Affiliation:
1Hepatology, University Clinic of Visceral Surgery and Medicine, Inselspital, University Hospital Bern, 3010 Bern, Switzerland
Email: mariagabriela.delgado@insel.ch
ORCID: https://orcid.org/0000-0002-3967-8893
Affiliation:
2ASST Santi Paolo e Carlo, Hepatology and Gastroenterology Unit, 20153 Milano, Italy
ORCID: https://orcid.org/0000-0003-0495-0188
Affiliation:
3Institute of Pathology, Inselspital, University Hospital Bern, 3008 Bern, Switzerland
ORCID: https://orcid.org/0000-0001-5377-0875
Affiliation:
4Clinic of Gastroenterolgy and Hepatology, Bürgerspital Solothurn, 4500 Solothurn, Switzerland
ORCID: https://orcid.org/0000-0003-4185-0151
Affiliation:
1Hepatology, University Clinic of Visceral Surgery and Medicine, Inselspital, University Hospital Bern, 3010 Bern, Switzerland
ORCID: https://orcid.org/0000-0003-2707-765X
Affiliation:
1Hepatology, University Clinic of Visceral Surgery and Medicine, Inselspital, University Hospital Bern, 3010 Bern, Switzerland
5Hepatology, Department of Biomedical Research, University of Bern, 3008 Bern, Switzerland
ORCID: https://orcid.org/0000-0003-4562-9016
Affiliation:
1Hepatology, University Clinic of Visceral Surgery and Medicine, Inselspital, University Hospital Bern, 3010 Bern, Switzerland
5Hepatology, Department of Biomedical Research, University of Bern, 3008 Bern, Switzerland
Email: jean-Francois.Dufour@insel.ch
ORCID: https://orcid.org/0000-0002-8062-1346
Explor Med. 2021;2:122–134 DOI: https://doi.org/10.37349/emed.2021.00037
Received: October 28, 2020 Accepted: March 17, 2021 Published: April 30, 2021
Academic Editor: Amedeo Lonardo, Azienda Ospedaliero-Universitaria di Modena, Italy
The article belongs to the special issue Exploring Chronic Liver Disease
Drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome is a potentially life-threatening drug reaction, which can affect multiple organs. Patients with DRESS syndrome and hepatic manifestations may present alterations ranging from mild hepatitis to acute liver failure. The diagnosis might be difficult, and the management of these patients is challenging. This report analyzes a series of five cases reporting the clinical presentation, which ranged from acute hepatitis to liver failure, and discussed their treatment.
Drug-induced hypersensitivity reaction with systemic symptoms and eosinophilia syndrome is an infrequent but potentially lethal cause of liver injury [1, 2]. The first description of cases was in the 1950s related to the use of anticonvulsants [3, 4]. In 1996 Bocquet et al. [1] introduced the term drug rash with eosinophilia and systemic symptoms (DRESS) syndrome. After that, the term “rash” was replaced by “reaction” because skin involvement is not mandatory for the diagnosis [1]. The terminology of this syndrome has changed over time, including other names such as drug-induced hypersensitivity syndrome (DIHS), drug-induced delayed multiorgan hypersensitivity syndrome (DIDMOHS), or anticonvulsant hypersensitivity syndrome (HSS) [5].
The clinical pattern is diverse and includes variable cutaneous and systemic symptoms such as skin rash, fever, enlarged lymph nodes, hypereosinophilia and involvement of at least one organ among liver, lung, kidney, heart, pancreas, and nervous system. Based on these observations, a diagnostic scoring system has been developed (RegiSCAR score) [6, 7]. The severity of this syndrome is related to systemic involvement, which can result in multi-organ failure. The liver is the most frequently affected visceral organ in patients with DRESS syndrome [2, 8–10]. Hepatic involvement has been described in up to 75–95% of patients, which can range from a slight elevation of transaminases to liver failure, being associated with a mortality of around 10% [1, 8, 11–14]. It has been postulated that the clinical features may be related to the class of drug taken [7].
Severe DRESS syndrome cases are challenging and usually present with significant alteration of liver enzymes or even liver failure. For this reason, this syndrome requires an extensive workup to rule out other possible causes of acute hepatitis and acute liver failure. Most of these patients respond to steroid treatment, but a small percentage may require rescue therapy with liver transplantation [15]. We present a series of five cases of DRESS syndrome and liver involvement.
