganic form with a half-life between 39–70 days [130]. In addition to the blood-brain barrier, methylmercury can pass through the placenta, potentially exposing women of childbearing age and children in general to harmful consequences [131]. Indeed, several studies have reported total Hg concentration in maternal blood during pregnancy being associated with reduction in anthropometry, although at somewhat higher blood Hg concentrations [132–135]. Furthermore, prenatal exposure to methylmercury or total Hg was associated with the onset of NTD both in animal [136, 137] and human models [41, 138, 139].
As for the potential role of Hg on CHD onset, a recent meta-analysis based on three studies, reported weak evidence of association, with a pooled OR of 1.22 (95% CI = 0.98–1.53), while no significant association was observed for CTD and septal defects [22]. Conversely, the multicenter case-control study by Wang et al. [13] estimated an OR of 2.88 (95% CI = 1.22–6.77) between maternal Hg blood concentration and the risk of CHD in newborns.
Although for many years Hg toxic effects were mainly associated with the central nervous and renal systems, Hg may also produce cardiotoxicity [14]. Numerous experimental and human studies have demonstrated the ability of Hg to increase free radical production and, consequently, oxidative stress, inactivate antioxidant enzymes (e.g., SOD, catalase, paraoxonase), induce immune and mitochondrial dysfunction, bind to Se to form Se-Hg-complex-mercury-selenide, which reduces Se availability for glutathione peroxidase, increase oxidation of low‐density lipoprotein and platelet aggregation, decrease NO bioavailability, induce apoptosis [140, 141]. The consequences of such actions might result in hypertension, coronary heart disease, myocardial infarction, cardiac arrhythmias, sudden death, reduced heart rate variability, increased carotid intima-media thickness and carotid artery obstruction [141]. Although yet to be confirmed, a dose-response function relating methylmercury to myocardial infarction exposures has been proposed [142]. Additionally, a recent systematic review and meta-analysis found a significant association of Hg exposure with nonfatal ischemic heart disease, but none with stroke [143].
Emerging evidence has suggested that epigenetic changes may be a critical regulator of the mechanisms associated with Hg exposure and the development of a variety of adverse effects in humans [144]. Higher prenatal maternal Hg exposure measured during the second trimester of pregnancy was associated with lower levels of global 5-hydroxymethylcytosine, one of the most common epigenetic modifications, in cord blood DNA after adjusting for potential confounders, and this association was persistent in early childhood (2.9 years to 4.9 years) [145]. More recently, a case-control study reported gender-specific changes in DNA methylation in cord tissues related to prenatal exposure to total Hg [146]. Therefore, the prenatal toxicity of Hg could be mediated by fetal epigenetic programming, as changes in DNA methylation play a critical role in embryogenesis and cell lineage commitment [145].
Given the key role of oxidative stress in the molecular mechanism of As-induced toxicity and related diseases, one of preventive and therapeutic strategy for As poisoning is based on the use of antioxidants that may reduce ROS generation, enhance the antioxidant capacity and regulate ROS-related signaling pathways [68].
Besides GSH, which can mitigate toxicity resulting from methylation-mediated As detoxification-excretion process, and metformin, which decreases intracellular ROS levels by inhibiting mitochondrial respiratory chain complex I, there are other antioxidants involved in regulating signaling pathways such as Nrf2, NF-κB, mitogen-activated protein kinases (that regulate proliferation, gene expression, differentiation, mitosis, survival, and apoptosis in the cell) [68]. Nrf2 is a potential target in disease prevention given its critical role in scavenging excessive ROS levels and restoring redox homeostasis [75]. On the other hand, a chronic activation of Nrf2 can lead to reductive stress, which is equally harmful than oxidative stress [75]. Hence, since the understanding of mechanisms underlying As toxicity is still to be fully elucidated, the application of antioxidants should be carefully evaluated.
