Affiliation:
3Department of Pathology, Aretaieion Hospital, Medical School, National and Kapodistrian University of Athens, 11528 Athens, Greece
4Translational and Clinical Research Institute, Faculty of Medical Sciences, Newcastle University, Framlington Place, NE2 4HH Newcastle upon Tyne, UK
Email: dtiniak@med.uoa.gr; dina.tiniakos@ncl.ac.uk
ORCID: https://orcid.org/0000-0003-4657-7780
Affiliation:
51st Department of Pathology, Medical School, National and Kapodistrian University of Athens, Goudi, 11527 Athens, Greece
ORCID: https://orcid.org/0000-0002-3639-6807
Explor Med. 2022;3:181–187 DOI: https://doi.org/10.37349/emed.2022.00084
Received: January 06, 2022 Accepted: February 23, 2022 Published: April 24, 2022
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
The article belongs to the special issue Exploring Chronic Liver Disease
A case of combined acute and chronic liver injury related to consumption of multi-ingredient nutritional oral supplements containing Aloe Vera gel and vitamin A among other vitamins, minerals and dietary elements such as fish and calamari oil in a 59-year-old female with unexplained hypertransaminasemia is reported. A unique complex liver injury was diagnosed on liver biopsy combining histological features of protracted acute hepatitis, mild manifestation of hypervitaminosis A and lipogranulomatous reaction attributed to Aloe Vera, vitamin A and lipids, respectively. Normalization of liver tests was achieved after discontinuation of all nutritional supplements. Updated Roussel Uclaf Causality Assessment Method (RUCAM) score (+8, probable) further supported herb-induced liver injury. The present case highlights the increasing incidence of complex histological liver injury linked to the constantly growing consumption of multi-ingredient dietary supplements and alternative medications.
The use of dietary supplements and alternative medications is rapidly increasing with over 50% of the US adult population and 18.5% of the European using herbal or dietary supplements [1]. The growing popularity of herbal and nutritional supplements is reflected in the higher incidence of side effects focusing primarily on their frequent responsibility for undiagnosed acute and chronic liver injury.
Patients turn to herbal medications because they are perceived as a safer, more affordable treatment choice with fewer side effects. However, these substances are taken without medical advice or monitoring and their safety, efficacy and dosage are not always well defined. Furthermore, they do not require prescription, so many physicians are unaware of their patients’ use of such supplements. Herbal medicines and traditional plant food supplements are defined under different legal frameworks. According to Directive 2004/24/EC for herbal medicines, herbal medicines are defined as “medicinal” if presented as having properties for treating or preventing disease in humans or if they have a pharmacological, immunological or metabolic action [2]. In the Directive 2002/46/EC on food supplements regulation No. 1924/2006 European Food Safety Authority, “food supplements” should be marketed as such [3].
The incidence of herbal and dietary supplements (HDS)-related hepatotoxicity is increasing worldwide. In a prospective study of drug-induced liver injury which was accomplished between 2004 and 2014 from the NIH-funded Drug-Induced Liver Injury Network (DILIN), the incidence of liver toxicity related to HDS and body building products rose from 7% to 20% [4]. The majority of cases were attributed to body building agents that contained anabolic steroids and the remaining were attributed to multi-ingredient products, herbal products (e.g., green tea, kratom, black cohosh), traditional botanical mixtures (e.g., “Chinese herbs”, “Korean herbs”, “Ayurvedic medications”), simple vitamins or minerals or dietary supplements (e.g., niacin, multivitamins, levocarnitine). In the Spanish Drug-Induced Liver Injury (DILI) Registry, the incidence of HDS-related liver injury increased from 2% of all DILI in 2006 to 13% between 2010 and 2013 [5].
Proportion of herb-induced liver injury (HILI) varies greatly in Asia with reported percentages 70% in Singapore, 73% in Korea, 18.6% in China and 2.5% in India [6]. Best estimate in a population-based survey in Iceland (2011–2012), 19/100,000 persons have been diagnosed with DILI, 16% attributed to HDS (incidence 3/10,000 persons) [7].