A 34-year-old man was referred to the emergency department for cough, fever, and a severely impaired liver function test. The symptoms started three weeks before the admission. After seven days of treatment with amoxicillin/clavulanic acid (3 g/day), paracetamol (4 g/day), and metamizole (4 g/day), he recovered completely. Two weeks later, fever (39–40°C), and cough recurred, and the patient was admitted to the hospital with the suspicion of sepsis and liver failure. On admission, the patient was febrile (39.3°C), his pulse regular at 99 beats per minute, his blood pressure was 107/68 mmHg (1 mmHg = 0.133 kPa), respiratory rate was 20 per minute, and his oxygen saturation was normal. The examination of the skin, heart, and abdomen was unremarkable. Lung examination displayed basal percussion dullness. There were no skin or mucosal lesions or palpable lymphadenopathy. The neurologic exam was normal. The main laboratory findings are shown in Table 1. The C-reactive protein (CRP) was 337 mg/L, the procalcitonin 5.59 μg/L (< 0.1 μg/L) and the D-dimer-test was markedly elevated (11,590 μg/L; standard value: ≤ 500 μg/L), Fibrinogen and thrombocytes were normal. Toxicological screening, including paracetamol, was negative. Urine and blood cultures were repeatedly negative. Screening tests for autoimmune liver disease and standard virologic and parasitological tests were negative (Table 1).
Characteristics of the patients
| Case | Clinical presentation | Drug possibly involved | Skin rash present | Day of definite diagnosis | Total WBC/Eosinoph (G/L) | AST/ALT (IU/L) | ALP pANCA/GGT (IU/L) | Bilirubin (umol/L) | INR | Negative screening Tests | Day starting steroids | Clinical outcome | RegiSCAR Score (points) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Male 34 years old | Cough, fever (39.3°C) and severely impaired liver function test | Oral amoxicillin/clavulanic acid for 7 days 2 weeks before admission | Not on onsetPruritic rash is seen by day 10 (thorax and legs) | 5, by histology findings based on a transjugular liver biopsy + rising eosinophilia (10.7 G/L) | 14.60/1.53 (max. 18.41) | 2,124/2,504 | 113/180 | 26 | 1.9 | HAV, HBV, HCV, HEV, HSV-1, HSV-2 VZV, CMV, EBV, HIV 1 + 2, Parvovirus B 19, HHV-6, HHV-7, Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila (Urine), Influenza A, Influenza B, RSV, Strongyloides, intestinal protozoa and helminths.ANA, pANCA, SMA, SLA, LKM-1, AMA, IgG and IgM, ceruloplasmin, alpha-1-antitrypsin | Day 5; prednisone60 mg and tapered | It was rapidly improved on steroids.Flare at day 10: increase of steroids.Discharged on day 12. | 4 (fever = 0, rash = 1, eosinophilia = 1, liver involved = 1, other causes excluded = 1) |
| 2. Male61 years old | Fever (39°C), exanthema, severely impaired liver function test | Ciprofloxacin | Diffuse maculopapular rash | 5, based on liver biopsy | 12.0/0.8 | 650 (max. 18.473)/680 (max. 6,745) | 180/423 | 46 | 1.8 | HAV, HBV, HCV, HEV, HSV-1, HSV-2 VZV, CMV, EBV, HIV 1 + 2, HHV-6, Parvovirus B 19, Strongyloides, intestinal protozoa and helminths. ANA, pANCA, SMA, SLA, LKM-1, AMA, IgG and IgM, ceruloplasmin, alpha-1-antitrypsin, Aspergillus (Galactomannan antigen), β-d-Glucan | Day 5; prednisone 25 mgDay 17; methylprednisolone, 250 mg i.v. | Rapidly progressive deterioration of liver function and multiorgan failure.Liver trans-plantation at day 20.Exitus letalis 16 days after LT. | 4 (fever = 0, rash = 1, eosinophilia = 1, liver involved = 1, other causes excluded = 1) |