In addition to chelation therapy, with meso-2,3-dimercaptosuccinic acid (DMSA) and calcium trisodium diethylene triaminepentaacetate combined effectively used in acute oral Cd, coenzyme Q10, vitamin C and vitamin E, naturally occurring antioxidants, and in particular their synergistic interaction, might have a beneficial effect in protecting from Cd-induced hepatotoxicity and be used as a preventive against acute Cd intoxication [147, 148]. Combination of ascorbic acid, alpha-tocopherol, and Se can be effective against Cd toxicity in rat intestine [149]. Magnesium and Zn also have many clinical applications facilitating immune function and preventing free radical generation [150]. Polyphenolic phytochemicals appear to be able to modulate lipid and lipoprotein abnormalities and thereby reducing the risk of cardiovascular disease by stabilizing antioxidant defense systems [151].
Chelation therapy has been widely recognized as the best treatment to reduce blood Pb levels [105]. While the previously recommended therapy of severe Pb poisoning with encephalopathy was a combination of calcium ethylenediamine tetraacetate (CaEDTA) given intravenously and 2,3-demercaptopropanol-1 (BAL) given intramuscularly to facilitate the brain-to-blood transfer and induce urinary excretion, DMSA is the most efficient and safe chelation treatment regimen presently available in moderately Pb-poisoned cases [152, 153]. DMSA can be administered either orally or intravenously and is characterized by lower toxicity than CaEDTA [152, 154]. As DMSA is unable to remove Pb from the intracellular sites because of its lipophobic nature, more severe cases can be treated either with a DMSA-monensin combination or DMSA combined with low-dosed BAL administered intramuscularly [152, 155].
Hg intoxication can be counteracted with both DMSA and 2,3-dimereaptopropanesulfonate (DMPS) treatment, which significantly decreases the burden of Hg in fetal liver and brain, whereas only DMPS treatment is able to reduce Hg renal levels [153, 156]. For severe inorganic or elemental Hg poisoning, a combined DMPS-BAL-regimen can be recommended [152]. Notably, Se supplementation may play a central role in biological response to Hg as Se has been suggested to have a protective effect against Hg toxicity by reducing absorption from the gastrointestinal tract, redistributing the toxic element to less sensitive target organs, facilitating demethylation of organic Hg to inorganic Hg, in turn binding to inorganic Hg to form insoluble, stable and inert Hg:Se complex, and finally restoring target selenoprotein activity and the intracellular redox environment [157].
A number of anthropogenic activities (e.g., ore mining, smelting processes, fossil fuels combustion, transport emissions, excessive pesticide and fertilizer application, electronic waste and wood preservatives, sewage and solid waste) have notably contributed to the excessive accumulation of toxic metals in agricultural soils and, as a result, have negatively impacted both food security and the health of millions of people globally [158–160]. In addition, due to their non-degradability, toxic metals persist in the soil for a long period of time [161]. In particular, the sources of Zn, Cd, and Pb have been mainly associated with mining activities, responsible for the increased accumulation of toxic metals in farmland soil and in food crops [162]. Zn and Cd are also commonly present in phosphate fertilizers, while other pesticides used in agriculture have toxic elements such as Hg, As, and Pb, too [158, 161]. Metal accumulation in crops mainly occurs via absorption by roots from contaminated soils, however the uptake of metals by plant roots is not linear with their soil concentration but vary across crop species, soil properties, e.g., pH, redox potential, content of organic matter and hydrous ferric oxide, moisture content and water holding capacity, soil aeration, microbial activity, and mineral composition), and metal species [159, 163, 164]. In particular, soil metal bioavailability is defined as the relationship between the amount of metals absorbed by plant tissues and the relative fractions bound to soil components or free in solution [159].
In recent years, physical, chemical, and biological treatment options have been employed to remediate toxic metal contaminated soil, water, and sediments. Such methods include thermal treatment, adsorption, electrokinetics, soil washing, chlorination, solvent extraction, filtration, ion-exchange, membrane separation, bioleaching, etc. [158]. Most of the physicochemical methods, in addition to their high costs, high energy requirements, low efficiency, and unpredictable metal ion removal, make sludge disposal difficult and, at the same time, pose the possibility of a secondary pollution problem [165, 166]. In contrast, bioremediation, which can be classified into in situ or ex situ categories based on the strategies involved, is a natural, cost-effective, environmentally friendly approach in which the polluted environment is biologically purified by using microorganisms (bacteria or fungi) or plants or the combination of both [161, 165, 166]. Nevertheless, in bioremediation, toxic metals are not eliminated but only transformed from one organic complex or oxidation state to another, sometimes producing more toxic species than the original elements [165, 166]. Furthermore, bioremediation is time consuming and may be limited by irregularity and uncertainty of completeness as well as influenced by the climatic and geological conditions of the site to be remediated [164, 166].