Hepatotoxicity due to HDS has often been reported in medical literature usually manifested as acute hepatitis (AH) and cholestatic syndromes. The spectrum of HDS-induced liver injury includes anabolic steroid-related jaundice due to illicit synthetic derivatives of androgens, acute liver injury due to identifiable specific botanical product or traditional herbal medicine i.e. most common: green tea (Camellia sinensis) or acute liver injury associated with a multi-ingredient nutritional supplement (68% of HDS-DILI) [5]. The identification of the responsible component is often challenging with regard to the main complicating factor such as synthetic chemicals or unknown toxic botanical.
A case of combined liver injury related to consumption of multi-ingredient nutritional oral supplements including Aloe Vera and vitamin A among other vitamins, minerals and dietary elements is presented.
A 59-year-old woman, with a 12-year history of rheumatoid arthritis, presented as a private patient in 2013 with fatigue and abdominal discomfort in the epigastrium that extended to the periumbilical area without fever or pruritus. There was no history of alcohol misuse and no signs of metabolic syndrome. Clinical examination revealed normal body-mass index, normal abdomen in palpation with increased intestinal sounds and a slightly enlarged liver. The remainder of the physical examination was unremarkable.
The patient had been treated with D-penicillamine and prednisolone (1991–1995) and with methotrexate (2001–2003) for her rheumatoid arthritis but had not received any treatment since 2003. In 2005, she started using daily nutritional supplements for healthy joints, specifically “Aloe Vera juice” containing 0.958L/L Aloe Vera and “Aloe berry nectar” with the same consistency. She also had been using supplements containing fish and calamari oil and a compound containing vitamin A from beta-carotene, vitamin E and mineral selenium.
Detailed laboratory test follow up showed a gradual increase in alanine aminotransferase/aspartate aminotransferase (ALT/AST) levels during the period 2013–2015 with peak values of ALT 227 U/L (n < 38) and AST 153 U/L (n < 40). Serum alkaline phosphatase (ALP), gamma-glutamyl transferase (γGT), bilirubin, complete blood count, urea, creatinine and total protein electrophoresis were all normal. Serologic examinations for viral screening and autoantibodies were negative. A liver ultrasound was normal.
A liver biopsy was performed in 2015 because of continuing unexplained hypertransaminasemia. Histological examination revealed AH characterized by zone 3 confluent necrosis with immature collagen deposition and mild portal inflammation. In zone 3, multiple lipogranulomas and ceroid laden macrophages were also seen. Additional findings were mild steatosis, hepatic stellate cell (HSC) hypertrophy and hyperplasia occasionally with intracytoplasmic lipid droplets and focal perisinusoidal fibrosis (Figure 1). Histological diagnosis suggested complex liver injury combining acute hepatitis with protracted course, lipogranulomatous reaction and possible hypervitaminosis A in keeping with a drug-induced/toxic etiology. Nutritional supplements containing Aloe Vera gel, vitamin A, fish and calamari oil were aetiologically implicated. The updated Roussel Uclaf Causality Assessment Method (RUCAM) was used to determine causality resulting to a +8 score supporting probable HILI (Table 1).