| 3. Male 60 years old | Fever, rash, cough, and vomiting | PhenytoinLevetiracetam | Pruritic maculopapular rash | 4, on histology findings | 12.0/4.24 (max. 13.6) | 135/308 | 317/1,688 | 75 | 1.16 | HAV, HBV, HCV, HEV, HSV-1, HSV-2, VZV, CMV, EBV, HIV 1 + 2, Parvovirus B 19, HHV-6, HHV-7, Mycoplasma pneumoniae, Chlamydia pneumonia, TBC, Strongyloides, intestinal protozoa and helminths.ANA, pANCA, SMA, SLA, LKM-1, AMA, IgG and IgM, ceruloplasmin, alpha-1-antitrypsin | Day 4; prednisone 1 mg/kg | Progressive improved on steroids. | 6 (fever = 0, rash = 2, eosinophilia = 1, atypical lymphocytes = 1, liver involved = 1, other causes excluded = 1) |
| 4. Female 62 years old | Jaundice and rash | Cefuroxime | Diffuse maculopapular Rash | 2, on histology findings based on a liver biopsy | 6.3/1.31 | 383/497 | 756/550 | 217 | < 1.0 | HAV, HBV, HCV, HEV, HSV-1, HSV-2 VZV, CMV, EBV, HIV 1 + 2, HAV, HHV6, intestinal protozoa and helminths.ANA, pANCA, SMA, SLA, LKM-1, AMA, IgG and IgM, ceruloplasmin, alpha-1-antitrypsin | Day 2; prednisone40 mg and tapered) | Rapidly improved on steroids. | 4 (rash = 1, eosinophilia = 1, liver involved = 1, other causes excluded = 1) |
| 5. Male65 years old | Progressive exanthema and elevation of ASAT, ALAT, GGT and AP | Lamotrigine | Diffuse maculopapular rash | The diagnosis was suspected by clinical mani-festations, it was not confirmed by biopsy | 10.8/1.18 | 264/659 | 219/106 | 8 | 1.2 | HBV, HCV, HEV, HSV-1, HSV-2 VZV, CMV, EBV, HIV 1 + 2, HAV, intestinal protozoa and helminths.ANA, pANCA, SMA, SLA, LKM-1, AMA, IgG and IgM, ceruloplasmin, alpha-1-antitrypsin | Day 14; 125 mg methylprednisolone i.v., then prednisone 60 mg and tapered | Progressive improved on steroids. | 4 (rash = 1, eosinophilia = 1, liver involved = 1, other causes excluded = 1) |
WBC: white blood cells; AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; GGT: gamma-glutamyl transferase; INR: international normalized ratio; LT: Liver transplantation; i.v: intravenous; HAV: hepatitis A virus; HBV: hepatitis B virus; HCV, hepatitis C virus; HEV: hepatitis E virus; HSV: herpes simplex virus; VZV: varicella-zoster virus; CMV: cytomegalovirus; EBV: Epstein-Barr virus; HIV: human immunodeficiency virus; HHV: human herpes virus; ANA: antinuclear antibodies; SMA: smooth muscle antibodies; SLA: anti-soluble liver antigen antibodies; LKM-1: liver kidney microsome type 1 antibodies; pANCA: anti-neutrophil cytoplasmic antibodies; AMA: antimitochondrial antibodies; IgG: total immunoglobulin G; IgM: total immunoglobulin M
Empiric antibiotic therapy with piperacillin/tazobactam was initiated. Computed tomography (CT) scan was performed and showed interstitial pneumonia, bilateral pleural effusions, and ascites. The patient further deteriorated, worsening leukocytosis with 25.8 G/L (eosinophils: 10.6%) and CRP of 285 mg/L at day 5 of the antibiotic therapy. A transjugular liver biopsy was performed. The histology showed an extensive liver cell necrosis with marked eosinophilic infiltrates, indicating drug-induced hepatitis (Figure 1). The liver histology, the eosinophilia, and the persistence of fever despite antibiotic treatment raised the high suspicion of DRESS syndrome. RegiSCAR score was 4 (probable case). Therefore, piperacillin/tazobactam was discontinued after five days, and prednisone 1 mg/kg was started. The patient had a rapid clinical and laboratory improvement after initiation of the treatment. Interestingly, ten days after the reduction of steroids, the patient developed a non-pruritic, non-painful rash over the patient’s chest, abdomen, and back in parallel with fever and rising of the eosinophils (18 G/L). These findings were interpreted as a DRESS syndrome flare, so the dose of steroids was increased. Within the first 24 h, he ameliorated significantly. The patient was discharged after two weeks, and prednisone was further slowly tapered (by 5 mg per week). Repeated polymerase chain reactions (PCRs) in the follow-up showed no signs of HHV-6 or HHV-7 reactivation (12 days and 4 weeks). At follow-up, the patient normalized the entire laboratory tests and remained asymptomatic.