Phytoremediation, in particular, is an emerging technology that involves plants for the removal, degradation, or containment of toxic metals from soils via different mechanisms, i.e., phytoextraction, phytostabilization, phytodegradation, and phytovolatilization [159, 167]. Phytoextraction, the most common form of phytoremediation, uses hyper-accumulators able to extract 50–500 times more pollutants than normal plants, and genetic engineering has allowed to transfer hyper-accumulator genes from plants having low biomass to others with high biomass such as Brassica species [161]. Several Brassica species were reported to moderately accumulate quantities Cd, Pb, and As [167–169]. Furthermore, the use of chelating agents such as synthetic ethylenediaminetetraacetic acid, increasing the solubility of the metals in soils, improves plant metal uptake and, consequently, soil decontamination [161, 164]. On the other hand, the increased availability of metals in the soil solution facilitated by chelators may result in the contamination of groundwater via leaching, and the same chelating agents, if used at high concentration, may be toxic to plants, soil microbes, and soil enzyme activities [164, 170]. Although phytoremediation currently represents the cheapest and fastest technique to decontaminate soil from toxic metals and the most appropriate method when the remediated site is used for crop production, its effectiveness depends on numerous factors including properties and concentration of metals, type of soil, depth of contamination and natural processes occurring at the site [161, 164].
Nano-bioremediation is a novel approach for the elimination of environmental pollutants (heavy metals, organic and inorganic compounds) using biosynthesized nanoparticles formed by plants, fungi, and bacteria with the help of nanotechnology [171]. Nanoparticles are characterized by high absorption, interaction, and reaction capabilities and can penetrate deeper in soils, enhancing the efficiency of phytoremediation, also representing a cost-effective and eco-friendly alternative to existing physical and chemical methods [165, 171]. Reported examples of nano-bioremediation include the application of nano‑titanium dioxide that increased uptake of Cd and reduced Cd toxicity to soybean plants [172] or by that of nano-hydroxyapatite and nano-carbon black, which significantly mitigated the phytotoxicity of Pb to the ryegrass and increased phytoextraction potential of the plant [173]. While studies on remediation applications and fate of nanoparticles in soil remain scarce and are mainly conducted at a laboratory scale, growing evidence supports that nanotechnology has the potential to revolutionize existing technologies used in various sectors, including pollution control [171, 174]. On the other hand, the safety of nanomaterials is a major concern for their full application and studies examining their toxicity to flora and fauna, along with their stability in the bioremediation of toxic metals at commercial and industrial levels, should be performed prior to their use [175].
The selection of crops with higher tolerance to metal toxicity is another strategy to tackle metal contamination in soils [159]. In addition to breeding interventions that identify and transfer useful traits to develop new crop varieties, e.g., Pb tolerant watermelon varieties [176], Cd-tolerant mutants of tomato [177], the identification of genetic regions associated with metal tolerance and the use of “omics” technologies (genomics, transcriptomics, proteomics, metabolomics, ionomics) has made it possible to mitigate toxic metal stress in crop plants [159]. Besides, the recent technique of clustered regularly interspaced short palindromic repeats (CRISPR)—CRISPR associated protein 9 (Cas9) systems has demonstrated a considerable potential for genome editing and can be employed for engineering genomes of several crop plants against various abiotic stresses, including crop species tolerant to toxic metals, by activating or repressing target genes/transcription factors [178, 179]. Although CRISPR/Cas system is still in the exploratory phase, this editing technology not only shows high efficacy in modifying template DNA sequences but promises to increase the phytoremediation ability of plants [180, 181].