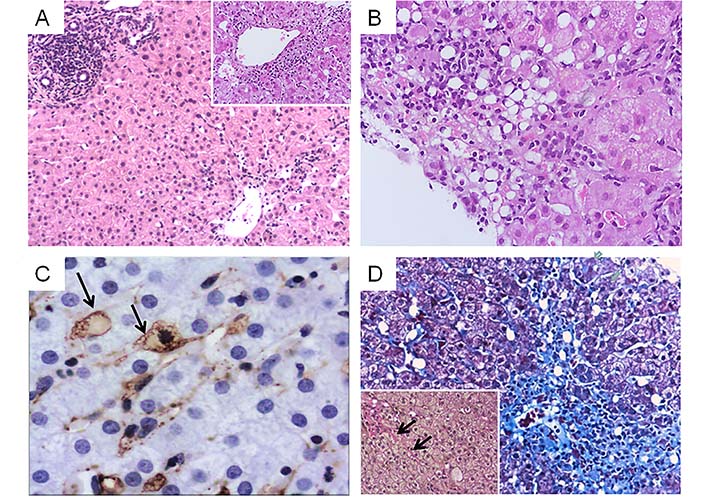
Combined liver injury related to consumption of multi-ingredient nutritional oral supplements containing Aloe Vera gel and vitamin A. A. Acute hepatitis pattern (HE, ×100) with confluent necrosis in zone 3 (inset, ×200) B. Lipogranulomatous reaction (HE, ×200) C. Hypertrophic HSC containing lipid droplets (arrows) highlighted by α-smooth muscle actin immunohistochemistry (3’3’ diaminobenzidine chromagen, ×400) D. Zone 3 necrosis and collapse with immature fibrosis (Masson trichrome, ×100). Inset: van Gieson stain highlights focal perisinusoidal fibrosis, ×200 (arrows)
Score results of the present case according to the updated RUCAM for hepatocellular injury
| Items for hepatocellular injury score result | Score | Results |
|---|---|---|
| 1. Time to onset from the beginning of the drug/herb | ||
| • 5–90 days (rechallenge: 1–15 days) | +2 | +2 |
| • < 5 or > 90 days (rechallenge: > 15 days) | +1 | |
| Alternative: time to onset from cessation of the drug/herb | ||
| • 15 days (except for slowly metabolized chemicals: > 15 days) | +1 | |
| 2. Course of ALT after cessation of the drug/herb. % difference between ALT peak & N | ||
| • Decrease ≥50% within 8 days | +3 | +3 |
| • Decrease ≥50% within 30 days | +2 | |
| • No information or continued drug use | 0 | |
| • Decrease ≥50% after the 30th day | 0 | |
| • Decrease < 50% after the 30th day or recurrent increase | −2 | |
| 3. Risk factors | ||
| • Alcohol use (current drinks/d: > 2 for women, > 3 for men) | +1 | |
| • Alcohol use (current drinks/d: ≤2 for women, ≤3 for men) | 0 | |
| • Age ≥55 years | +1 | +1 |
| • Age < 55 years | 0 | |
| 4. Concomitant drug(s)/herb(s) | ||
| • None or no information | 0 | 0 |
| • Concomitant drug/herb with incompatible time to onset | 0 | |
| • Concomitant drug/herb with compatible or suggestive time to onset | −1 | |
| • Concomitant drug/herb known as hepatotoxin and with compatible or suggestive time to onset | −2 | |
| • Concomitant drug/herb with evidence for its role in this case (positive rechallenge or validated test) | −3 | |
| 5. Search for alternative causes | ||
| Group I (7 causes) | ||
| • HAV: Anti-HAV-IgM | ||
| • Hepatobiliary sonography/colour Doppler | ||
| • HCV: Anti-HCV, HCV-RNA | ||
| • HEV: Anti-HEV-IgM, anti-HEV-IgG, HEV-RNA | ||
| • Hepatobiliary sonography/colour Doppler sonography of liver vessels/endosonography/CT/MRC | ||
| • Alcoholism (AST/ALT ≥2) | ||
| • Acute recent hypotension history (particularly if underlying heart disease) | ||
| Group II (5 causes) | ||
| • Complications of underlying disease(s) such as sepsis, metastatic malignancy, autoimmune hepatitis, chronic hepatitis B or C, PBC or PSC, genetic liver diseases (-) | ||
| • Infection suggested by PCR and titer change for | ||
| • CMV (anti-CMV-IgM, anti-CMV-IgG) | ||
| • EBV (anti-EBV-IgM, anti-EBV-IgG) | ||
| • HSV (anti-HSV-IgM, anti-HSV-IgG) | ||
| • VZV (anti-VZV-IgM, anti-VZV-IgG) | ||
| Evaluation of groups I and II | ||
| • All causes-groups I and II—reasonably ruled out | +2 | |
| • The 7 causes of group I ruled out | +1 | +1 |
| • 6 or 5 causes of group I ruled out | 0 | |
| • Less than 5 causes of group I ruled out | −2 | |
| • Alternative cause highly probable | −3 | |
| 6. Previous hepatotoxicity of the drug/herb | ||