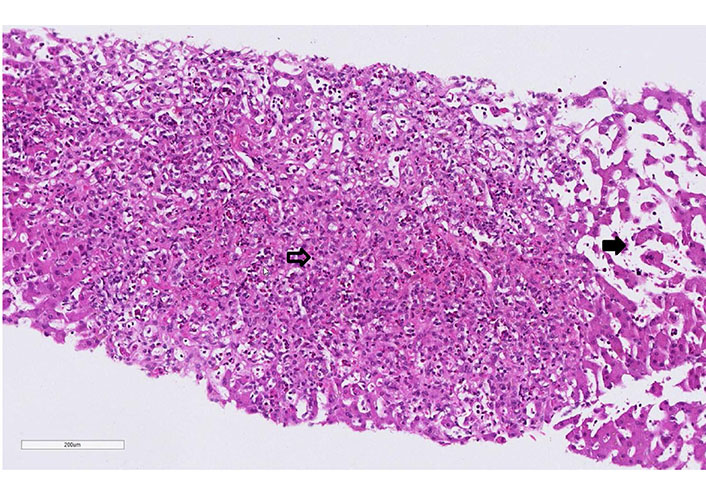
Case 1 liver biopsy: extensive centrilobular (zone 3 to 2) necrosis with fiber collapse and prominent eosinophilia (empty arrow). Presence of sinusoid dilatation (full arrow, hematoxylin-eosin, 20x)
A 61-year-old man with a history of polyarthritis under therapy with prednisone 5 mg and sulfasalazine 500 mg Tid was admitted due to persistent fever and rash. The patient reported that he had already suffered from fever and progressive asthenia 16 days before admission. At that time, he had received ambulant treatment with ciprofloxacin for suspected urinary tract infection. After five days of treatment, the fever persisted, and a diffuse rash appeared. The patient was admitted to the hospital, and treatment with amoxicillin/clavulanic and prednisone 25 mg was started. The workup showed leukocytosis, light eosinophilia without atypical lymphocytes. Liver tests were elevated and INR prolonged. Screening for autoimmune liver disease and standard virologic and parasitological tests were negative (Table 1). A Staphylococcus aureus grew in the blood culture and switched the antibiotic to vancomycin. The abdominal ultrasound did not show signs of liver or spleen enlargement. RegiSCAR score was 4 (probable case). On day 3 of hospitalization, the patient developed liver failure, and a transjugular liver biopsy was performed. The histology showed panacinar necrosis/apoptosis of the lobular zones 2 and 3, cholestasis, damage to the remaining hepatocyte population, lymphocytic and plasma cellular reactions in the portal fields with portal eosinophilic infiltrated. The findings were concluded as compatible with DRESS syndrome (Figure 2). Following clinical worsening, an urgent LT was performed. The biopsy of the explant confirmed the diagnosis of DRESS syndrome (Figure 3). The patient received treatment with prednisone, mycophenolate mofetil, and cyclosporine. The alteration of the hepatic profile persisted. A percutaneous liver biopsy was performed, which showed signs of rejection (Banff criteria: 6 points). Treatment with a high dose of methylprednisolone was started. Sixteen days after LT, the patient developed unspecific symptoms with neurologic impairment and metabolic acidosis. Within hours, he presented cardiorespiratory arrest that did not respond to cardiovascular resuscitation. The liver histology on the autopsy showed a pattern compatible with DRESS syndrome with moderated eosinophilic infiltrate and diffuse necrosis.
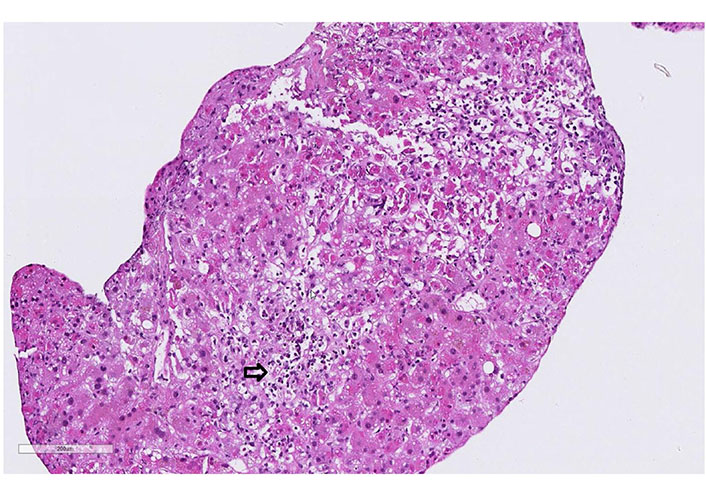
Case 2 liver biopsy: portal fields has expanded, due to a mixed cell infiltrate dominated by lymphocytes and eosinophils. Extensive interface lesions. Bile duct alterations and florid endothelial changes (arrow, hematoxylin-eosin, 20x)
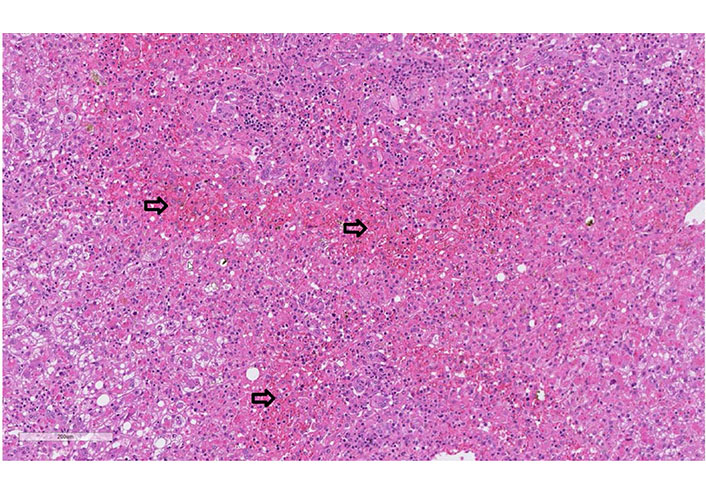
Case 2 biopsy of liver explant: liver explant (I; 1,764 g heavy) with extensive panacinar necrosis/apoptosis of the lobular zones 2 and 3 (especially pericentral, arrows), cholestasis, damage of the remaining hepatocyte population (with ballooning) as well as lymphocytic and plasmacellular reactions in the portal fields (hematoxylin-eosin, 20x)