Finally, some home processing methods such as boiling, roasting, and frying were reported to reduce the levels of As, Cd, Hg, and Pb in food crops grown in and around mining centers [182]. Specifically, the decrease in toxic metal contents occurred best in frying, followed by boiling and then roasting, while reductions in Pb levels were more pronounced than for other metals [182]. Additionally, root vegetables constitute a group particularly vulnerable to the presence of contaminants, including certain metals like Pb and Cd, which migrate from soil and accumulate in roots depending on pH, ionic strength, soil texture, organic matter content, and time [183, 184]. Therefore, it is highly recommended that root vegetables require at least washing, and often peeling, blanching, boiling, or frying, before they can be eaten or processed [183].
As reported above, a non-negligible number of heavy metals is conveyed to the human body due to contaminated food intake. Contamination of foods originates by bedrock weathering, air pollution, soil irrigation with contaminated water and polluting groundwater [185], as well as contaminated compost [186]. One of the strategies to challenge such issues is represented by the attention towards the use of pesticides. In fact, pesticides, especially inorganic ones, are known to contain heavy metals. Some phosphate and micronutrient fertilizers, and liming materials contain higher levels of As, Cd, and Pb than other fertilizers, like nitrogen, potash, gypsum, making the first object of investigation in this specific framework [187, 188]. In fact, despite the efforts by regulatory bodies, including those specifically working into pesticides [or plant protection products (PPPs)], wide amounts of these contaminants are currently in use, especially in developing countries—where normative guidance is often lacking—with direct effects on workers, consumers, and citizens both in the country of origin and abroad, due to massive export of contaminated products, where the regulations are not particularly strict on this specific topic. Fortunately, the European Union (EU) is at the forefront in this field, with a cautious, attentive pathway for the distribution of edible compounds on the market. This consists of a clear definition of the characteristics of a compound to be considered a PPP [see the Regulation (EC) No 1107/2009] [189] and of a two-tiered approach for the approval and authorization of PPP use. At first, to enter the European market, the active compound of the pesticide must be approved for the EU. Subsequently, a PPP approval procedure can take place in each of the member states individually, followed, in case of positive outcome, by a monitoring program aimed at verifying that pesticide residues in the food stay below the EFSA limits. Overall, the use of PPPs in the EU follows the above-mentioned Regulation (EC) No 1107/2009 [189] in cooperation with the Regulation (EC) No 396/2005 [190] on maximum residue levels in food and the Directive 2009/128/EC on sustainable use of pesticides [191]. The bodies in charge for the relationships with pesticide regulations in the EU include the European Commission, EFSA, and the European Chemicals Agency. In this regard, several research activities have been proposed to develop new strategies and products, having similar properties than PPPs, even reducing the possible effects, direct or indirect, PPPs could possibly have on human health. Therefore, the application of aromatic plant essential oils has been seen as a fruitful, promising alternative in such a framework to prevent post-harvest losses of stored food, as they appear to be environmentally friendly, biodegradable, non-polluting for soil and water, and have a quite low toxicity [192].
The use of essential oils as an alternative to PPPs carries on several advantages but is also challenged by a bulk of significant drawbacks. Among these, several issues are identified when it comes to their sensory properties and characteristics. This fact is largely related to the variability in their composition, mirrored by the function of biotic and abiotic factors acting on the treated plant and the bioactivity of the single oil components. Under these premises, a small number of pure chemical components are commonly used to build up these compounds, often with the production of strong odors, as in the case of using monoterpenes and sesquiterpenes, in turn making their application to edible products cumbersome [193, 194]. Alternatives in this regard have been studied, including the use of eugenol and other derivatives to reduce the volatility of essential oils and its odor fingerprints, maintaining, as much as possible, the organoleptic properties of the edible compound [195]. The referenced article, together with related literature, highlights the importance of considering the sensory maintenance as the cornerstone for implementing promising, well accepted alternatives to pesticides. Until now, sensory assessment is normally performed through large, well-grounded sensory panels, providing descriptive, semi-quantitative responses to several items dedicated to different parts of the sensory domains, including visual, olfactory, gustatory characteristics of a given food. This approach is well accepted by most of the sensory community, although important methodological drawbacks are still present. Indeed, according to the common belief, the presence of trained, skilled panelists makes the results of this analysis poorly generalizable to the general population, in turn characterized by a completely different approach and mindset with respect to the problem demanded [196]. In addition, by relying on questionnaires, such approach makes use of the subjective judgment towards one or more characteristics of the compound evaluated, without an objective counterpart to be monitored. Therefore, efforts are made in this direction, and consensus has been started in the purpose to support the typical approach of sensory investigation panels with technological tools, including electronic noses, electronic tongues, and wearable sensors [197].