| • Reaction labelled in the product characteristics | +2 | |
| • Reaction published but unlabelled | +1 | +1 |
| • Reaction unknown | 0 | |
| 7. Response to unintentional reexposure | ||
| • Doubling ALT with the drug/herb alone, provided ALT below 5N before reexposure | +3 | |
| • Doubling of ALT with the drug(s)/herb(s) already given at the time of first reaction | +1 | |
| • Increase of ALT but less than N in the same conditions as for the first administration | −2 | |
| • Other situations | 0 | 0 |
| Total score for the case | +8 |
Individual items related to the case and corresponding results are shown in bold font; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HAV: hepatitis A virus; HCV: hepatitis C virus; HEV: hepatitis E virus; HSV: herpes simplex virus; IgG: immunoglobulin G; IgM: immunoglobulin M; MRC: magnetic resonance cholangiography; N: upper limit of the normal range; PBC: primary biliary cholangitis; PCR: polymerase chain reaction; PSC: primary sclerosing cholangitis; VZV: varicella zoster virus
The patient was advised to stop all nutritional supplements and liver tests (LTs) gradually returned to normal over a five-month period. She did not attend any subsequent medical appointments.
Complex histological liver injury due to drugs and toxins is observed more frequently nowadays due to the consumption of multi-ingredient products. The agent that was implicated for the development of AH in our case is Aloe Vera since neither the additional ingredients of the supplements nor the other used supplement compounds she was consuming have known hepatotoxicity. Aloe Vera is extracted from a cactoid member of the Liliaceae family and is most frequently used for body weight management or constipation relief [8]. Hepatic toxicity attributed to Aloe Vera preparations is becoming more and more apparent, with 22 cases reported in a recent review [8]. These concerned 17 women and 5 men (mean age 50 years) and a latency period ranging from 3 to 24 weeks. Symptoms included jaundice, fatigue, abdominal discomfort and nausea. Hepatocellular injury was the most common liver manifestation (86.3%). Poor prognosis was associated with multiple herb consumption while in most cases liver injury was self-limited with normalization of liver tests following discontinuation of the drug [8]. In a previous literature review of Aloe Vera acute liver injury. 9 cases were studied; these concerned 7 women and 2 men, 21–68 year-old with a latency ranging from 2 weeks to 5 months [9]. Symptoms included jaundice (n = 4), fatigue (n = 2), pruritus (n = 3), abdominal discomfort (n = 4), nausea (n = 2), vomiting (n = 1) and fever (n = 1). Although age, daily dose and latency period varied, histological findings were invariably consistent with AH and hepatocellular type of liver injury. Following discontinuation of Aloe Vera ingestion, AH resolved in almost all cases with LFTs decreasing within 8 days to 30 days and normalizing within 51 days to 1 year. In one case positive re-challenge after retaking the same extract one month after discharge provided evidence of Aloe Vera pathogenetic effect [9]. Another patient with multiple sclerosis who was receiving intramuscular Interferon beta and Aloe supplements developed AH after several years of treatment [10].