A 60-year-old man was treated for years with phenytoin and levetiracetam for epilepsy. He was admitted to the hospital due to progressive asthenia, fever, cough, dark urine, rash, and severely elevated liver tests with clinical suspicion of DRESS syndrome. On admission, the patient was febrile (39.9°C), with tachycardia, and normal blood pressure (115/70 mmHg). A diffuse erythematous maculopapular rash was present on his face, upper torso, back, and upper and lower extremities without mucosal involvement. Cardiovascular, lungs, abdomen, and neurological examination were normal. No lymphadenopathy was palpable. The laboratory findings showed leukocytosis, eosinophilia, and atypical lymphocytes. Liver tests were elevated (Table 1). INR was prolonged, and factor V was in the normal range. The CRP was 134 mg/L. Toxicological screening, including paracetamol, was negative. Urine and blood cultures were repeatedly negative. Screening for autoimmune liver disorders, virologic and parasitological tests were negative (Table 1). Abdominal ultrasound was normal. A skin biopsy was performed and showed a superficial and deep perivascular dermatitis with interface changes and eosinophilic infiltration. The liver histology showed massive eosinophil infiltrates (Figure 4). RegiSCAR score was 6 (definite case). Levetiracetam was stopped, and the patient started 1 mg/kg prednisone daily. All the symptoms and laboratory findings rapidly improved. The patient was discharged with tapering prednisone therapy (by 10 mg weekly; at the 20 mg dose: reduction by 5 mg per week). During the follow-up, the patient remained asymptomatic.
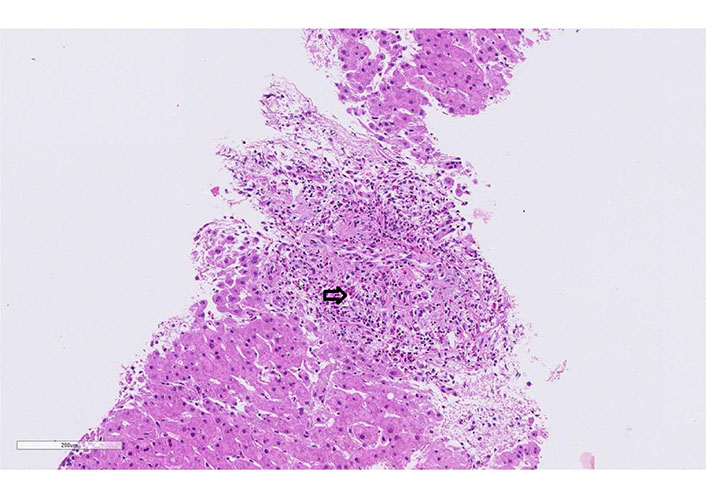
Case 3 liver biopsy: eosinophilic portal field infiltrate (arrow, hematoxylin-eosin, 20x)
A 62-year-old woman developed after 15 days with cefuroxime treatment (7 days) a diffuse maculopapular rash. The patient was admitted by clinical suspicion of DRESS syndrome (rash, elevated eosinophils, and abnormal liver test). At the moment of admission, the vital signs were under the normal range. Diffuse erythematous maculopapular rash was present on her upper torso, back, and upper and lower extremities. No mucosal involvement was described. Cardiovascular, lungs, abdomen, and neurological examination were normal. There was no palpable lymphadenopathy. The laboratory tests showed normal leukocytes, eosinophilia without atypical lymphocytes. Liver tests were elevated, but the liver function was preserved (Table 1). Urine and blood cultures were negative. The toxicological, virologic, and parasitological tests were negative. The autoimmune liver screening did not show abnormalities (Table 1). The sonographic findings of the abdomen were also normal. Liver biopsy demonstrated portal infiltrates with eosinophilic granulocytes, low portal edema, minor reactive changes in the bile duct, and focal accentuated canalicular cholestasis, and low predominantly microvesicular centrally emphasized steatosis (5–10% of hepatocytes, Figure 5). All these findings were compatible with DRESS syndrome. RegiSCAR score was 4 (probable case). Treatment with 40 mg daily of prednisone was started. All the symptoms and laboratory tests rapidly improved. The patient was discharged with gradual tapering of steroids therapy (by 5 mg per week). During the follow-up, she remained asymptomatic.