The global spread of consumer technologies is noticeable in any sector of our everyday life, from gaming and entertainment to healthcare and well-being. Food industry is just one of the possible use cases of this approach, taking advantage of the opportunities and close connection with the healthcare and well-being domain. Yet, it is technically feasible and cost affordable to monitor oneself well-being status through smartwatches or wearables, merging low invasiveness and acceptable reliability, especially when used along with artificial intelligence-based algorithms. Through wearables, one may detect changes in terms of the central and autonomic nervous system (ANS) activation by observing and analyzing in real-time signals captured from the human body. As such, galvanic skin response, related to sympathetic arousal, can be studied through the application of soft electrodes to fingers of one’s non-dominant hand, whereas the photoplethysmographic and electrocardiographic signals normally detected through smartwatches or chest straps are gaining reliability and momentum in sports applications and beyond, making these tools capable of informing about the sympathetic and vagal activity of the ANS, as well as of their overall balance. The use of these methodologies and materials in food-related studies has been recently increased, with works carried out from the laboratory settings up to non-structured or semi-structured environments [198, 199]. According to the literature, chemosensory pleasantness is closely related to familiarity towards a given compound [200, 201], but just recently the relationship of odor pleasantness with autonomic variations was observed [198, 202] and, interestingly, recent investigations have shed a light into the consequentiality between those two domains, with familiarity being the driver for pleasantness. This observation was carried out in healthy individuals; however, its retrieval in clinically relevant population could potentially have important consequences on the possible development of functional foods, with particular ingredients and organoleptic features for given clinical groups, including pregnant mothers and very young children, or eventually for promoting healthy attitudes towards food consumption, overall, also taking into account the environmental and economic sustainability of the food production chain, reducing the pressure put on the planet from food companies (Figure 1).
The future perspective of food choices: a journey between physiology, emotions, and sustainability, powered by artificial intelligence
Overall, findings from observational studies support the risk association of maternal exposure to toxic elements—As and Pb—with increased risk for CHD, although significant associations have only been observed for total and/or specific subgroups of CHD. As for Cd and Hg, a few studies have assessed this relationship and the evidence of association with CHD remains, yet, quite speculative (Table 1). On the other hand, a number of cellular processes support a cardiotoxic action of all these elements on the developing heart (Figure 2).