The underlying mechanisms of Aloe Vera-induced hepatotoxicity are not well defined due to the small number of reported cases and the complex biochemical reaction of the substance, as it contains several alkaloids that may induce or block hepatic enzymes [11]. Both direct cytotoxicity of aloe or its biotransformed constituents and idiosyncratic reaction [11] have been implicated, the latter showing delayed presentation in humans [12]. A secondary reaction to contamination with Bacillus subtilis and/or chemical additives has also been suggested [13]. Our case also showed HSC hypertrophy and hyperplasia occasionally with intracellular lipid droplets and focal perisinusoidal fibrosis, most likely as a result of hypervitaminosis A. The relative mild lesions despite the long-term consumption may be ascribed to the low risk of vitamin A overdose in relation to beta-carotene use.
Several cases of toxic liver injury due to hypervitaminosis A have been described and their frequency is increasing due to the wide consumption of vitamin A supplements [14]. Chronic hepatotoxicity may exist for many years without recognition resulting in portal hypertension and finally cirrhosis [15]. In excessive dietary vitamin A intake, vitamin A accumulates in HSCs as retinyl palmitate within lipid droplets. The number, size and vitamin A (retinyl ester) content of the latter increases in response to greater dietary vitamin A [16]. Histological hallmark is HSC hyperplasia accompanied by patchy zone 3, periportal and sinusoidal fibrosis with narrowing of the space of Disse [13]. In a study examining clinical associations of liver biopsies with HSC hyperplasia, only a minority had documented hypervitaminosis A while it was most commonly ascribed to multimedicated patients [14].
The pathogenesis of the concomitant lipogranulomatous reaction in our case, could be attributed to endogenous lipid release from steatotic hepatocytes and lipid-laden HSCs due to zone 3 confluent necrosis as well as to possible exogenous lipid origin from the consumed oil products [17].
It is worth mentioning that in the present case histopathology highlighted the etiology of liver injury. Moreover, the updated RUCAM, a commonly used tool for clinical estimation of hepatic toxicity related to drugs or herbs, was indicative of probable herb-induced liver injury supporting the implication of Aloe Vera in the aetiology of liver injury in our case [18].
In summary, our patient’s clinical and histological findings suggest combined acute and chronic liver injury of different aetiology due to multi-ingredient supplements use. The updated RUCAM score in combination with liver biopsy are critical tools in reaching the diagnosis in suspected HILI. Our case emphasizes the need to take into account the use of supplements as a probable cause of complex liver injury. It is proposed that nutritional and herbal supplements should undergo the same toxicological studies and surveillance measures as synthetic drugs.
AH: acute hepatitis
ALT: alanine aminotransferase
AST: aspartate aminotransferase
CMV: cytomegalovirus
DILI: drug-induced liver injury
EBV: Epstein-Barr virus
HCV: hepatitis C virus
HDS: herbal and dietary supplements
HEV: hepatitis E virus
HILI: herb-induced liver injury
HSC: hepatic stellate cell
HSV: herpes simplex virus
IgG: immunoglobulin G
IgM: immunoglobulin M
RUCAM: Roussel Uclaf Causality Assessment Method
VZV: varicella zoster virus
KD was responsible for drafting the manuscript, literature review; EM clinical responsibility, clinico-pathological review; DT interpretation of histological findings, critical review of the final manuscript; SS interpretation of histological findings, critical review of the final manuscript. All authors contributed to manuscript revision, read and approved the submitted version.
The authors declare that they have no conflicts of interest.
Not applicable.
A written informed consent was obtained from the patient when liver biopsy was performed, with which the tissue might be used for scientific research but did not relate to patient’s privacy.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2022.
Copyright: © The Author(s) 2022. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
View: 13071
Download: 66
Times Cited: 0
Maria Gabriela Delgado ... Jean François Dufour
Maria Giulia Cornacchia ... Gaetano Serviddio
Alessandro Mantovani ... Andrea Dalbeni
Sheikh Mohammad Fazle Akbar ... Yoichi Hiasa
Amedeo Lonardo
Chunye Zhang, Ming Yang
Chunye Zhang ... Ming Yang