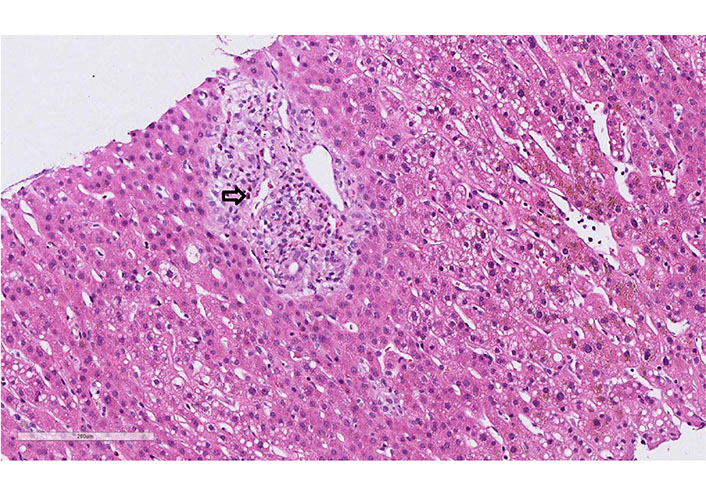
Case 4 liver biopsy: eosinophilic portal field infiltrate (arrow, hematoxylin-eosin, 20x)
A 65-year-old man was treated since February 2018 with lamotrigine and levetiracetam for structural epilepsy without proper compliance. In March 2019, the patient was admitted in the hospital after a car accident. During the hospitalization, the level of lamotrigine in serum was not detectable. Lamotrigine was reinitiated. After four weeks from discharge, the patient was readmitted, presenting a non-itching progressive exanthema for two weeks. At the moment of admission, the vital signs were under normal range. Diffuse erythematous maculopapular rash was present with swelling of lips, hands, and feet. Cardiovascular, lungs, abdomen, and neurological examination were normal. There was no palpable lymphadenopathy. The laboratory tests showed normal leukocytes, eosinophilia, and lymphocytosis. Liver tests were elevated, but the liver function was preserved. The toxicological, virologic, and parasitological tests were negative (Table 1). The abdominal ultrasound was normal. The patient was admitted by clinical suspicion of DRESS syndrome (rash, elevated eosinophils, and abnormal liver test). The RegiSCAR score was 4 (probable case). He received a single dose of prednisolone i.v. (125 mg) in the emergency room, then started treatment with 60 mg of prednisone daily. The patient was discharged with gradual tapering of steroids therapy (by 10 mg per week; at the 20 mg dose: reduction by 5 mg per week). The symptoms and laboratory tests progressively improved.
DRESS syndrome is an idiosyncratic adverse drug reaction characterized by a maculopapular rash, high fever, lymphadenopathy, visceral involvement, eosinophilia, and presence of atypical lymphocytes. An incidence ranging from 1/1,000 to 1/10,000 patients/year per drug exposures has been reported [16, 17]. The annual prevalence ranges from 2.8 [18] to 9.6 cases per 100,000 inpatients [13]. DRESS syndrome is a potentially fatal condition with mortality approximately of 10% [2, 12]. This syndrome is usually characterized by late-onset (up to 10 weeks after the first drug intake), but there have been described cases developing symptoms within first days after the drug exposure [14]. Flares after drug discontinuation have also been described [10]. Many drugs have been related to this syndrome and the most frequent associated are anticonvulsants, allopurinol, anti-tuberculosis drugs, antibiotics, sulfonamides, non-steroidal anti-inflammatory drugs (NSAIDs), and diuretics [2, 12, 14, 19–21]. The drugs involved in our cases were amoxicillin/clavulanic, phenytoin, levetiracetam, lamotrigine, ciprofloxacin, and cefuroxime. Multiple hypotheses have been proposed to explain the pathogenesis of DRESS syndrome, such as genetic predisposition in important genes for drug metabolism and reactivation of viral infections such as HHV-6 and 7, cytomegalovirus (CMV), Epstein-Barr virus (EBV) [2, 14, 22]. A study involving 100 patients demonstrated that HHV-6 viremia usually appears between day 10 to day 28, and detection of anti-HHV-6 in serum occurs simultaneously or later. HHV-6 reactivation was associated with poor prognosis [22]. Reactivation of CMV, EBV, and HHV-6 was ruled out by serological antibodies assay and quantitative real-time PCR within two weeks of onset symptoms in four patients in our series. HHV-7 was tested in two patients. Antibodies against HHV-6 and HHV-7 were detected by Immunofluorescence Assay.