Clues and pitfalls for the role of toxic metals in the risk of CHD
| Clues | References | Pitfalls | References |
|---|---|---|---|
| As | |||
| Associated with developmental and cardiovascular outcomes in humans | [40] | Few observational studies conducted | [13, 22, 45, 46] |
| Potential teratogen | [39–44] | Inconsistent results due to differences in the study design, sample size, and methods of exposure assessment | [13, 22, 45, 46] |
| Significant associations with total CHD and septal defects | [22, 45, 46] | Lack of information on As levels in food and possibility of recall bias on dietary habits | [46] |
| Cd | |||
| Associations with outcomes in the cardiovascular system | [84] | Few and inconsistent data because of heterogeneity between studies | [14, 22, 89] |
| Association with congenital anomalies and other adverse reproductive outcomes | [85–88] | ||
| Association observed between co-exposure to Cd and other toxic elements and CHD | [90] | ||
| Pb | |||
| Associated with adverse effects at birth and somewhat with certain subgroups of congenital anomalies | [107, 108] | Heterogeneity among studies due to different measurement sources | [22] |
| Small sample size of studies | [109] | ||
| Hg | |||
| Evidence for an association birth outcomes | [132–135] | Few studies performed with conflicting results | [13, 22] |
| Association with NTD in human and animal studies | [41, 136–139] | ||
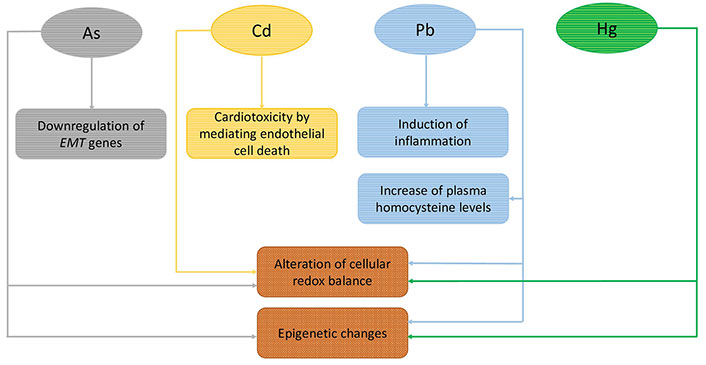
A summary of the proposed underlying mechanisms in the association of As, Cd, Pb, and Hg exposure with the onset of CHD. See text for details
Prospective cohort studies involving biological specimen collection in early pregnancy (preferably during the first 3–8 weeks of pregnancy, when the heart develops) as well as in the newborns on blood, hair, and umbilical serum, investigations on larger populations and the adjustment of more potential confounders in the models are warranted to explore and/or verify these findings. Of note, the choice of suitable biomarkers to assess metal exposure is of crucial importance, for primary prevention, health-care management, and decision-making in public health. Furthermore, it would be useful to investigate both physiological and biochemical processes of the metals and whether the genetic susceptibility may influence the metabolism and distribution of metal elements in the human body. Additionally, the associations between exposure to toxic metals individually and in combination and CHD are not fully elucidated and warrant further research.
Primary prevention—the removal of toxic elements from the environment and the maintenance of a sufficient diet—is the most effective way to reduce possibility of direct exposure. Analytical methods to measure the levels of elements in different food products, should also be developed and validated to prevent health hazards. Furthermore, screening tests on several biological matrices (blood, urine, hair) and secondary prevention remain an essentially safe choice for pregnant women who can be potentially exposed to harmful contaminants. The legislation within the food production chain aimed at reducing harmful compounds, such as pesticides, carrying on important environmental hazards also related to heavy metals, is helping to this end. The use of technologies in assessing the organoleptic characteristics of a given food and the psychophysiological reactions of consumers during feeding also represents a promising and ambitious perspective for enhancing food safety and sustainability, fostering the reduction of food-related spread of heavy metals from the environment to the table and, ultimately, promoting safe habits in the overall population and particularly in fragile groups as pregnant mothers and newborns.
As(III): arsenite
As: arsenic
BAL: 2,3-demercaptopropanol-1
bw: bodyweight
Cd: cadmium
CHD: congenital heart defects
CI: confidence interval
CRISPR: clustered regularly interspaced short palindromic repeats
CTD: conotruncal defects
DMPS: 2,3-dimereaptopropanesulfonate
DMSA: meso-2,3-dimercaptosuccinic acid
EFSA: European Food Safety Authority
EMT: epithelial to mesenchymal transition
EU: European Union
GSH: reduced glutathione
Hg: mercury
LVOTO: left ventricular outflow tract obstruction
miRNA: microRNA
NO: nitric oxide
Nrf2: nuclear factor-erythroid 2-related factor 2
NTD: neural tube defects
OR: odds ratio
Pb: lead
PPPs: plant protection products
ROS: reactive oxygen species
Se: selenium
SOD: superoxide dismutase
TGFβ: transforming growth factor beta
TWI: tolerable weekly intake
Zn: zinc
FG and AT: Conceptualization, Investigation, Writing—original draft, Writing—review & editing. Both authors read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
Not applicable.
© The Author(s) 2023.
Copyright: © The Author(s) 2023. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Maria Grazia Andreassi
Cristina Vassalle