One of the most frequent signs described in patients with DRESS syndrome is fever, up to 90% of recorded patients [2, 6, 14, 19], and usually precedes the skin rash. Pruritus could appear before the skin lesions [2, 6, 14, 19]. The most common cutaneous manifestation is the diffuse erythematous maculopapular rash (85% of cases) [19] and usually affects the face, trunk, and upper limbs, extending later to the rest of the body. Facial edema appears in 76% of cases [19]. Mucosal involvement is reported up to 50% of cases [6, 10, 19]. Four of our patients presented fever at the moment of diagnosis and maculopapular rash. One of the patients developed the cutaneous lesions after the diagnosis, during the tapering of the steroids, with recurrence of fever, leukocytosis, and eosinophilia, which was interpreted as DRESS syndrome flare. This episode was controlled with an increase in the dose of steroids. The detection of HHV-6 was performed by PCR at day 12 and 4 weeks after the onset of symptoms in our patient. The symptomatology during the flares is usually more severe and improves after the increase of the steroid dose [1, 10].
The most frequent hematological findings described in DRESS syndrome are leukocytosis (95%), eosinophilia (52–96%), lymphocytosis (25–52%), thrombocytosis (19%), thrombocytopenia (7–25%), and the presence of atypical lymphocytes (57–63%) [6, 12, 19]. Patients in our series only presented leukocytes and eosinophilia, and only one atypical lymphocytes without further hematological alteration. One of the patients had elevated levels of D-dimer, and thrombosis was ruled out. It has been reported that patients with DRESS syndrome may have elevated levels of plasma prothrombin fragments, but not D-dimer [23]. Several researchers have suggested a relationship between eosinophil activation, increased vascular permeability, and thrombin generation in these patients [23–25]. Another hematologic alteration described in these patients is hemophagocytic syndrome [9].
Different organs and systems could be affected by DRESS syndrome. Bocquet [1] described that visceral damage could be related to eosinophilia. Studies have described the involvement of a single organ in 35–75% [6, 13] of cases and two or more organs in 56% [6]. An association between the clinical pattern and the type of drug has been described [2, 6, 9, 19]. The liver is the most common organ involved (75–95%) [6, 12, 13, 20]. The spectrum of liver damage ranges from mild hepatitis to fulminant liver failure. Elevation of transaminases has been described in up to 90% of cases [10].
DRESS syndrome could be included in one of the phenotypes of drug-induced liver injury (DILI) [26]. Some authors recommend the DILI severity index to evaluate the degree of liver injury [14, 27]. In our series, two cases were moderate, one was severe, one was mild, and one was fatal, according to this index. The liver biopsy is not mandatory but may provide diagnostic information by matching known patterns of DILI [28, 29].
A retrospective multicenter study including 16 patients with severe acute liver damage described an overall survival of 75% and after liver transplantation of 60% [15]. Hepatic encephalopathy, Factor V level (≤ 40% or higher with a reduction at day 2), and prothrombin time (PT) were the predictors of poor prognosis. Five patients were transplanted, and 60% presented a recurrence of DRESS syndrome. Mean delay between LT and recurrence was 75 ± 91 days [15]. In our series, only two patients had prolongation of the INR and none Factor V below 40% or hepatic encephalopathy. Four patients evolved satisfactorily with medical treatment, and one patient received a liver transplant as rescue therapy and died 16 days after. The liver biopsy showed acute rejection (Banff 6) and no DRESS-compatible findings, although signs of recurrent DRESS syndrome were subsequently described in the autopsy. Despite liver transplantation represents the rescue therapy option in some severe cases, the recurrence of the disease is possible regardless of a high dose of immunosuppressants and may lead to graft loss or death.
Due to the diversity of clinical and laboratory features that DRESS syndrome patients may present, a scoring system denominated RegiSCAR score was created to facilitate the diagnosis. This scoring system classifies the cases as indefinite, probable, possible, or not based on the history of drug exposure and presence of rash, fever, organ dysfunction, enlarged adenopathy, and hematologic abnormalities [6]. The leucocyte transformation/activation test (LTT) could be used to confirm the causative drug of DRESS syndrome. Sensibility of 50% and specificity of 92% have been reported in the acute phase of DRESS syndrome [14]. However, both parameters improve dramatically when the test is performed in the recovery phase (4–8 weeks after the reaction). A positive LTT is useful to confirm the diagnosis due to the low rate of false-positive results (only 2%). However, a negative test cannot exclude the diagnosis [30].
The initial management of DRESS syndrome consists of the withdrawal of the causative drug. Steroids constitute the first therapy line in patients with hepatic manifestation (0.5–1 mg/kg daily tapered in 8–12 weeks) [14, 31]. The duration of the therapy depends on the severity of the symptoms and the organ involved but should be at least 2 to 3 months [19]. The recently Spanish DRESS syndrome Guidelines recommend in patients with a liver injury, start with oral methylprednisolone 60–120 mg/day or prednisone 40–60 mg/day and taper by 5–10 mg weekly [14]. In severe cases of hepatitis, human immunoglobulin could be added to the treatment [32]. Other immunosuppressants such as cyclosporine, mycophenolate mofetil, and rituximab have been used in patients who do not have an adequate response to steroids [14, 31]. All our patients received steroids treatment, four improved progressively, and one of them required urgent LT. Patients with DRESS syndrome and severe liver injury without liver transplantation have estimated mortality of 75% [15].
DRESS syndrome can present with heterogeneous manifestations and can lead to fulminant liver failure. Early recognition, withdrawal of the causative drug and steroids therapy could avoid irreversible damage of the liver. The response to treatment should be evaluated very strictly. The patients who do not show improvement of hepatic function should be referred to a tertiary center for eventual urgent liver transplantation.
AMA: antimitochondrial antibodies
ANA: antinuclear antibodies
CRP: C-reactive protein
CT: computerized tomography
CMV: cytomegalovirus
DILI: drug-induced liver injury
DRESS: drug rash with eosinophilia and systemic symptoms
EBV: Epstein-Barr virus
GGT: gamma-glutamyl transferase
HAV: hepatitis A virus
HBV: hepatitis B virus
HCV: hepatitis C virus
HEV: hepatitis E virus
HHV-6: human herpesvirus virus-6
HHV-7: human herpesvirus virus-7
HIV: human immunodeficiency virus
HSV: herpes simplex virus
IgG: total immunoglobulin G
IgM: total immunoglobulin M
INR: international normalized ratio
i.v.: intravenous
LKM-1: liver kidney microsome type 1 antibodies
LT: liver transplantation
NSAID: non-steroidal anti-inflammatory drugs
pANCA: anti-neutrophil cytoplasmic antibodies
PCRs: polymerase chain reactions
SLA: anti-soluble liver antigen antibodies
SMA: smooth muscle antibodies
VZV: varicella-zoster virus
The authors confirm contribution to the paper as follows: study conception and design: MGD, FB, AB, JFD. Data collection: MGD, FB, SC, MM. Analysis and interpretation of results: MGD, JFD, AB. Draft manuscript preparation: MGD, JFD, AB, SC, FB. All authors reviewed the results and approved the final version of the manuscript.
No author has any conflict of interest.
All patients provided written consent to the use of their health-related data for research. The hospital ethics committee approved collection of patient information and the study protocol, which was consistent with the principles of the current version of the Declaration of Helsinki, Good Clinical Practice (GCP) guidelines, and local regulatory requirements.
All patients provided written consent to the use of their health-related data for research.
Not applicable.
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Not applicable.
© The Author(s) 2021.
Copyright: © The Author(s) 2021. This is an Open Access article licensed under a Creative Commons Attribution 4.0 International License (https://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, sharing, adaptation, distribution and reproduction in any medium or format, for any purpose, even commercially, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
Maria Giulia Cornacchia ... Gaetano Serviddio
Alessandro Mantovani ... Andrea Dalbeni
Sheikh Mohammad Fazle Akbar ... Yoichi Hiasa
Amedeo Lonardo
Chunye Zhang, Ming Yang
Chunye Zhang ... Ming Yang
Katerina Delladetsima ... Stratigoula Sakellariou
